Abstract
Background
Many novel therapeutics have failed to reduce all-cause mortality associated with severe sepsis. Eukaryotic translation initiation factor 5A (eIF5A) is a regulator of apoptosis as well as inflammatory cell activation, making it a potential target for sepsis therapy.
Methods
In a murine model of severe sepsis, mice were intraperitoneally challenged with lipopolysaccharide (LPS). Mice were treated both before and after LPS challenge with liposome complexes containing either an eIF5A-specific or control small interference RNA (siRNA), and both survival and serum concentrations of inflammatory cytokines were monitored. The ability of eIF5A siRNA to reduce inflammatory cytokines was also tested in a model of acute lung injury established by intranasal administration of LPS to mice.
Results
There was a statistically significant increase in the rate of survival for mice intraperitoneally challenged with LPS that received eIF5A siRNA, compared with that noted for mice that received control siRNA (71% vs. 5%; P < .001), as well as a reduction in cytokine expression in serum. Concentrations of proinflammatory cytokines were also reduced in the lung homogenates and serum of mice that were intranasally challenged with LPS and received eIF5A siRNA (P ≤ .05).
Conclusions
eIF5A siRNA-liposome complexes reduced inflammation and contributed to increased survival in a model of severe sepsis, decreased inflammation in a model of acute lung injury, and should be considered for clinical use.
Approximately 900,000 cases of sepsis occur annually in the United States, causing roughly 210,000 deaths and resulting in costs of almost 17 billion dollars [1]. Sepsis is characterized by an initial hyperinflammatory state, which is followed by a hypoinflammatory, immunosuppressive state during which apoptosis occurs [2]. Because of the dearth of therapeutics for sepsis, new strategies and tools are needed for sepsis management.
Polyamines are important mediators of inflammation [3], and studies have indicated that polyamine biosynthesis is increased during sepsis [4, 5]. Although the functions of the up-regulated polyamines during sepsis and inflammation are not clear, we hypothesize that polyamine levels may increase during inflammation to help fuel hypusine biosynthesis, which is a polyamine-dependent reaction. Eukaryotic translation initiation factor 5A (eIF5A) is an abundant, constitutively expressed protein, and it is the only known protein to contain the unique amino acid hypusine.
The hypusine residue is formed posttranslationally in a 2-step process that results in the mature, active form of eIF5A. The first step, catalyzed by deoxyhypusine synthase (DHS), involves the transfer of a butylamine group from the polyamine spermidine to a conserved lysine on eIF5A [6]. The second hydroxylation step, which is mediated by deoxyhypusine hydroxylase (DOHH), results in the formation of the mature hypusine-modified eIF5A [7]. Inhibitors of DHS and DOHH that block the hypusination reaction have been found to have anti-inflammatory properties [8, 9]. eIF5A is widely believed to have a function in regulated mRNA transport [10–12], protein translation [13, 14], and cell proliferation [15–21]. It also appears to be involved in the activation and/or proliferation of T lymphocytes [22] and seems to be required for the maturation and function of dendritic cells [23], thereby suggesting that eIF5A may be an important target in inflammatory diseases.
eIF5A also has an important role in apoptosis [24–26], and small interference RNA (siRNA) targeting eIF5A can protect cells from apoptosis caused by tumor necrosis factor (TNF)–α [24] and genotoxic stress [26], thus making it a potential target for sepsis therapy. Many studies have identified apoptosis, particularly apoptosis of lymphocytes, as a risk factor for death due to sepsis [2, 27, 28]. Recent therapeutic strategies have used siRNA to knock down the genes that are involved in apoptosis and up-regulated during sepsis. For example, Fas ligand and caspases have been targeted in mice with sepsis, resulting in increased survival [29]. To determine whether a reduction in eIF5A expression may have anti-inflammatory effects, an siRNA targeting eIF5A was tested in murine models of severe sepsis and acute lung injury, and the effects on proinflammatory cytokine production and animal survival were monitored.
MATERIALS AND METHODS
Mice
Female C57BL/6 and BALB/c mice (body weight, ≈20 g; Jackson Laboratories) were housed at 68°F–72°F with a 12-h light/dark cycle, fed standard laboratory food and water ad libitum, and kept under specific pathogen-free conditions. The protocols used in the present study were approved by the animal care and use committees of the University of Virginia and the University of Colorado.
Reagents and Drugs
Lipopolysaccharide (Escherichia coli O111:B4) was purchased from Sigma. 1,2-dioleoyl-3-trimethylammonium propane (DOTAP) was obtained from Roche Diagnostics. Ketamine and xylazine were purchased from Vedco. In all cases, the vehicle used was PBS.
siRNA
The siRNAs used in the present study were synthesized by Dharmacon. An siRNA directed against mouse eIF5A (Gen-Bank accession no. NM 181582) suppresses eIF5A expression in the mouse cell line L929 (R. Fayad, G. Fantuzzi, L. L. Reznikov, C. A. Taylor, J. E. Thompson, and C. A. Dinarello, unpublished data). The target sequence for the eIF5A siRNA was 5'-AACGGAATGACTTCCAGCTGA-3'. A search of BLAST (the Basic Local Alignment Search Tool) revealed no mouse transcripts with significant homology to the eIF5A siRNA. A control siRNA with the reverse sequence of the eIF5A-specific siRNA, which does not target eIF5A or any other known mouse gene product, was also synthesized by Dharmacon. The target sequence for the control siRNA was 5'-AAAGTCGACCTTCAGTAAGGC-3'.
The Severe Sepsis Model: Intraperitoneal Challenge with LPS
Rationale
DOTAP:siRNA complexes delivered by intraperitoneal injection are able to efficiently transfect murine peritoneal macrophages [30, 31], and anti-TNF siRNAs delivered using this method are able to protect mice from LPS-induced septic shock [30]. Interleukin (IL)–1 siRNA delivered in a complex with DOTAP also transfected cultured murine alveolar macrophages and reduced liver-mediated pulmonary inflammation after intraperitoneal delivery [32]. In the present study, the effect of eIF5A siRNA:DOTAP complexes on inflammation was determined in a murine model of sepsis.
Survival
Inoculation of C57BL/6 and BALB/c mice with intraperitoneally administered E. coli O111:B4 LPS (25 mg/kg and 5 mg/kg, respectively) caused death among 95% of controls. Animals received either eIF5A siRNA- (n = 78) or control siRNA-liposome complexes (n = 20). A 50-µg dose of eIF5A siRNA was given intraperitoneally in conjunction with 100 µg of transfection micelle comprising sterile DOTAP at a volume of 1 mL per mouse. The timing of siRNA-liposome complex dosing varied for the subsets of animals tested. To assess differential survival between treatment groups, animals were followed until death, but they were not euthanized unless clearly moribund, and then euthanasia was performed for ethical reasons.
Cytokine quantification after intraperitoneal challenge with LPS
In cytokine quantification experiments, the eIF5A and control siRNA were dosed at t = −48 h and t = −24 h before administration of LPS. Mice were euthanized at 90 min or 8 h after LPS administration, and blood samples were obtained via intracardiac puncture. The timing of sample collection allowed evaluation of early- and late-appearing cytokines. A protein bead–based multiplex immunoassay system (Bio-Rad Laboratories) used flow cytometry technology to quantify serum cytokines. We measured IL-1α and IL-1β, TNF-α, interferon (IFN)–γ, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12 p40 and p70, IL-17, granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage inflammatory protein (MIP)–1α, and RANTES (regulated on activation, normally T cell expressed and secreted). The selection of these analytes allowed for the evaluation of early- and late-appearing, as well as proinflammatory and anti-inflammatory, cytokines.
Comparison of eIF5A siRNA with dexamethasone
Mice were treated with dexamethasone (10 mg/kg) administered intraperitoneally; 1 h later, they received a nonlethal intraperitoneally administered dose of LPS (3.75 mg/kg). Blood samples were then obtained 90 min later. For comparison, mice were treated intraperitoneally with 50 µg of control or eIF5A siRNA, and, after 24 h, LPS was injected intraperitoneally. Ninety minutes after LPS administration, all mice were euthanized, and blood samples were obtained for measurement of the TNF-α concentration.
The Acute Lung Injury Model: Intranasal Challenge with LPS
Rationale
Intranasal administration of uncomplexed siRNA has been shown to have good uptake in the lung tissue of mice [33, 34], and it has been used as an antiviral agent against respiratory viruses [34]. In the present study, intranasally administered eIF5A siRNA was evaluated for its ability to suppress inflammatory markers in mice challenged with intranasal LPS.
Measurement of inflammation after intranasal challenge with LPS
Once the animals were anesthetized with isoflurane, 50 µL of a solution containing 50 µg of eIF5A or control siRNA was applied to the external nares of C57BL/6 mice until the solution was inhaled. Forty-eight h later, the mice were again anesthetized, and 75 µg of LPS (3.75 mg/kg) was similarly instilled by use of the external nares route. For one experiment, to assess the effect of systemically administered eIF5A on pulmonary inflammation, eIF5A or control siRNA was delivered intraperitoneally before intranasal challenge with LPS. Twenty-four h after LPS challenge, mice were euthanized by inhalation of isoflurane, followed by cervical dislocation, and the lungs and/or blood was removed.
Both lungs were minced and placed in a homogenizer (Tissue-Tearor; BioSpec Products) with 1 mL of ice-cold extraction buffer containing 20 mmol/L HEPES (pH 7.4), 20 mmol/L glycerolphosphate, 20 mmol/L sodium pyrophosphate, 0.2 mmol/L Na3VO4, 2 mmol/L EDTA, 20 mmol/L sodium fluoride, 10 mmol/L benzamidine, 1 mmol/L dithiothreitol, 20 ng/mL leupeptin, 0.4 mmol/L Pefabloc SC, and 0.02% Tween. Lung homogenates were divided into 2 sets of aliquots. One aliquot was incubated at room temperature for 1 h; a second aliquot was incubated with 0.5% (vol/vol) Triton X-100, and all samples were frozen at −70°C
Myeloperoxidase was measured as described elsewhere [35]. For cytokine assays, the homogenates were thawed at room temperature and then were centrifuged at 14,000 g at 4°C for 15 min. The supernatant was collected, and the protein concentration was determined using the bicinchoninic acid assay (Pierce). Data were expressed as the number of cytokines per milliliter of homogenate or per milligram of protein. Both methods revealed the same differences.
We compared lung concentrations of TNF-α after administration of LPS with or without Triton X-100 extraction, which lyses membranes releasing protein components. The TNF-α concentration was significantly higher when the Triton extraction method was used (data not shown). Therefore, after homogenization with Triton, lung tissue was assayed for TNF-α, IL-1α, IL-6, and MIP-1α, by use of the electrochemiluminescence bead method (BioVeris), as described elsewhere [36]. The antibody pairs and standards were from R&D Systems.
Statistical Analysis
Statistical comparisons of cytokine values were calculated using a 2-tailed Student’s t test (Microsoft Excel software, version 2003; Microsoft). Fisher’s exact test was used when expected numbers were <5. Survival was plotted and data were compared using a log-rank test (Prism software, version 4; GraphPad). Data are expressed as the mean ± SE, unless otherwise indicated. Differences were considered to be statistically significant at P ≤ .05.
RESULTS
The Severe Sepsis Model
eIF5A siRNA and survival after intraperitoneal challenge with LPS
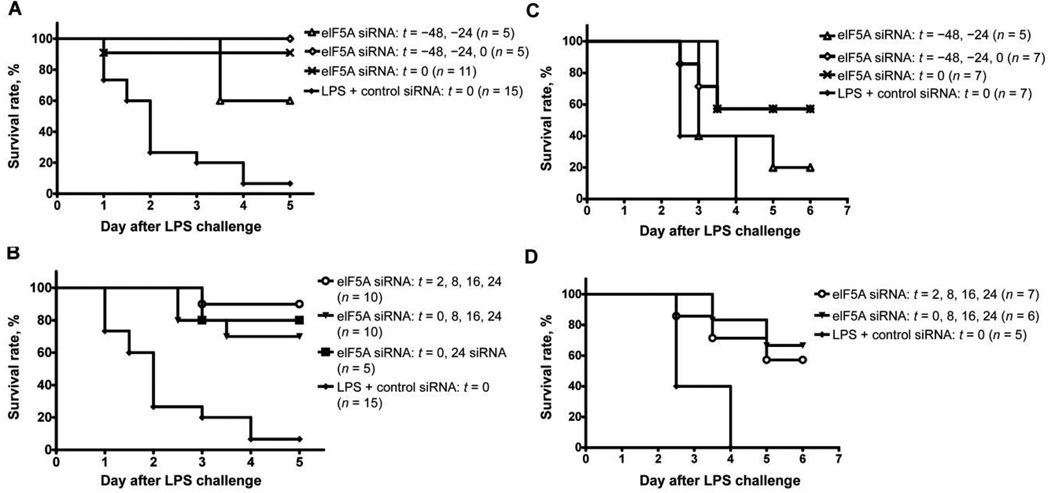
Greater survival of all treated animals, regardless of the dosing regimen, was statistically significant, compared with survival of controls (71% vs. 5%; P < .001) (figure 1). Survival was greatest among BALB/c animals when the eIF5A siRNA-liposome complex was administered 48 and 24 h before and at the time of LPS administration (100% vs. 7%; P = .013) (figure 1A). Importantly, the survival benefit remained significant when the eIF5A siRNA-liposome complex was administered after injection of LPS (P < .001) (figure 1B and 1D). A significant survival benefit was also noted for C57BL/6 mice under similar conditions. The best C57BL/6 response was noted in animals treated at 0, 8, 16, and 24 h after LPS inoculation (survival rate, 67% vs. 0%; P = .0079) (figure 1D).
Figure 1.
Effect of eukaryotic translation initiation factor 5A (eIF5A) small interference RNA (siRNA) on the survival of BALB/c and C57BL/6 mice after intraperitoneal challenge with lipopolysaccharide (LPS). BALB/c mice were treated intraperitoneally with 50 µg of eIF5A siRNA at the time of and/or before (A) or at the time of and/or after (B) intraperitoneal challenge with 5 mg/kg LPS (n = 5–11 mice per group). C57BL/6 mice were treated intraperitoneally with 50 µg of eIF5A siRNA at the time of and/or before (C) or at the time of and/or after (D) intraperitoneal challenge with 25 mg/kg LPS (n = 5–7 mice per group).
eIF5A siRNA and serum cytokine concentrations after intraperitoneal challenge with LPS
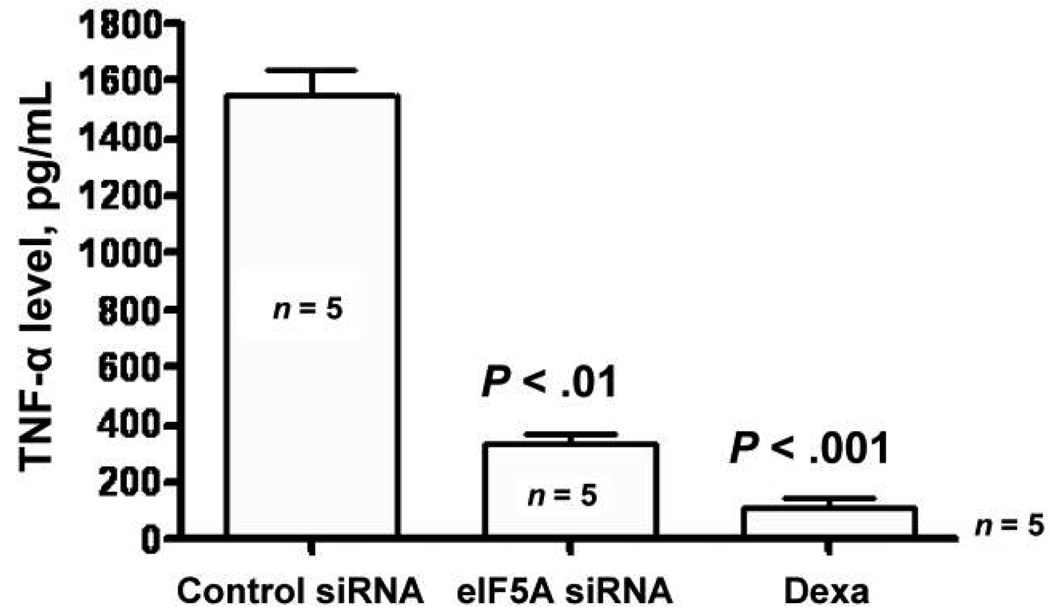
There was a statistically significant decrease in the serum concentrations of IL-2, IL-4, IL-5, GM-CSF, IL-3, IL-6, IL-12 p40, IL-12 p70, MIP-1α, and RANTES at 90 min after LPS administration in mice treated with the eIF5A siRNA-liposome complex at 48 and 24 h before LPS inoculation (table 1). At 8 h after LPS administration, there was also a statistically significant decrease in IL-1β, IL-12, and IL-10 in siRNA-liposome treated animals, compared with control mice (table 2). There was no significant difference in the concentration of other cytokines tested at either 90 min or 8 h after LPS administration (table 1 and table 2). Finally, a comparison of eIF5A siRNA and dexamethasone revealed similarly decreased concentrations of TNF-α in blood at 90 min after intraperitoneal challenge with LPS (figure 2).
Table 1.
Effect of eukaryotic translation initiation factor 5A (eIF5A) on serum cytokine concentrations 90 min after intraperitoneal challenge with lipopolysaccharide (LPS).
| Cytokine | Serum concentration, mean ± SE, pg/mL | P | |
|---|---|---|---|
| After receipt of eIF5A siRNAa |
After receipt of control siRNAa |
||
| IL-1α | 457.6 ± 80.7 | 1024.7 ± 351.8 | .1 |
| IL-1β | 970.2 ± 210.5 | 1001.9 ± 166.6 | .2 |
| IL-2 | 68.3 ± 10.9 | 99.3 ± 12.9 | .006 |
| IL-3 | 37.9 ± 6.3 | 57.3 ± 2.8 | .001 |
| IL-4 | 7.8 ± 1.0 | 12.7 ± 0.7 | .001 |
| IL-5 | 43.6 ± 6.1 | 65.4 ± 4.0 | .009 |
| IL-6 | 306,613.4 ± 180,002.4 | 2,041,862 ± 809,364.4 | .05 |
| IL-10 | 708.1 ± 156.8 | 1001.9 ± 166.6 | .2 |
| IL-12 (p40) | 26,429.6 ± 5416.0 | 96,886.1 ± 19,577.9 | .003 |
| IL-12 (p70) | 2410.9 ± 514.4 | 3906.9 ± 485.5 | .05 |
| IL-17 | 1029.4 ± 103 | 1086.4 ± 128.8 | .7 |
| IFN-γ | 273.2 ± 59.2 | 361.4 ± 27.0 | .3 |
| GM-CSF | 388.9 ± 42.8 | 541.2 ± 17.6 | .004 |
| MIP-1α | 5061.5 ± 1416.7 | 13,577.7 ± 2922.7 | .02 |
| RANTES | 1276.7 ± 256.3 | 4121.9 ± 788.4 | .003 |
| TNF-α | 28,111.5 ± 8383.5 | 23,424.7 ± 351.8 | .1 |
NOTE. BALB/c mice were treated with eIF5A small interference (siRNA) 48 and 24 h before challenge with 5 mg/kg LPS. The mice were euthanized 90 min after challenge with LPS for evaluation of serum cytokine concentrations. GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normally T cell expressed and secreted; siRNA, small interference RNA; TNF, tumor necrosis factor.
There were 10 mice per experimental group.
Table 2.
Effect of eukaryotic translation initiation factor 5A (eIF5A) on serum cytokine concentrations 8 h after intraperitoneal challenge with lipopolysaccharide (LPS).
| Cytokine | Serum concentration, mean ± SE, pg/mL | P | |
|---|---|---|---|
| After receipt of eIF5A siRNAa |
After receipt of control siRNAa |
||
| IL-1α | 580.468 ± 67.804 | 859.7 ± 139.3 | .09 |
| IL-1β | 1003.4 ± 171.9 | 1994.4 ± 326.7 | .01 |
| IL-2 | 78.925 ± 11.5 | 115.429 ± 14.6 | .06 |
| IL-3 | 48.2 ± 7.8 | 66.7 ± 8.4 | .1 |
| IL-4 | 10.6 ± 1.5 | 12.8 ± 1.3 | .3 |
| IL-5 | 82.972 ± 18.6 | 87.8 ± 14.0 | .8 |
| IL-6 | 246,335.3 ± 119,250.6 | 674,670.4 ± 20,9941.0 | .08 |
| IL-10 | 241.0 ± 14.3 | 471.0 ± 84.6 | .03 |
| IL-12 (p40) | 57,345.7 ± 13,116.8 | 112,535.7 ± 20,938.3 | .04 |
| IL-12 (p70) | 1992.6 ± 342.7 | 3292.1 ± 687.4 | .1 |
| IL-17 | 1193.8 ± 168.6 | 1827.7 ± 403.1 | .2 |
| IFN-γ | 644.5 ± 225.9 | 940.3 ± 172.1 | .3 |
| GM-CSF | 447.3 ± 48.6 | 519.0 ± 56.4 | .3 |
| MIP-1α | 783.4 ± 118.7 | 1175.7 ± 162.9 | .07 |
| RANTES | 23,853.3 ± 7903.5 | 43,711.0 ± 12,594.7 | .2 |
| TNF-α | 1007.7 ± 135.9 | 1424.9 ± 191.7 | .09 |
NOTE. Mice were treated with eIF5A siRNA 48 and 24 h before challenge with 5 mg/kg LPS. The mice were euthanized 8 h after challenge with LPS for evaluation of serum concentrations of cytokines. GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normally T cell expressed and secreted; TNF, tumor necrosis factor.
There were 10 mice per experimental group.
Figure 2.
Comparison of eukaryotic translation initiation factor 5A (eIF5A) small interference RNA (siRNA) with dexamethasone (Dexa). Mice were injected intraperitoneally with 50 µg of control or eIF5A siRNA. After 24 h, 75 µg of lipopolysaccharide (LPS) was injected intraperitoneally, and, 90 min later, blood samples were obtained for determination of serum concentrations of tumor necrosis factor (TNF)–α. In another study, mice were pretreated intraperitoneally with 10 mg/kg Dexa, and, after 1 h, they received 75 µg of LPS. Blood samples were obtained 90 min after administration of LPS. Data are the mean ± SE. n = 5 mice in each group.
The Acute Lung Injury Model
eIF5A siRNA and inflammation after intranasal challenge with LPS
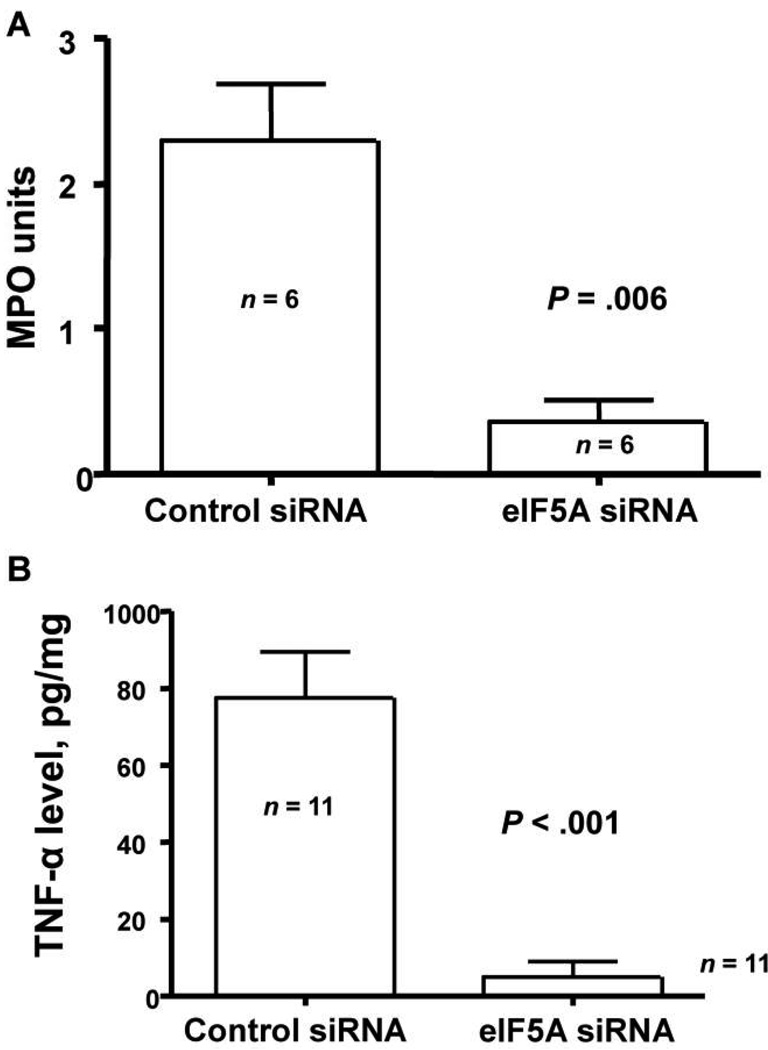
As shown in figure 3A, myeloperoxidase (MPO) activity was markedly (>80%) reduced in mice treated with 50 µg of eIF5A siRNA 48 h before intranasal administration of LPS, compared with MPO activity noted in controls. In a second experiment, siRNA was administered 24 h before the LPS challenge. The reduction in total lung MPO activity was also reduced (by 42%) in mice receiving eIF5A siRNA, compared with controls (data not shown). Because eIF5A siRNA reduced the neutrophilic response in the lungs, we next examined the change in lung cytokine concentrations. Mice were again pretreated with either eIF5A or control siRNA 48 h before intranasal LPS challenge. Twenty-four h after the LPS challenge, mice were euthanized and their lungs homogenized. There was a near total absence of total lung TNF-α in mice treated with eIF5FA siRNA (figure 3B).
Figure 3.
Effect of eukaryotic translation initiation factor 5A (eIF5A) small interference RNA (siRNA) on lung inflammation. A, Myeloperoxidase (MPO) activity in mice treated with 50 µg in 50 µL of control or eIF5A siRNA by means of nasal administration. After 48 h, 75 µg of lipopolysaccharide (LPS) was given intranasally, and, 24 h later, the lungs were removed, weighed, and frozen. MPO assays were performed on the thawed tissue (see Materials and Methods). Data are mean ± SE. n = 6 mice in each group. B, Tumor necrosis factor (TNF)–α concentrations in mice treated with 50 µg in 50 µL of control or eIF5A siRNA by means of nasal administration. After 48 h, 75 µg of LPS was given intranasally, and, 24 h later, the lungs were removed, weighed, and homogenized. Triton X-100 was added, and the samples were frozen until they were thawed for TNF-α quantification (see Materials and Methods). Data are mean ± SE. n = 11 mice in each group.
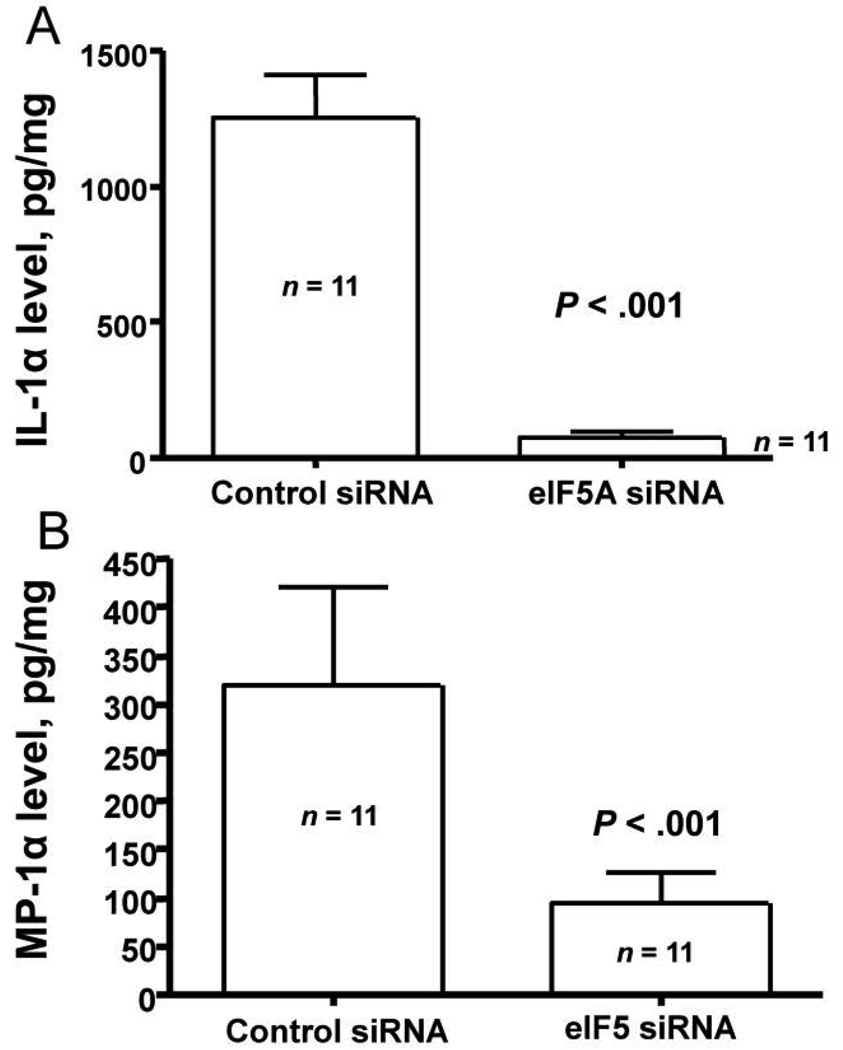
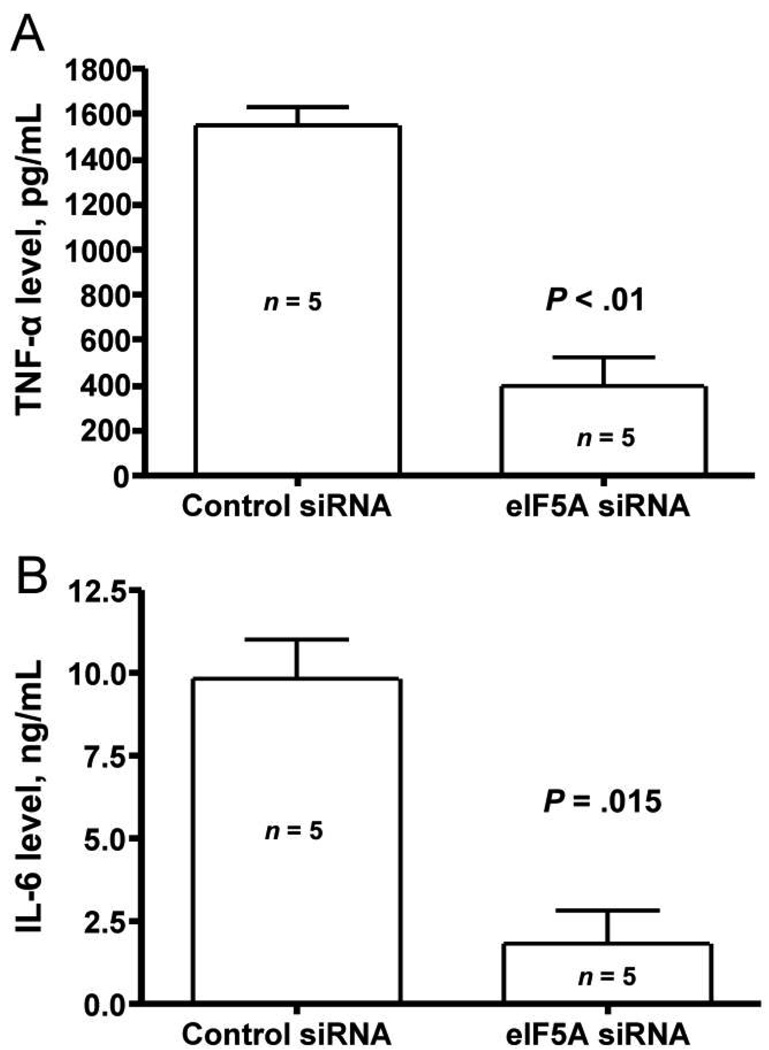
Similar to the near total reduction in lung concentrations of TNF-α, total lung concentrations of IL-1α were also nearly absent (figure 4A). We next measured the concentrations of the chemokine MIP-1α in the same samples. There was a 71% reduction in the lung tissue concentration of this chemokine (figure 4B). We also administered eIF5A and control siRNA intraperitoneally, and, after 24 h, we challenged the mice with intranasally administered LPS. There was a statistically significant reduction in the serum concentrations of TNF-α (figure 5A) and IL-6 (figure 5B) at 4 h.
Figure 4.
Effect of eukaryotic translation initiation factor 5A (eIF5A) small interference RNA (siRNA) on interleukin (IL)–1α and macrophage inflammatory protein (MIP)–1α concentrations in the lung. Mice were treated with 50 µg in 50 µL of control or eIF5A siRNA by means of nasal administration. After 48 h, 75 µg of lipopolysaccharide (LPS) was given intranasally, and, 24 h later, the lungs were removed, weighed, and homogenized. Triton X-100 was added, and the samples frozen until they were thawed to undergo IL-1α (A) and MIP-1α (B) assays (see Materials and Methods). Data are mean ± SE. n = 11 mice in each group.
Figure 5.
Effect of eukaryotic translation initiation factor 5A (eIF5A) small interference RNA (siRNA) on lipopolysaccharide (LPS)–induced serum concentrations of tumor necrosis factor (TNF)–α and interleukin (IL)–6. A, TNF-α concentrations in mice injected intraperitoneally with 50 µg of control or eIF5A siRNA. After 24 h, 75 µg of LPS was given intranasally, and, 4 h later, blood samples were obtained for determination of serum concentrations of TNF-α. Data are mean ± SE. n = 5 mice in each group. B, IL-6 concentrations in mice pretreated intraperitoneally for 24 h with 50 µg of control or eIF5A siRNA. A total of 75 µg of LPS was given intranasally, and, after 4 h, blood samples were obtained for determination of serum concentrations of IL-6. Data are mean ± SE. n = 5 mice in each group.
DISCUSSION
The findings of these studies are important because we have shown that it is possible to improve the survival of mice with sepsis by administration of an siRNA complex that targets eIF5A. Furthermore, of clinical importance is the finding that survival occurs even when the siRNA complex is administered after inoculation with LPS. In addition, we confirmed the antiinflammatory effect of eIF5A siRNA by use of a model of pulmonary inflammation in which LPS is administered via an intranasal route. Finally, in both the lung injury model and the sepsis model, proinflammatory markers in lung homogenate and serum are significantly attenuated by eIF5A siRNA treatment.
Expression of eIF5A is constitutive in a wide variety of tissues and cell lines, but it appears to be more tightly regulated in human blood cells. Specifically, human peripheral blood mononuclear cells (PBMCs) [22] and immature dendritic cells [23] express low levels of eIF5A. However, increased levels of hypusine, which are required for eIF5A expression, have been associated with activation of lymphocyte growth [37], and expression of eIF5A has been found to be strongly up-regulated in PBMCs in response to stimulators of T cell activation and/or proliferation [22]. Dendritic cells also increase eIF5A expression during maturation [23]. Hypusine-modified eIF5A is thought to be required for the nuclear export CD83 mRNA, because an inhibitor of DHS was found to block CD83 surface expression and inhibit the ability of dendritic cells to activate T lymphocytes [23]. Collectively, these data suggest that eIF5A may be an important factor in the activation of leukocytes and, therefore, an essential component of a normal inflammatory response.
Inhibitors of DHS, such as CNI-1493 [38], and those of DOHH, such as mimosine, have anti-inflammatory properties. Mimosine reduced the inflammatory index and expression of the chemokines monocyte chemoattractant protein (MCP)–1 and MIP-2, in mice infected with the parasite Trichinella spiralis [8], and it suppressed production of the cytokines TNF-α and IL-6 in a model of chronic inflammation [9]. Although these inhibitors may affect pathways other than hypusine biosynthesis, the reduction in hypusinated eIF5A may contribute to the anti-inflammatory properties of these compounds.
Polyamines are known to be endogenous regulators of inflammation, and the polyamine spermidine is important as a substrate for the formation of the hypusine residue on eIF5A [39]. In fact, a critical function of spermidine in the support of yeast cell growth is to act as a substrate for hypusine modification of eIF5A [40]. Sepsis and endotoxemia have been found to stimulate polyamine biosynthesis, resulting in the accumulation of putrescine and spermidine in the liver [4] and jejunal mucosa [5]. In further support of this finding, Soulet et al. [41] found that polyamines, including spermidine, accumulated in the brain after LPS challenge in mice and that inhibition of polyamine synthesis reduced the ability of LPS to trigger TNF and Toll-like receptor 2 transcriptional activation, as well as increased the survival of mice challenged with LPS. Although the role of polyamines in the regulation of inflammation is yet unclear, one possible reason for increased spermidine levels may be to fuel hypusine biosynthesis and the formation of mature eIF5A.
Several studies have also found a role for eIF5A in the regulation of apoptosis. Overexpression of eIF5A has been found to induce apoptosis in lung [25] and colon [26] cancer cell lines. Silencing of eIF5A by use of siRNAs was also found to protect human lamina cribrosa cells from TNF-α–induced apoptosis [24], suggesting that eIF5A may be involved in the TNF-α apoptotic pathway. Cytokines, such as IFN-α [21], IFN-γ [26], and TNF-α [24] stimulate eIF5A expression, and TNF-α triggers a rapid nuclear accumulation of eIF5A in colon cells before apoptotic cell death [26]. These data collectively suggest that eIF5A may be involved in apoptosis triggered by cytokine exposure and that suppression of eIF5A could lead to enhanced cellular survival during acute inflammation.
Taken together, these findings suggest that eIF5A is involved in regulation of the inflammatory response to sepsis via both the initial proinflammatory response and the subsequent hypoinflammatory apoptotic response. Given the lack of efficacy of therapies targeting only the inflammatory phase of sepsis, targeting eIF5A provides a novel, multifactorial approach to modifying the deleterious inflammatory response to sepsis via exploiting siRNA technology to block cell death and through inhalational administration [42]. Further studies are needed to extrapolate these findings to other animal models of sepsis, including cecal ligation and puncture, and to further characterize the role of apoptosis inhibition in the survival benefit associated with eIF5A siRNA complexes.
Acknowledgments
Financial support: National Institutes of Health (grants CAD AI 15614 [to L.L.R.] and 1K08AI65461-01A1 [to C.C.M.]); Senesco Technologies (grant G111587).
Footnotes
Potential conflicts of interest: C.T., R.D., and J.T. own stock or stock options in Senesco Technologies. All other authors: no conflict.
Presented in part: 45th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, D.C., 16–19 December 2005 (abstract B-1990); 44th Annual Meeting of the Infectious Diseases Society of America, Toronto, Ontario, Canada, 12–15 October 2006 (abstract 1088).
References
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Tinsley KW, Karl IE. Role of apoptotic cell death in sepsis. Scand J Infect Dis. 2003;35:585–592. doi: 10.1080/00365540310015692. [DOI] [PubMed] [Google Scholar]
- 3.Peulen O, Deloyer P, Deville C, Dandrifosse G. Polyamines in gut inflammation and allergy. Current Medicinal Chemistry Anti-Inflammatory and Anti-Allergy Agents. 2004;3:1–8. [Google Scholar]
- 4.Tiao G, Noguchi Y, Lieberman MA, Fischer JE, Hasselgren PO. Sepsis stimulates polyamine biosynthesis in the liver and increases tissue levels of ornithine decarboxylase mRNA. Shock. 1995;4:403–410. [PubMed] [Google Scholar]
- 5.Noguchi Y, Meyer TA, Tiao G, Ogle CK, Fischer JE, Hasselgren PO. Influence of sepsis and endotoxemia on polyamine metabolism in mucosa of small intestine in rats. Metabolism. 1996;45:28–33. doi: 10.1016/s0026-0495(96)90196-1. [DOI] [PubMed] [Google Scholar]
- 6.Wolff EC, Park MH, Folk JE. Cleavage of spermidine as the first step in deoxyhypusine synthesis: the role of NAD. J Biol Chem. 1990;265:4793–4799. [PubMed] [Google Scholar]
- 7.Park MH, Cooper HL, Folk JE. The biosynthesis of protein-bound hypusine (N epsilon -(4-amino-2-hydroxybutyl)lysine). Lysine as the amino acid precursor and the intermediate role of deoxyhypusine (N epsilon -(4-aminobutyl)lysine) J Biol Chem. 1982;257:7217–7222. [PubMed] [Google Scholar]
- 8.Conti P, Frydas S, Reale M, et al. Inhibition of MCP-1 and MIP-2 transcription and translation by mimosine in muscle tissue infected with the parasite Trichinella spiralis. Mol Cell Biochem. 2002;229:129–137. doi: 10.1023/a:1017989014906. [DOI] [PubMed] [Google Scholar]
- 9.Frydas S, Papazahariadou M, Papaioannou N, et al. Effect of the compound l-mimosine in an in vivo model of chronic granuloma formation induced by potassium permanganate (KMNO4) Int J Immunopathol Pharmacol. 2003;16:99–104. doi: 10.1177/039463200301600202. [DOI] [PubMed] [Google Scholar]
- 10.Rosorius O, Reichart B, Kratzer F, Heger P, Dabauvalle MC, Hauber J. Nuclear pore localization and nucleocytoplasmic transport of eIF-5A: evidence for direct interaction with the export receptor CRM1. J Cell Sci. 1999;112:2369–2380. doi: 10.1242/jcs.112.14.2369. [DOI] [PubMed] [Google Scholar]
- 11.Ruhl M, Himmelspach M, Bahr GM, et al. Eukaryotic initiation factor 5A is a cellular target of the human immunodeficiency virus type 1 Rev activation domain mediating trans-activation. J Cell Biol. 1993;123:1309–1320. doi: 10.1083/jcb.123.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipowsky G, Bischoff FR, Schwarzmaier P, et al. Exportin 4: a mediator of a novel nuclear export pathway in higher eukaryotes. EMBO J. 2000;19:4362–4371. doi: 10.1093/emboj/19.16.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanauske-Abel HM, Slowinska B, Zagulska S, et al. Detection of a subset of polysomal mRNAs associated with modulation of hypusine formation at the G1-S boundary: proposal of a role for eIF-5A in onset of DNA replication. FEBS Lett. 1995;366:92–98. doi: 10.1016/0014-5793(95)00493-s. [DOI] [PubMed] [Google Scholar]
- 14.Jao DL, Chen KY. Tandem affinity purification revealed the hypusine-dependent binding of eukaryotic initiation factor 5A to the translating 80S ribosomal complex. J Cell Biochem. 2006;97:583–598. doi: 10.1002/jcb.20658. [DOI] [PubMed] [Google Scholar]
- 15.Schnier J, Schwelberger HG, Smit-McBride Z, Kang HA, Hershey JW. Translation initiation factor 5A and its hypusine modification are essential for cell viability in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:3105–3114. doi: 10.1128/mcb.11.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakus J, Wolff EC, Park MH, Folk JE. Features of the spermidine-binding site of deoxyhypusine synthase as derived from inhibition studies. Effective inhibition by bis- and mono-guanylated diamines and polyamines. J Biol Chem. 1993;268:13151–13159. [PubMed] [Google Scholar]
- 17.Chen ZP, Yan YP, Ding QJ, et al. Effects of inhibitors of deoxyhypusine synthase on the differentiation of mouse neuroblastoma and erythroleukemia cells. Cancer Lett. 1996;105:233–239. doi: 10.1016/0304-3835(96)04287-5. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki K, Abid MR, Miyazaki M. Deoxyhypusine synthase gene is essential for cell viability in the yeast Saccharomyces cerevisiae. FEBS Lett. 1996;384:151–154. doi: 10.1016/0014-5793(96)00310-9. [DOI] [PubMed] [Google Scholar]
- 19.Shi XP, Yin KC, Ahern J, Davis LJ, Stern AM, Waxman L. Effects of N1-guanyl-1,7-diaminoheptane, an inhibitor of deoxyhypusine synthase, on the growth of tumorigenic cell lines in culture. Biochim Biophys Acta. 1996;1310:119–126. doi: 10.1016/0167-4889(95)00165-4. [DOI] [PubMed] [Google Scholar]
- 20.Park MH, Joe YA, Kang KR. Deoxyhypusine synthase activity is essential for cell viability in the yeast Saccharomyces cerevisiae. J Biol Chem. 1998;273:1677–1683. doi: 10.1074/jbc.273.3.1677. [DOI] [PubMed] [Google Scholar]
- 21.Caraglia M, Marra M, Giuberti G, et al. The eukaryotic initiation factor 5A is involved in the regulation of proliferation and apoptosis induced by interferon-α and EGF in human cancer cells. J Biochem. 2003;133:757–765. doi: 10.1093/jb/mvg097. [DOI] [PubMed] [Google Scholar]
- 22.Bevec D, Klier H, Holter W, et al. Induced gene expression of the hypusine-containing protein eukaryotic initiation factor 5A in activated human T lymphocytes. Proc Natl Acad Sci USA. 1994;91:10829–10833. doi: 10.1073/pnas.91.23.10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruse M, Rosorius O, Krätzer F, et al. Inhibition of CD83 cell surface expression during dendritic cell maturation by interference with nuclear export of CD83 mRNA. J Exp Med. 2000;191:1581–1590. doi: 10.1084/jem.191.9.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor CA, Senchyna M, Flanagan J, et al. Role of eIF5A in TNF-α-mediated apoptosis of lamina cribrosa cells. Invest Ophthalmol Vis Sci. 2004;45:3568–3576. doi: 10.1167/iovs.03-1367. [DOI] [PubMed] [Google Scholar]
- 25.Li AL, Li HY, Jin BF, et al. Anovel eIF5A complex functions as a regulator of p53 and p53-dependent apoptosis. J Biol Chem. 2004;279:49251–49258. doi: 10.1074/jbc.M407165200. [DOI] [PubMed] [Google Scholar]
- 26.Taylor CA, Sun Z, Cliche DO, et al. Eukaryotic translation initiation factor 5A induces apoptosis in colon cancer cells and associates with the nucleus in response to tumour necrosis factor α signalling. Exp Cell Res. 2007;313:437–449. doi: 10.1016/j.yexcr.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 27.Le Tulzo Y, Pangault C, Gacouin A, et al. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock. 2002;18:487–494. doi: 10.1097/00024382-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol. 2005;78:325–337. doi: 10.1189/jlb.0105017. [DOI] [PubMed] [Google Scholar]
- 29.Wesche-Soldato DE, Chung CS, Lomas-Neira J, Doughty LA, Gregory SH, Ayala A. In vivo delivery of caspase-8 or Fas siRNA improves the survival of septic mice. Blood. 2005;106:2295–2301. doi: 10.1182/blood-2004-10-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sørensen DR, Leirdal M, Sioud M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol. 2003;327:761–766. doi: 10.1016/s0022-2836(03)00181-5. [DOI] [PubMed] [Google Scholar]
- 31.Sioud M, Sørensen DR. Cationic liposome-mediated delivery of siRNAs in adult mice. Biochem Biophys Res Commun. 2003;312:1220–1225. doi: 10.1016/j.bbrc.2003.11.057. [DOI] [PubMed] [Google Scholar]
- 32.Glasgow SC, Ramachandran S, Blackwell TS, Mohanakumar T, Chapman WC. Interleukin-1β is the primary initiator of pulmonary inflammation following liver injury in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L491–L496. doi: 10.1152/ajplung.00009.2007. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Shan P, Jiang D, et al. Small interfering RNA targeting heme oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. J Biol Chem. 2004;279:10677–10684. doi: 10.1074/jbc.M312941200. [DOI] [PubMed] [Google Scholar]
- 34.Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nat Med. 2005;11:50–55. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- 35.Abraham E, Kaneko DJ, Shenkar R. Effects of endogenous and exogenous catecholamines on LPS-induced neutrophil trafficking and activation. Am J Physiol. 1999;276:L1–L8. doi: 10.1152/ajplung.1999.276.1.L1. [DOI] [PubMed] [Google Scholar]
- 36.Lewis EC, Shapiro L, Bowers OJ, Dinarello CA. α1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci USA. 2005;102:12153–12158. doi: 10.1073/pnas.0505579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper HL, Park MHl, Folk JE. Posttranslational formation of hypusine in a single major protein occurs generally in growing cells and is associated with activation of lymphocyte growth. Cell. 1982;29:791–797. doi: 10.1016/0092-8674(82)90441-x. [DOI] [PubMed] [Google Scholar]
- 38.Hauber I, Bevec D, Heukeshoven J, et al. Identification of cellular deoxyhypusine synthase as a novel target for antiretroviral therapy. J Clin Invest. 2005;115:76–85. doi: 10.1172/JCI21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chattopadhyay MK, Tabor CW, Tabor H. Spermidine but not spermine is essential for hypusine biosynthesis and growth in Saccharomyces cerevisiae: spermine is converted to spermidine in vivo by the FMS1-amine oxidase. Proc Natl Acad Sci USA. 2003;100:13869–13874. doi: 10.1073/pnas.1835918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chattopadhyay MK, Park MH, Tabor H. Hypusine modification for growth is the major function of spermidine in Saccharomyces cerevisiae polyamine auxotrophs grown in limiting spermidine. Proc Natl Acad Sci USA. 2008;105:6554–6559. doi: 10.1073/pnas.0710970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soulet D, Rivest S. Polyamines play a critical role in the control of the innate immune response in the mouse central nervous system. J Cell Biol. 2003;162:257–268. doi: 10.1083/jcb.200301097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deans KJ, Haley M, Natanson C, Eichacker PQ, Minneci PC. Novel therapies for sepsis: a review. J Trauma. 2005;58:867–874. doi: 10.1097/01.ta.0000158244.69179.94. [DOI] [PubMed] [Google Scholar]