Abstract
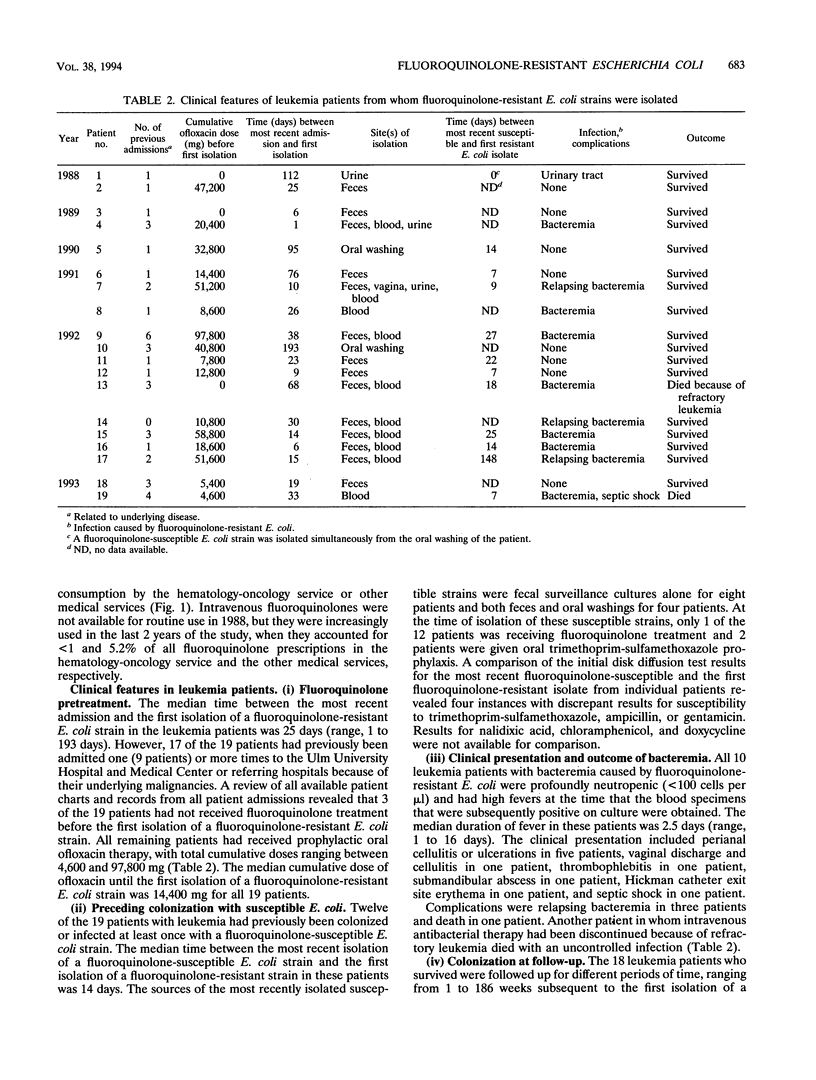
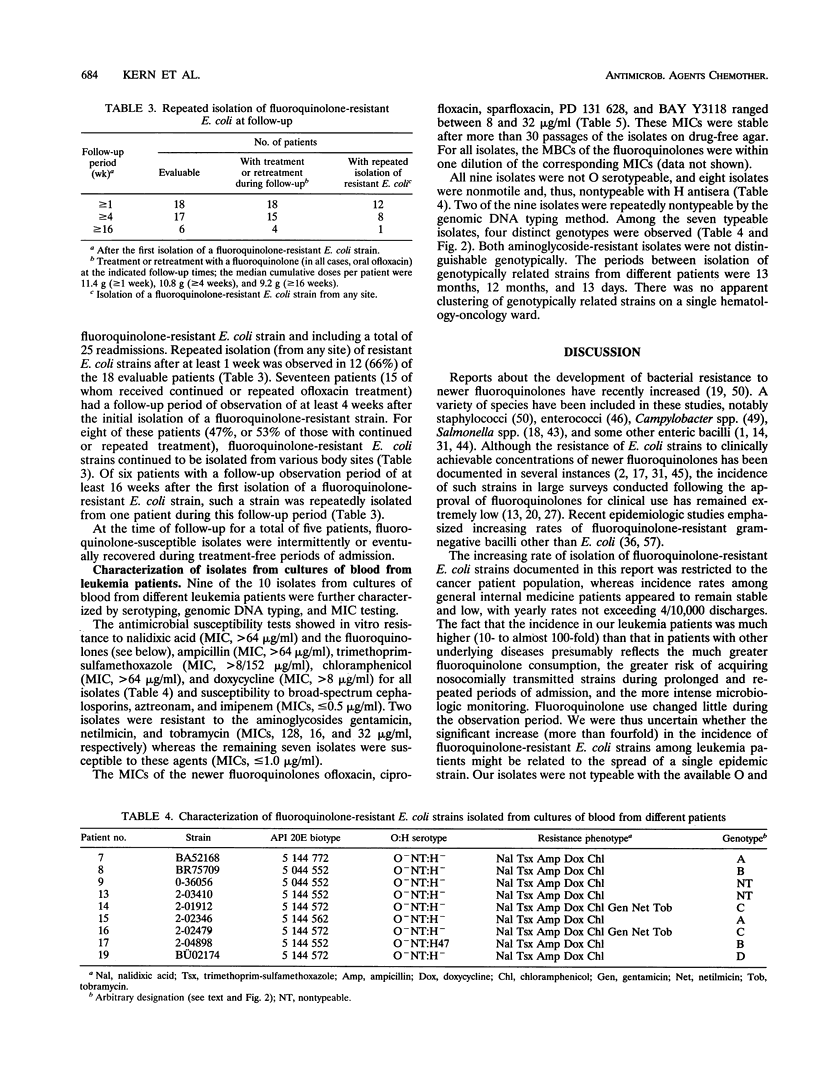
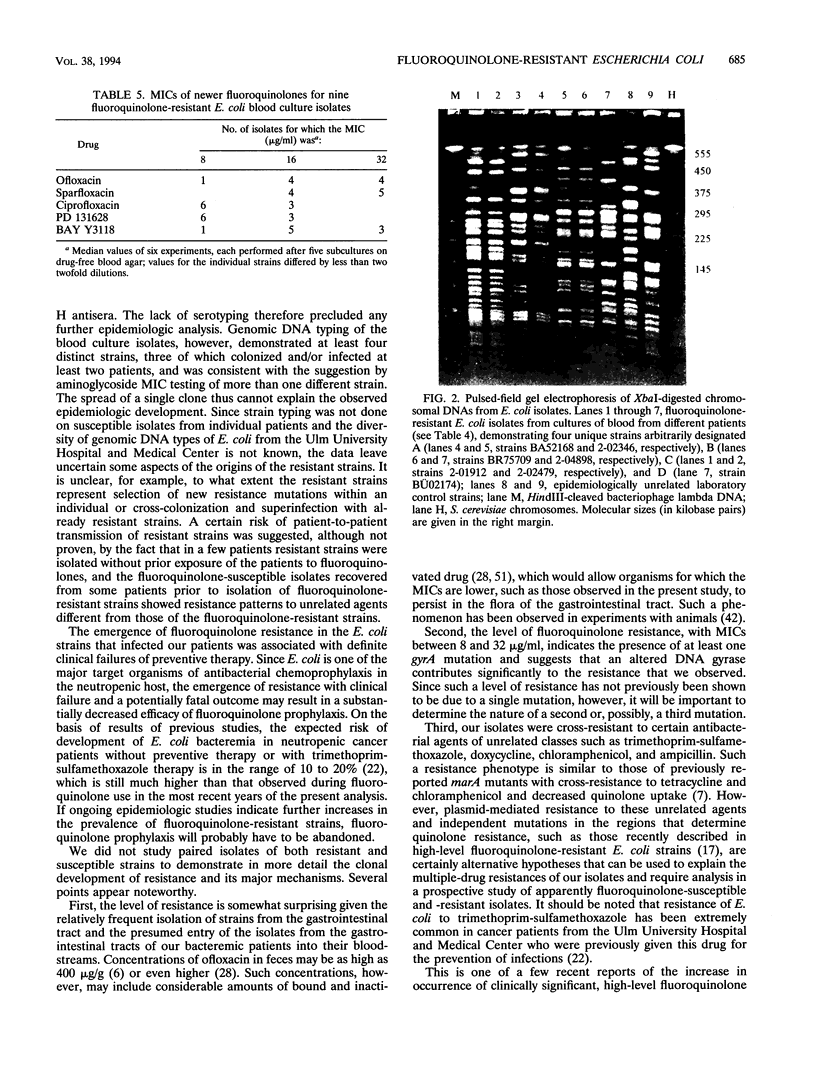
Prophylactic treatment with fluoroquinolones of patients with profound neutropenia has been found to be useful for preventing gram-negative bacteremia and has become a standard preventive-therapy strategy in many cancer centers, but the development of bacterial resistance is a cause of concern. During the past few years, we have observed an increasing number of patients with leukemia from whom fluoroquinolone-resistant strains of Escherichia coli were isolated. The increase was significant in this patient population, and among patients with other underlying diseases, the rates of isolation of such strains per number of discharges were significantly lower and did not increase. Most of the leukemia case patients (16 of 19) had been pretreated with an oral quinolone (ofloxacin), with cumulative doses until the first isolation of a resistant E. coli strain ranging from 0 to 97.8 g (median, 14.4 g). Repeated isolation of such strains was seen in 8 of 17 patients during a follow-up period of > or = 4 weeks and in 1 of 6 patients during a follow-up period of > or = 16 weeks. Ten patients developed bacteremia (mortality, 1 of 10). On the basis of the number of patients with leukemia admitted to the hematology-oncology service, the incidence of bacteremia caused by fluoroquinolone-resistant E. coli increased from < 0.5% in 1988-1989 and 0.8% in 1990-1991 to 4.5% in 1992-1993 (P < 0.01). MICs for nine isolates obtained from cultures of blood from different patients ranged between 8 and 16 microgram/ml (ciprofloxacin and PD 131628), 8 and 32 microgram/ml (ofloxacin and BAY Y 3118), and 16 and 32 microgram/ml (sparfloxacin) and indicated resistance to trimethoprim-sulfamethoxazole, ampicillin, doxycycline, and chloramphenicol. Of nine isolates obtained from cultures of blood from different patients and that were subjected to genomic DNA typing by pulsed-field gel electrophoresis of XbaI digests, seven were typeable. Among these, four different genotypes were identified, suggesting both the independent development and the horizontal spread of resistant clones of E. coli.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama H., Fujimaki K., Sato K., Fujii T., Inoue M., Hirai K., Mitsuhashi S. Clinical isolate of Citrobacter freundii highly resistant to new quinolones. Antimicrob Agents Chemother. 1988 Jun;32(6):922–924. doi: 10.1128/aac.32.6.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama H., Sato K., Kato T., Hirai K., Mitsuhashi S. Norfloxacin resistance in a clinical isolate of Escherichia coli. Antimicrob Agents Chemother. 1987 Oct;31(10):1640–1641. doi: 10.1128/aac.31.10.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arning M., Wolf H. H., Aul C., Heyll A., Scharf R. E., Scheider W. Infection prophylaxis in neutropenic patients with acute leukaemia--a randomized, comparative study with ofloxacin, ciprofloxacin and co-trimoxazole/colistin. J Antimicrob Chemother. 1990 Nov;26 (Suppl 500):137–142. doi: 10.1093/jac/26.suppl_d.137. [DOI] [PubMed] [Google Scholar]
- Cohen S. P., McMurry L. M., Hooper D. C., Wolfson J. S., Levy S. B. Cross-resistance to fluoroquinolones in multiple-antibiotic-resistant (Mar) Escherichia coli selected by tetracycline or chloramphenicol: decreased drug accumulation associated with membrane changes in addition to OmpF reduction. Antimicrob Agents Chemother. 1989 Aug;33(8):1318–1325. doi: 10.1128/aac.33.8.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciani M., Concia E., Navarra A., Perversi L., Bonetti F., Aricò M., Nespoli L. Prophylactic co-trimoxazole versus norfloxacin in neutropenic children--perspective randomized study. Infection. 1989 Mar-Apr;17(2):65–69. doi: 10.1007/BF01646878. [DOI] [PubMed] [Google Scholar]
- De Pauw B. E., Donnelly J. P., De Witte T., Nováková I. R., Schattenberg A. Options and limitations of long-term oral ciprofloxacin as antibacterial prophylaxis in allogeneic bone marrow transplant recipients. Bone Marrow Transplant. 1990 Mar;5(3):179–182. [PubMed] [Google Scholar]
- Dornbusch K. Resistance to beta-lactam antibiotics and ciprofloxacin in gram-negative bacilli and staphylococci isolated from blood: a European collaborative study. European Study Group on Antibiotic Resistance. J Antimicrob Chemother. 1990 Aug;26(2):269–278. doi: 10.1093/jac/26.2.269. [DOI] [PubMed] [Google Scholar]
- Fujimaki K., Fujii T., Aoyama H., Sato K., Inoue Y., Inoue M., Mitsuhashi S. Quinolone resistance in clinical isolates of Serratia marcescens. Antimicrob Agents Chemother. 1989 May;33(5):785–787. doi: 10.1128/aac.33.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano M., Pantosti A., Gentile G., Venditti M., Arcese W., Martino P. Effects on oral and intestinal microfloras of norfloxacin and pefloxacin for selective decontamination in bone marrow transplant patients. Antimicrob Agents Chemother. 1989 Oct;33(10):1709–1713. doi: 10.1128/aac.33.10.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisig P., Wiedemann B. Use of a broad-host-range gyrA plasmid for genetic characterization of fluoroquinolone-resistant gram-negative bacteria. Antimicrob Agents Chemother. 1991 Oct;35(10):2031–2036. doi: 10.1128/aac.35.10.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A. J., Joseph T. D., Bloodworth L. L., Frost J. A., Chart H., Rowe B. The emergence of ciprofloxacin resistance in Salmonella typhimurium. J Antimicrob Chemother. 1990 Aug;26(2):296–298. doi: 10.1093/jac/26.2.296. [DOI] [PubMed] [Google Scholar]
- Jones R. N. Fluoroquinolone resistance. An evolving national problem or just a problem for some physicians? Diagn Microbiol Infect Dis. 1992 Feb;15(2):177–179. doi: 10.1016/0732-8893(92)90046-v. [DOI] [PubMed] [Google Scholar]
- Kern W., Kurrle E., Schmeiser T. Streptococcal bacteremia in adult patients with leukemia undergoing aggressive chemotherapy. A review of 55 cases. Infection. 1990 May-Jun;18(3):138–145. doi: 10.1007/BF01642101. [DOI] [PubMed] [Google Scholar]
- Kern W., Linzmeier K., Kurrle E. Antimicrobial susceptibility of viridans group streptococci isolated from patients with acute leukemia receiving ofloxacin for antibacterial prophylaxis. Infection. 1989 Nov-Dec;17(6):396–397. doi: 10.1007/BF01645556. [DOI] [PubMed] [Google Scholar]
- Kotilainen P., Nikoskelainen J., Huovinen P. Emergence of ciprofloxacin-resistant coagulase-negative staphylococcal skin flora in immunocompromised patients receiving ciprofloxacin. J Infect Dis. 1990 Jan;161(1):41–44. doi: 10.1093/infdis/161.1.41. [DOI] [PubMed] [Google Scholar]
- Kresken M., Wiedemann B. Development of resistance to nalidixic acid and the fluoroquinolones after the introduction of norfloxacin and ofloxacin. Antimicrob Agents Chemother. 1988 Aug;32(8):1285–1288. doi: 10.1128/aac.32.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh D. A., Walsh B., Harris K., Hancock P., Travers G. Pharmacokinetics of ofloxacin and the effect on the faecal flora of healthy volunteers. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):115–125. doi: 10.1093/jac/22.supplement_c.115. [DOI] [PubMed] [Google Scholar]
- Liang R. H., Yung R. W., Chan T. K., Chau P. Y., Lam W. K., So S. Y., Todd D. Ofloxacin versus co-trimoxazole for prevention of infection in neutropenic patients following cytotoxic chemotherapy. Antimicrob Agents Chemother. 1990 Feb;34(2):215–218. doi: 10.1128/aac.34.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschmeyer G., Haralambie E., Gaus W., Kern W., Dekker A. W., De Vries-Hospers H. G., Sizoo W., König W., Gutzler F., Daenen S. Ciprofloxacin and norfloxacin for selective decontamination in patients with severe granulocytopenia. Infection. 1988 Mar-Apr;16(2):98–104. doi: 10.1007/BF01644312. [DOI] [PubMed] [Google Scholar]
- Menichetti F., Felicini R., Bucaneve G., Aversa F., Greco M., Pasquarella C., Moretti M. V., Del Favero A., Martelli M. F. Norfloxacin prophylaxis for neutropenic patients undergoing bone marrow transplantation. Bone Marrow Transplant. 1989 Sep;4(5):489–492. [PubMed] [Google Scholar]
- Moniot-Ville N., Guibert J., Moreau N., Acar J. F., Collatz E., Gutmann L. Mechanisms of quinolone resistance in a clinical isolate of Escherichia coli highly resistant to fluoroquinolones but susceptible to nalidixic acid. Antimicrob Agents Chemother. 1991 Mar;35(3):519–523. doi: 10.1128/aac.35.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muder R. R., Brennen C., Goetz A. M., Wagener M. M., Rihs J. D. Association with prior fluoroquinolone therapy of widespread ciprofloxacin resistance among gram-negative isolates in a Veterans Affairs medical center. Antimicrob Agents Chemother. 1991 Feb;35(2):256–258. doi: 10.1128/aac.35.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim B. A., Hartley J. W., Lee W., Burnie J. P. Outbreak of coagulase negative staphylococcus highly resistant to ciprofloxacin in a leukaemia unit. BMJ. 1989 Jul 29;299(6694):294–297. doi: 10.1136/bmj.299.6694.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi E., Navarra A., Cruciani M., Caldera D., Morra E., Castagnola C., Concia E., Bernasconi C. Norfloxacin versus cotrimoxazole for infection prophylaxis in granulocytopenic patients with acute leukemia. A prospective randomized study. Haematologica. 1990 May-Jun;75(3):296–298. [PubMed] [Google Scholar]
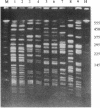
- Ott M., Bender L., Blum G., Schmittroth M., Achtman M., Tschäpe H., Hacker J. Virulence patterns and long-range genetic mapping of extraintestinal Escherichia coli K1, K5, and K100 isolates: use of pulsed-field gel electrophoresis. Infect Immun. 1991 Aug;59(8):2664–2672. doi: 10.1128/iai.59.8.2664-2672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecquet S., Andremont A., Tancrède C. Selective antimicrobial modulation of the intestinal tract by norfloxacin in human volunteers and in gnotobiotic mice associated with a human fecal flora. Antimicrob Agents Chemother. 1986 Jun;29(6):1047–1052. doi: 10.1128/aac.29.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddock L. J., Griggs D. J., Hall M. C., Jin Y. F. Ciprofloxacin resistance in clinical isolates of Salmonella typhimurium obtained from two patients. Antimicrob Agents Chemother. 1993 Apr;37(4):662–666. doi: 10.1128/aac.37.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Watanakunakorn C. Emergence of resistance to beta-lactams, aminoglycosides, and quinolones during combination therapy for infection due to Serratia marcescens. J Infect Dis. 1986 Mar;153(3):617–619. doi: 10.1093/infdis/153.3.617. [DOI] [PubMed] [Google Scholar]
- Sato K., Inoue Y., Fujii T., Aoyama H., Inoue M., Mitsuhashi S. Purification and properties of DNA gyrase from a fluoroquinolone-resistant strain of Escherichia coli. Antimicrob Agents Chemother. 1986 Nov;30(5):777–780. doi: 10.1128/aac.30.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg D. R., Dillon W. I., Terpenning M. S., Robinson K. A., Bradley S. F., Kauffman C. A. Increasing resistance of enterococci to ciprofloxacin. Antimicrob Agents Chemother. 1992 Nov;36(11):2533–2535. doi: 10.1128/aac.36.11.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeiser T., Kern W. V., Hay B., Hertenstein B., Arnold R. Single-drug oral antibacterial prophylaxis with ofloxacin in BMT recipients. Bone Marrow Transplant. 1993 Jul;12(1):57–63. [PubMed] [Google Scholar]
- Schmeiser T., Kurrle E., Arnold R., Wiesneth M., Bunjes D., Hertenstein B., Kern W., Heit W., Heimpel H. Norfloxacin for prevention of bacterial infections during severe granulocytopenia after bone marrow transplantation. Scand J Infect Dis. 1988;20(6):625–631. doi: 10.3109/00365548809035663. [DOI] [PubMed] [Google Scholar]
- Segreti J., Gootz T. D., Goodman L. J., Parkhurst G. W., Quinn J. P., Martin B. A., Trenholme G. M. High-level quinolone resistance in clinical isolates of Campylobacter jejuni. J Infect Dis. 1992 Apr;165(4):667–670. doi: 10.1093/infdis/165.4.667. [DOI] [PubMed] [Google Scholar]
- Trucksis M., Hooper D. C., Wolfson J. S. Emerging resistance to fluoroquinolones in staphylococci: an alert. Ann Intern Med. 1991 Mar 1;114(5):424–426. doi: 10.7326/0003-4819-114-5-424. [DOI] [PubMed] [Google Scholar]
- Venditti M., Baiocchi P., Santini C., Brandimarte C., Serra P., Gentile G., Girmenia C., Martino P. Antimicrobial susceptibilities of Streptococcus species that cause septicemia in neutropenic patients. Antimicrob Agents Chemother. 1989 Apr;33(4):580–582. doi: 10.1128/aac.33.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti M., Santini C., Serra P., Micozzi A., Gentile G., Martino P. Comparative in vitro activities of new fluorinated quinolones and other antibiotics against coagulase-negative Staphylococcus blood isolates from neutropenic patients, and relationship between susceptibility and slime production. Antimicrob Agents Chemother. 1989 Feb;33(2):209–211. doi: 10.1128/aac.33.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Guiney D. G. Failure of oral trimethoprim-sulfamethoxazole prophylaxis in acute leukemia: isolation of resistant plasmids from strains of Enterobacteriaceae causing bacteremia. N Engl J Med. 1982 Jan 7;306(1):16–20. doi: 10.1056/NEJM198201073060105. [DOI] [PubMed] [Google Scholar]
- Wimperis J. Z., Baglin T. P., Marcus R. E., Warren R. E. An assessment of the efficacy of antimicrobial prophylaxis in bone marrow autografts. Bone Marrow Transplant. 1991 Nov;8(5):363–367. [PubMed] [Google Scholar]
- Winston D. J., Ho W. G., Bruckner D. A., Gale R. P., Champlin R. E. Ofloxacin versus vancomycin/polymyxin for prevention of infections in granulocytopenic patients. Am J Med. 1990 Jan;88(1):36–42. doi: 10.1016/0002-9343(90)90125-w. [DOI] [PubMed] [Google Scholar]
- Yee Y. C., Muder R. R., Hsieh M. H., Lee T. C. Molecular epidemiology of endemic ciprofloxacin-susceptible and -resistant Enterobacteriaceae. Infect Control Hosp Epidemiol. 1992 Dec;13(12):706–710. doi: 10.1086/648343. [DOI] [PubMed] [Google Scholar]
- Young L. S. Antimicrobial prophylaxis in the neutropenic host: lessons of the past and perspectives for the future. Eur J Clin Microbiol Infect Dis. 1988 Feb;7(1):93–97. doi: 10.1007/BF01962191. [DOI] [PubMed] [Google Scholar]
- van Saene J. J., van Saene H. K., Lerk C. F. Inactivation of quinolone by feces. J Infect Dis. 1986 May;153(5):999–1000. doi: 10.1093/infdis/153.5.999. [DOI] [PubMed] [Google Scholar]