Abstract
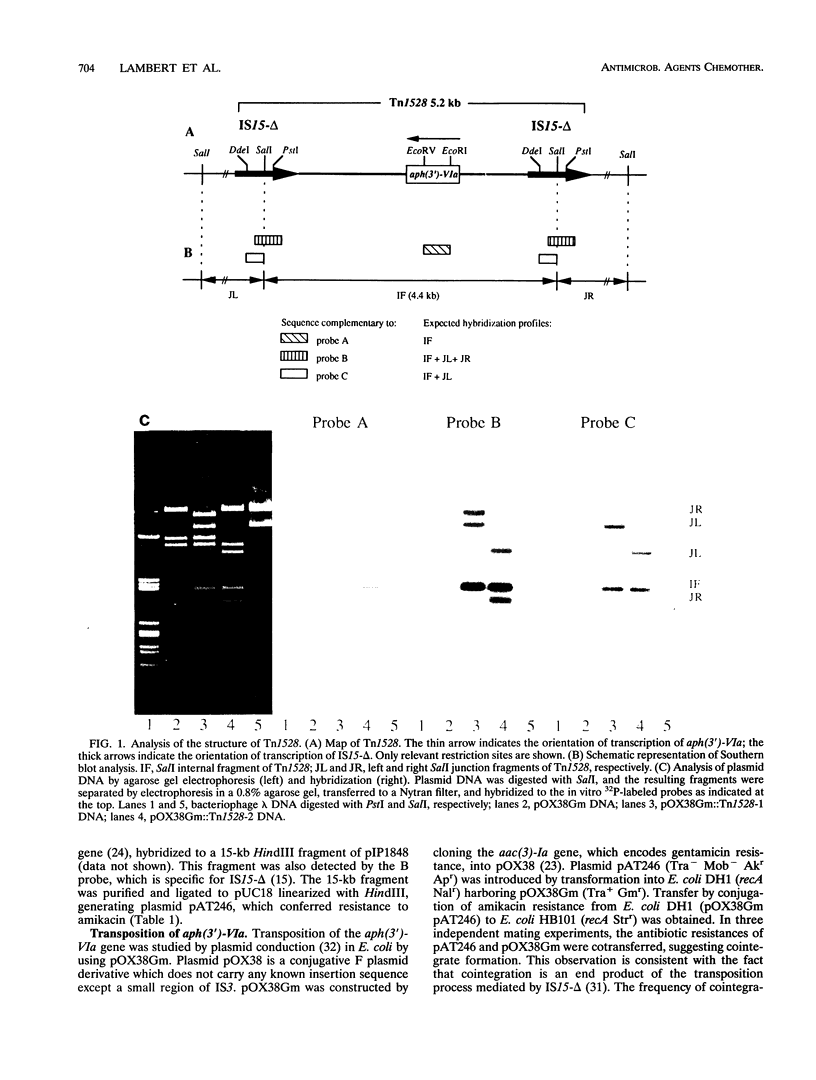
Providencia stuartii BM2667, which was isolated from an abdominal abscess, was resistant to amikacin by synthesis of aminoglycoside 3'-O-phosphotransferase type VI. The corresponding gene, aph(3')-VIa, was carried by a 30-kb self-transferable plasmid of incompatibility group IncN. The resistance gene was cloned into pUC18, and the recombinant plasmid, pAT246, was transformed into Escherichia coli DH1 (recA) harboring pOX38Gm. The resulting clones were mixed with E. coli HB101 (recA), and transconjugants were used to transfer pAT246 by plasmid conduction to E. coli K802N (rec+). Analysis of plasmid DNAs from the transconjugants of K802N by agarose gel electrophoresis and Southern hybridization indicated the presence of a transposon, designated Tn1528, in various sites of pOX38Gm. This 5.2-kb composite element consisted of aph(3')-VIa flanked by two direct copies of IS15-delta and transposed at a frequency of 4 x 10(-5). It therefore appears that IS15-delta, an insertion sequence widely spread in gram-negative bacteria, is likely responsible for dissemination to members of the family Enterobacteriaceae of aph(3')-VIa, a gene previously confined to Acinetobacter spp.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D. E., Davies J., Allet B., Rochaix J. D. Transposition of R factor genes to bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3628–3632. doi: 10.1073/pnas.72.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Coupland G. M., Willetts N. S. Characterization of IS46, an insertion sequence found on two IncN plasmids. J Bacteriol. 1984 Aug;159(2):472–481. doi: 10.1128/jb.159.2.472-481.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräu B., Piepersberg W. Cointegrational transduction and mobilization of gentamicin resistance plasmid pWP14a is mediated by IS140. Mol Gen Genet. 1983;189(2):298–303. doi: 10.1007/BF00337820. [DOI] [PubMed] [Google Scholar]
- Chabbert Y. A., Scavizzi M. R., Witchitz J. L., Gerbaud G. R., Bouanchaud D. H. Incompatibility groups and the classification of fi - resistance factors. J Bacteriol. 1972 Nov;112(2):666–675. doi: 10.1128/jb.112.2.666-675.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Chandler M. Structure and stability of Tn9-mediated cointegrates. Evidence for two pathways of transposition. J Mol Biol. 1982 Jan 15;154(2):245–272. doi: 10.1016/0022-2836(82)90063-8. [DOI] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Iida S., Meyer J., Linder P., Goto N., Nakaya R., Reif H. J., Arber W. The kanamycin resistance transposon Tn2680 derived from the R plasmid Rts1 and carried by phage P1Km has flanking 0.8-kb-long direct repeats. Plasmid. 1982 Sep;8(2):187–198. doi: 10.1016/0147-619x(82)90056-7. [DOI] [PubMed] [Google Scholar]
- Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972 Nov;112(2):917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A., Courvalin P. IS15, a new insertion sequence widely spread in R plasmids of gram-negative bacteria. Mol Gen Genet. 1983;189(1):102–112. doi: 10.1007/BF00326061. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A., Gerbaud G., Courvalin P. Translocation of sequences encoding antibiotic resistance from the chromosome to a receptor plasmid in Salmonella ordonez. Mol Gen Genet. 1981;182(3):390–408. doi: 10.1007/BF00293927. [DOI] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Bouvet P., Vieu J. F., Courvalin P. Dissemination of amikacin resistance gene aphA6 in Acinetobacter spp. Antimicrob Agents Chemother. 1990 Jun;34(6):1244–1248. doi: 10.1128/aac.34.6.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Courvalin P. Transferable amikacin resistance in Acinetobacter spp. due to a new type of 3'-aminoglycoside phosphotransferase. Antimicrob Agents Chemother. 1988 Jan;32(1):15–19. doi: 10.1128/aac.32.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Galimand M., Courvalin P. Characterization of Acinetobacter haemolyticus aac(6')-Ig gene encoding an aminoglycoside 6'-N-acetyltransferase which modifies amikacin. Antimicrob Agents Chemother. 1993 Oct;37(10):2093–2100. doi: 10.1128/aac.37.10.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg E. M. Plasmid reference center registry of transposon (Tn) allocations through July 1981. Gene. 1981 Dec;16(1-3):59–61. doi: 10.1016/0378-1119(81)90060-3. [DOI] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabilat C., Courvalin P. Development of "oligotyping" for characterization and molecular epidemiology of TEM beta-lactamases in members of the family Enterobacteriaceae. Antimicrob Agents Chemother. 1990 Nov;34(11):2210–2216. doi: 10.1128/aac.34.11.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris J. C., Nordmann P. L., Reznikoff W. S. Mutational analysis of insertion sequence 50 (IS50) and transposon 5 (Tn5) ends. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2224–2228. doi: 10.1073/pnas.85.7.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Jullien E., Courvalin P. Nucleotide sequence of Acinetobacter baumannii aphA-6 gene: evolutionary and functional implications of sequence homologies with nucleotide-binding proteins, kinases and other aminoglycoside-modifying enzymes. Mol Microbiol. 1988 Sep;2(5):615–625. doi: 10.1111/j.1365-2958.1988.tb00070.x. [DOI] [PubMed] [Google Scholar]
- Mollet B., Iida S., Shepherd J., Arber W. Nucleotide sequence of IS26, a new prokaryotic mobile genetic element. Nucleic Acids Res. 1983 Sep 24;11(18):6319–6330. doi: 10.1093/nar/11.18.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies B. A., Meyer J. F., Wiedemann B. Tn2440, a composite tetracycline resistance transposon with direct repeated copies of IS160 at its flanks. J Gen Microbiol. 1985 Sep;131(9):2443–2447. doi: 10.1099/00221287-131-9-2443. [DOI] [PubMed] [Google Scholar]
- Roussel A. F., Chabbert Y. A. Taxonomy and epidemiology of gram-negative bacterial plasmids studied by DNA-DNA filter hybridization in formamide. J Gen Microbiol. 1978 Feb;104(2):269–276. doi: 10.1099/00221287-104-2-269. [DOI] [PubMed] [Google Scholar]
- Shaw K. J., Hare R. S., Sabatelli F. J., Rizzo M., Cramer C. A., Naples L., Kocsi S., Munayyer H., Mann P., Miller G. H. Correlation between aminoglycoside resistance profiles and DNA hybridization of clinical isolates. Antimicrob Agents Chemother. 1991 Nov;35(11):2253–2261. doi: 10.1128/aac.35.11.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K. J., Rather P. N., Hare R. S., Miller G. H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993 Mar;57(1):138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu-Cuot P., Courvalin P. Nucleotide sequence of the transposable element IS15. Gene. 1984 Oct;30(1-3):113–120. doi: 10.1016/0378-1119(84)90111-2. [DOI] [PubMed] [Google Scholar]
- Trieu-Cuot P., Courvalin P. Transposition behavior of IS15 and its progenitor IS15-delta: are cointegrates exclusive end products? Plasmid. 1985 Jul;14(1):80–89. doi: 10.1016/0147-619x(85)90034-4. [DOI] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- Wrighton C. J., Strike P. A pathway for the evolution of the plasmid NTP16 involving the novel kanamycin resistance transposon Tn4352. Plasmid. 1987 Jan;17(1):37–45. doi: 10.1016/0147-619x(87)90006-0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]




