Abstract
Levodopa (l-DOPA) is the ‘gold standard’ to treat Parkinson's disease. Unfortunately, dyskinesias detract from its efficacy. Current dyskinesia treatments, including amantadine and dextromethorphan, are thought to work via N-methyl-d-aspartate (NMDA) antagonism, but this hypothesis has not been tested. The NMDA antagonists MK- 801and HA-966 failed to suppress expression of dyskinesias in the 6-hydroxydopamine rat. Dyskinesias, however, were suppressed by the NMDA and sigma (σ)-1receptor ligand dextromethorphan and by the σ-1 antagonist BMY-14802. Antidyskinetic effects of dextromethorphan may be mediated via mechanisms other than NMDA, including the σ-1 receptor and other binding sites common to dextromethorphan and BMY-14802.
Keywords: 6-hydroxydopamine, levodopa-induced dyskinesias, N-methyl-d-aspartate, Parkinson's disease, rat, sigma
Introduction
Although levodopa (l-DOPA) treats most symptoms of Parkinson's disease, its efficacy is reduced by dyskinesias. N-methyl-d-aspartate (NMDA) antagonists are thought to be promising treatments based on the antidyskinetic action of the NMDA antagonist amantadine in mouse [1], rat [2], and monkey [3] models of Parkinson's disease, and of both amantadine and the noncompetitive NMDA antagonist dextromethorphan in Parkinson's patients [4]. Amantadine, however, may lose efficacy after 8–12 months [5], and dextromethorphan has the potential to be abused or psychotomimetic [6]. Therefore, delineating other pharmacotherapies is crucial.
Although antidyskinetic effects of some NMDA antagonists, such as dextromethorphan and amantadine, have been clinically established, the hypothesized NMDA antagonist mechanism has yet to be pharmacologically evaluated. These drugs have comparatively weak affinity for the NMDA receptor ion channel, but comparable or higher affinity with other ligand-gated ion channels, including nicotinic acetylcholine receptors and the recently cloned sigma (σ) receptor [7], which utilizes both ligand-gated and alternative signal transduction mechanisms. These other sites could contribute to some or all of the antidyskinesia effects of weak NMDA antagonists.
We have attempted to confirm the hypothesized anti-dyskinetic role of NMDA antagonists in the unilateral 6-hydroxydopamine (6-OHDA) rat by evaluating the effects of structurally unrelated antagonists targeting different sites of the NMDA heterooligomeric protein. We tested MK-801, the most widely-studied noncompetitive NMDA antagonist, and HA-966, a frequently used antagonist with high affinity for the glycine site. We also explored the possible role of nicotinic and σ-1 receptors using mecamylamine and BMY-14802, respectively.
Materials and methods
Animals
Fifty-three (n=8–10/group) male Sprague–Dawley rats (Harlan, Indianapolis, Indiana, USA) weighing 300 g at surgery were used. Rats were housed at 21°C on a 12-h light : dark cycle (lights on at 0.700) and ad lib food and water. Testing was conducted between 09.00 and 15.00. Procedures were in accordance with the Institutional Animal Care and Use Committee.
Drugs
Drugs were dissolved in 0.9% saline, except BMY-14802, which was dissolved in distilled water. Drugs were administered at 1 ml/100 g of body weight via intraperitoneal (i.p.) injection 30 min before l-DOPA, except for mecamylamine, which was administered subcutaneously (s.c.) 20 min prior. l-DOPA methyl ester, benserazide hydrochloride, l-ascorbic acid, d-amphetamine sulfate, ( + )-MK-801 hydrogen maleate, dextromethorphan hydrobromide, and mecamylamine hydrochloride were obtained from Sigma-Aldrich (St Louis, Missouri, USA). (R)-( + )-HA-966 and BMY-14802 were obtained from Tocris (Ellisville, Missouri, USA). Doses were chosen based on efficacy to suppress behavioral effects of dopamine agonists (e.g. stereotypy, locomotion) without reducing basal activity levels. Rats were randomly assigned to receive up to three test compounds at least 1 week apart.
6-Hydroxydopamine lesions
Rats underwent stereotaxic surgery under isoflurane anesthesia. A 30-gauge infusion cannula, attached via PE20 tubing to a 10 μl Hamilton gastight syringe and driven by a microinfusion pump (Harvard Apparatus, Holliston, Massachusetts, USA) was used to infuse 6-OHDA (22.8 μg/ 2 μl of 0.9% saline with 0.02% ascorbic acid, 0.5 μl/min) into each of two sites within the right medial forebrain bundle (AP −4.3 and −4.8, ML±1.2, DV −8.6 in mm). Rats then received an injection of buprenorphen analgesic (0.05 mg/ kg, s.c.; Reckitt Benckiser Pharmaceuticals, Richmond, Virginia, USA) and supplemental soft food until regaining their presurgery weight.
Amphetamine-evoked rotational behavior
An a priori criterion of an average of ≥5 turns/min over 10 consecutive minutes in response to amphetamine (5 mg/kg, i.p.) was used to select rats with significant hemiparkinsonism [8].
l-DOPA treatment
At least 3 days after rotational screening, all rats received l-DOPA (7.5 mg/kg, i.p.), combined with benserazide (15 mg/kg, i.p.) and ascorbic acid (2.6 mg/kg, i.p.), once daily for 21 consecutive days, a regimen that induces dyskinesias [9]. Thereafter, rats received a maintenance regimen of two injections/week. All test drugs were administered during the maintenance phase to examine their efficacy to suppress dyskinesia expression.
Dyskinesia assessment
Dyskinesias were assessed by an investigator blind to treatment, using an adaptation of the Abnormal Involuntary Movement Scale described by Cenci et al. [9]. Limb, axial, and oral dyskinesias, as well as contraversive rotational behavior (an index of l-DOPA's motor activating effects), were rated on a 0–4 severity scale every 20th min for 3 h, beginning 20 min after l-DOPA. Total dyskinesia scores were computed as the sum of limb + axial + oral dyskinesias.
Lesion size quantification
Brains were exposed to immunohistochemistry or Western analysis, and average tyrosine hydroxylase loss was 92.50±4.19 and 92.95±2.63% of control, respectively.
For immunohistochemistry, rats were sacrificed under anesthesia by cardiac perfusion with 60 ml each of phosphate-buffered saline and 4% paraformaldehyde, then 40 μm coronal sections were cut. Free-floating sections were rinsed in phosphate-buffered saline (3×10 min) between steps: 1.5% hydrogen peroxide for 30 min, 4% horse serum and 0.1% Triton X-100 for 2 h, monoclonal primary antibody (1 : 10,000; Immunostar, Hudson, Wisconsin, USA) overnight at 4°C, horse antimouse antibody for 2 h (1 : 200; Vector Laboratories, Burlingame, California, USA), and avidin–biotin complex (Vector Laboratories) for 1 h. Tyrosine hydroxylase was visualized in 50 mM Tris buffer with diaminobenzidine (Vector Laboratories) using a Leica DM LFS microscope (Leica Microsystems, Wetzlar, Germany). Optical density was quantified using a Leica DFC 480 camera (Leica camera, Solms, Germany) and Image Pro-Plus 4.1 software (MediaCybernetics, Bethesda, Maryland, USA). Lesion size was calculated by comparing 1.4×1.0 mm areas of 6-OHDA-treated and untreated dorsolateral neostriatum with adjacent cerebral cortex as background.
For Westerns, rats were sacrificed by decapitation under anesthesia. Brains were removed and whole neostriatum dissected and frozen. Protein was extracted with lysis buffer (50 mM Tris, 10 mM ethylenediaminetetra-acetic acid, 1% Triton-X 100, 10 nM phenylmethylsulfonyl fluoride) containing Complete Protease Inhibitor Cocktail (Roche Diagnostics, Indianapolis, Indiana, USA), then quantified with a Pierce protein assay kit (Rockford, Illinois, USA). Samples (15 mg) were diluted 1 : 2 in Laemmli sample buffer (Sigma-Aldrich), heated at 100°C for 5 min, then separated on a 10% Criterion Tris–HCl polyacrylamide gel (Bio-Rad; 200 V, 1 h). Proteins were transferred to polyvinylidene diflouride membranes (30 V, 13 h), then washed in Tris-buffered saline with 1% Tween-20 (pH 7.4; 3×10 min) between steps: 5% nonfat dry milk for 1 h, monoclonal primary antibody (1 : 10 000; Immunostar) for 2 h, and goat antimouse antibody (1 : 3000; Bio-Rad) for 1 h. Proteins were visualized using ECF (Amersham, Piscataway, New Jersey, USA) according to supplier's instructions, then scanned and quantified using the Typhoon fluorescence detection system (Typhoon 9410 Variable Mode Imager, GE Healthcare, Piscataway, New Jersey, USA; Emission filter: 520 BP40, Laser: 488 Blue2).
Statistical analysis
Data were analyzed with SPSS (Chicago, Illinois, USA) with P≤0.05. A mixed design Time×Treatment analysis of variance was used to analyze dyskinesias over the 3-h sessions, and t-tests were used to analyze total dyskinesia scores in the 3-h session and the first hour after l-DOPA. When data violated homogeneity of variance assumptions, Greenhouse–Geisser corrections or t-tests in which equal variance was not assumed were used for repeated or between-subjects data, respectively, yielding fractional degrees of freedom.
Results
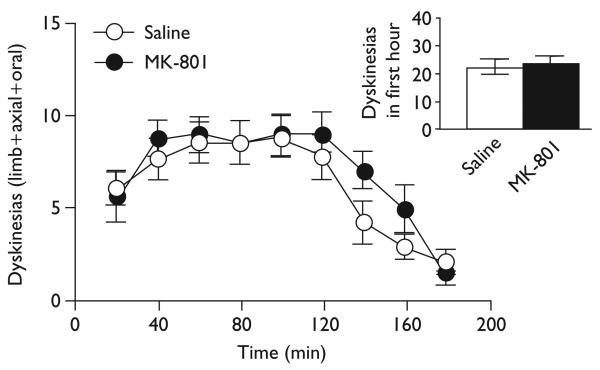
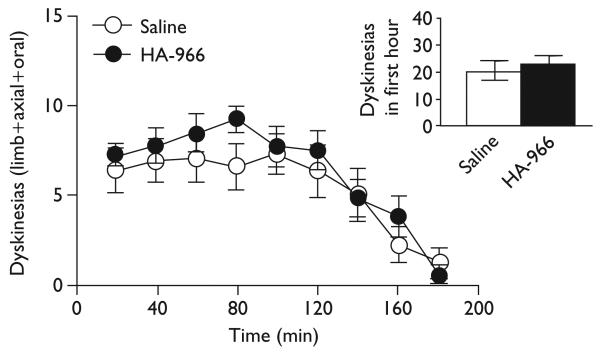
All rats expressed some degree of dyskinesia over time with a broad spectrum of severity (F≥11.74, P<0.001). Contrary to the NMDA hypothesis, dyskinesias were not reduced by traditional NMDA antagonists. MK-801 (0.1 mg/kg) did not affect expression of dyskinesias over time [F(1,17)=0.67, ns], nor did MK-801 affect total dyskinesias during the 3-h session or in the first hour after l-DOPA when the peak response occurs (inset) [t(17)≤0.82, ns], as shown in Fig. 1. HA-966 (15 mg/kg) did not affect dyskinesias over time [F(1,13)=3.98, ns], nor did HA-966 affect total dyskinesias during the 3-h session or in the first hour (inset) [t(13)≤2.00, ns], as shown in Fig. 2. Mecamylamine also did not affect dyskinesias over time [F(1,18)=0.71, ns], nor did it affect total dyskinesias during the 3-h session or in the first hour [t(9)≤0.96, ns] (data not shown).
Fig.1.
MK-801did not suppress dyskinesias, even in the first hour (inset).
Fig. 2.
HA-966 did not suppress dyskinesias, even in the first hour (inset).
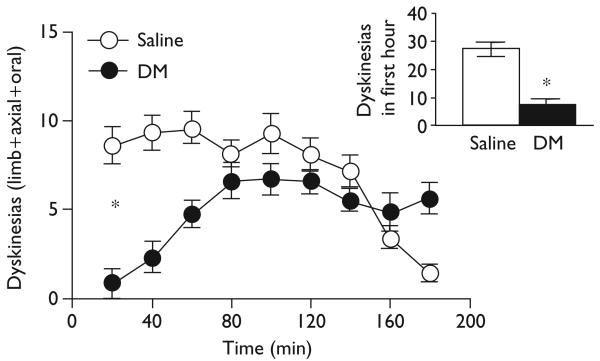
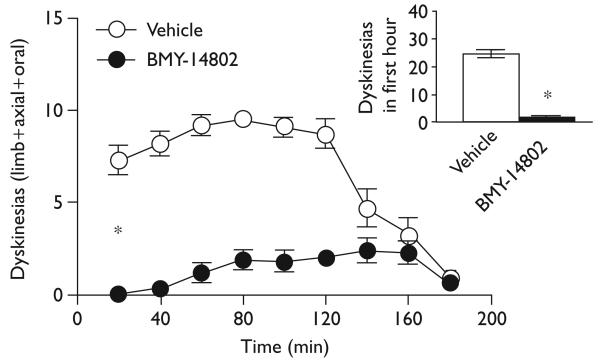
In stark contrast, dextromethorphan (45 mg/kg) significantly reduced dyskinesias over time [F(1,16)=7.83, P≤0.01], as well as total dyskinesias over the 3-h session and in the first hour (inset) [t(16)≥2.80, P≤0.01], as shown in Fig. 3. This effect during the first hour was significant for each of the subscales: limb, axial, and oral (data not shown). The σ-1 antagonist BMY-14802 (15 mg/kg, i.p.) showed a similar but even more striking effect, significantly reducing dyskinesias over time [F(1,18)=134.37, P<0.001], as well as total dyskinesias during the 3-h session and in the first hour (inset) [t(12.61–18)≥11.59, P<0.001], as shown in Fig. 4. This effect during the first hour was significant for each of the dyskinesia subscales (data not shown).
Fig. 3.
Dextromethorphan (DM) reduced dyskinesias, especially in the first hour (inset).
Fig. 4.
BMY-14802 reduced dyskinesias, especially in the first hour (inset).
All rats demonstrated some degree of contralateral rotation in response to l-DOPA with a broad spectrum of severity [F≥19.35, P≤0.001] (data not shown). MK-801, HA-966, mecamylamine, and dextromethorphan did not affect l-DOPA-induced contralateral rotation over time [F≤2.92, ns], but BMY-14802 reduced rotation [F(1,18)=61.96, P<0.001]. Neither MK-801 nor HA-966 affected total rotation during the 3-h session or in the first hour [t(13–17)≤1.22, ns]. Dextromethorphan did not affect total rotation during the 3-h session [t(16)=−0.70, ns], but did reduce rotation in the first hour [t(16)=4.45, P<0.001]. Mecamylamine and BMY-14801 reduced total rotation during o the 3-h session and in the first hour [t(9–18)≥2.33, P<0.05].
Discussion
We have demonstrated a procedure in which dyskinesias are reliably expressed in all rats to varying degrees. Significant modifications from Cenci et al. [9] include the use of males, altered lesion coordinates, desipramine to protect noradrenergic neurons, and a higher 6-OHDA dose. Our collaborators recently demonstrated that severe dopamine depletion is necessary but not sufficient to evoke dyskinesias and reviewed factors that may affect dyskinesia emergence [10].
In our model, the prototypical noncompetitive NMDA antagonist MK-801 does not reduce dyskinesias under conditions in which dextromethorphan is effective. These data do not support the hypothesis that NMDA antagonism mediates the antidyskinetic effect of dextromethorphan: this hypothesis predicts that MK-801, with higher affinity for the NMDA receptor and greater potency for NMDA antagonist-mediated behavior [11], would show equivalent or enhanced efficacy relative to dextromethorphan. Alternately, the complex behavioral effects mediated by MK-801 could interfere with its putative antidyskinetic mechanism or with dyskinesia assessment. As behavioral effects of NMDA antagonists seem to require blockade of the ligand-gated channel or the glutamate recognition site, we also evaluated HA-966, an NMDA antagonist at the glycine site. Like MK-801, HA-966 failed to suppress dyskinesias. Thus, further clinical trials of NMDA antagonists in dyskinesia may not be warranted without prior evaluation in animal models.
The nicotinic antagonist mecamylamine had no significant effect on dyskinesias. This is not surprising given the lack of reports of an antidyskinetic effect despite decades of clinical use for hypertension and smoking cessation. In contrast, the σ-1 antagonist BMY-14802 markedly suppressed dyskinesias. This is consistent with a wide range of preclinical studies demonstrating that σ antagonists block acute and sensitized extrapyramidal behavioral effects in response to dopamine and σ agonists. Acutely, BMY-14802 and other σ antagonists block dopaminergic stimulant-induced locomotion and stereotypy [12]. Interestingly, σ-1 antagonists also block dystonic behavior produced by σ agonists and vacuous chewing produced by microinfusion of σ agonists into cranial nerve motor nuclei that control oral dyskinesias [13,14].
Furthermore, repeated administration of σ antagonists, including BMY-14802, has been shown to block the development of behavioral sensitization to cocaine [15] and methamphetamine [16,17]. Dyskinesias have recently been hypothesized to develop by a ‘behavioral sensitization-like’ mechanism [18], raising the possibility that σ antagonists may block development of dyskinesias, in addition to suppressing their expression, as demonstrated here. Studies are currently underway in our laboratory to evaluate σ agents as antidyskinesia prophylactic agents.
Clinical studies also support a role for σ receptors in dyskinesia. Human patients with dyskinesias show enhanced σ binding in the cerebellum, and ablative surgery or deep brain stimulation decreases σ binding and improves parkinsonian symptoms and dyskinesias [19]. A strong positive correlation of binding potential with dyskinesias, but not symptom severity, suggests σ receptors may be involved in dyskinesia genesis.
BMY-14802 suppressed l-DOPA-induced rotation in this study. Although this is consistent with earlier studies showing similar antagonism of amphetamine-induced behavior, it raises the question of whether the antidyskinetic effects of BMY-14802 could result from nonspecific motoric suppression. This seems unlikely, as earlier studies clearly demonstrate that σ antagonists lack sedative effects, both in preclinical studies with nondopaminergic stimulants [20] and in clinical trials in schizophrenia [21]. Another concern is that l-DOPA-induced rotation has sometimes been used as a marker for l-DOPA's therapeutic effects, and thus its reduction by BMY-14802 could indicate suppression of l-DOPA's therapeutic effects. This also seems unlikely since BMY-14802 has been demonstrated to counteract dystonia induced in rodents by σ agonists, including haloperidol [13]. Similarly, no parkinsonian effects were observed in clinical trials of BMY-14802 in schizophrenia [21]. Studies are underway in our laboratory to evaluate the consequences of BMY-14802 on l-DOPA-mediated improvements of forelimb use.
Our findings are difficult to reconcile with a common σ mechanism of dyskinesia. Dextromethorphan is classified as a σ agonist and BMY-14802 as a σ antagonist, yet both reduce dyskinesias. Furthermore, we have found no effect of the commonly used σ agonists 1,3-di-(2-tolyl)guanidine and dehydroepiandrosterone on dyskinesias (unpublished observations). Although perplexing, this pattern is consistent with several past studies that demonstrated blockade of dopamine agonist-induced behavioral effects by σ ligands. Separate groups have independently concluded that their results did not correlate with known σ classification schemas [22,23]. Thus, it is possible that dextromethorphan may be a partial σ-1 agonist, for example. Alternately, reported nonsigma mechanisms of BMY-14802 could contribute to its antidyskinetic effects [24,25]. Future studies with other σ-1 antagonists or with antisense oligonucleotides are needed to confirm that BMY-14802's antidyskinetic effects are mediated by σ-1. It would be fortuitous if past investments used to bring σ agents to unsuccessful clinical trials in schizophrenia could be leveraged toward developing a dyskinesia pharmacotherapy.
Conclusion
Our findings raise considerable doubt that NMDA antagonism contributes to the antidyskinetic effects of dextromethorphan and amantadine. These data also suggest caution before concluding that dextromethorphan and BMY-14802 exert therapeutic effects via a σ mechanism.
Acknowledgements
Funding was provided by NIDA DA007262-16 (M.A.P.), NINDS N538715 (S.J.W.), and Veterans Affairs Merit Review 385 (S.P.B.). No conflicts of interest are present.
Footnotes
Address where the work was carried out: Department of Veterans Affairs Medical Center, Portland, OR 97239.
References
- 1.Lundblad M, Usiello A, Carta M, Hkanssond K, Fisoned G, Cenci MA. Pharmacological validation of a mouse model of l-DOPA-induced dyskinesia. Exp Neurol. 2005;194:66–75. doi: 10.1016/j.expneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson's disease. Eur J Neurosci. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- 3.Blanchet PJ, Konitsiotis S, Chase TN. Amantadine reduces levodopa-induced dyskinesias in Parkinsonian monkeys. Mov Disord. 1998;13:798–802. doi: 10.1002/mds.870130507. [DOI] [PubMed] [Google Scholar]
- 4.Verhagen Metman L, Blanchet PJ, van den Munckhof P, Del Dotto P, Natte R, Chase TN. A trial of dextromethorphan in parkinsonian patients with motor response complications. Mov Disord. 1998;13:414–417. doi: 10.1002/mds.870130307. [DOI] [PubMed] [Google Scholar]
- 5.Thomas A, Iacono D, Luciano AL, Armellino K, Di Iorio A, Onofrj M. Duration of amantadine benefit on dyskinesia of severe Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75:141–143. [PMC free article] [PubMed] [Google Scholar]
- 6.Miller SC. Dextromethorphan psychosis, dependence and physical withdrawal. Addict Biol. 2005;10:325–327. doi: 10.1080/13556210500352410. [DOI] [PubMed] [Google Scholar]
- 7.Seth P, Leibach FH, Ganapathy V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem Biophys Res Commun. 1997;18:535–540. doi: 10.1006/bbrc.1997.7840. [DOI] [PubMed] [Google Scholar]
- 8.Chang JW, Wachtel SR, Young D, Kang UJ. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson's disease: studies on medial forebrain bundle and striatal lesions. Neuroscience. 1999;88:612–628. doi: 10.1016/s0306-4522(98)00217-6. [DOI] [PubMed] [Google Scholar]
- 9.Cenci MA, Lee CS, Bjorklund A. l-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10:2694–2706. [PubMed] [Google Scholar]
- 10.Putterman DB, Munhall AC, Kozell LB, Belknap JK, Johnson SW. Evaluation of levodopa dose and magnitude of dopamine depletion as risk factors for levodopa-induced dyskinesia in a rat model of Parkinson's disease. JPET. 2007;323:277–284. doi: 10.1124/jpet.107.126219. [DOI] [PubMed] [Google Scholar]
- 11.Ishmael JE, Franklin PH, Murray TF. Dextrorotatory opioids induce stereotyped behavior in Sprague–Dawley and Dark Agouti rats. Psychopharmacology. 1998;140:206–216. doi: 10.1007/s002130050759. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto RR, McCracken KA, Friedman MJ, Pouw B, De Costa BR, Bowen WD. Conformationally restricted analogs of BD1008 and an antisense oligodeoxynucleotide targeting sigma1 receptors produce anti-cocaine effects in mice. Eur J Pharmacol. 2001;419:163–174. doi: 10.1016/s0014-2999(01)00968-2. [DOI] [PubMed] [Google Scholar]
- 13.Okumura K, Ujike H, Akiyama K, Kuroda S. BMY-14802 reversed the sigma receptor agonist-induced neck dystonia in rats. J Neural Transm. 1996;103:1153–1161. doi: 10.1007/BF01271200. [DOI] [PubMed] [Google Scholar]
- 14.Tran TT, de Costa BR, Matsumoto RR. Microinjection of sigma ligands into cranial nerve motor nuclei produces vacuous chewing in rats. Pharmacology. 1998;137:191–200. doi: 10.1007/s002130050609. [DOI] [PubMed] [Google Scholar]
- 15.Ujike H, Kuroda A, Orauki A. σ Receptor antagonists block the development of sensitization to cocaine. Eur J Pharmacol. 1996;296:123–128. doi: 10.1016/0014-2999(95)00693-1. [DOI] [PubMed] [Google Scholar]
- 16.Ujike H, Kanzaki A, Okumura K, Akiyama K, Otsuki S. Sigma (σ) antagonist BMY 14802 prevents methamphetamine-induced sensitization. Life Sci. 1992;50:PL129–PL134. doi: 10.1016/0024-3205(92)90466-3. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama K, Kanzaki A, Tsuchida K, Ujike H. Methamphetamine-induced behavioral sensitization and its implications for relapse of schizophrenia. Schiz Res. 1994;12:251–257. doi: 10.1016/0920-9964(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 18.Nutt JG. Clinical pharmacology of levodopa-induced dyskinesia. Ann Neurol. 2000;47(4 Suppl 1):S160–S164. [PubMed] [Google Scholar]
- 19.Nimura T, Ando T, Yamaguchi K, Nakajima T, Shirane R, Itoh M, Tominaga T. The role of sigma-receptors in levodopa-induced dyskinesia in patients with advanced Parkinson disease: a positron emission tomography study. J Neurosurg. 2004;100:606–610. doi: 10.3171/jns.2004.100.4.0606. [DOI] [PubMed] [Google Scholar]
- 20.Matthews RT, McMillen BA, Sallis R, Blair D. Effects of BMY 14802, a potential antipsychotic drug, on rat brain dopaminergic function. J Pharmacol Exp Ther. 1986;239:124–131. [PubMed] [Google Scholar]
- 21.Gewirtz GR, Gorman JM, Volavka J, Macaluso J, Gribkoff G, Taylor DP, Borison R. BMY 14802, a sigma receptor ligand for the treatment of schizophrenia. Neuropsychopharmacology. 1994;10:37–40. doi: 10.1038/npp.1994.5. [DOI] [PubMed] [Google Scholar]
- 22.Wettstein JG, Roman FJ, Rocher MN, Junien JL. Effects of sigma receptor ligands on schedule-controlled behavior of rats: relation to sigma and PCP receptor binding affinity. Psychopharmacology. 1991;104:157–163. doi: 10.1007/BF02244171. [DOI] [PubMed] [Google Scholar]
- 23.Maj J, Rogóz Z, Skuza G. Some behavioral effects of 1,3-di-o-tolylguanidine, opipramol and sertraline, the sigma site ligands. Pol J Pharmacol. 1996;48:379–395. [PubMed] [Google Scholar]
- 24.Moison D, De Deurwaerdère P, Cagnotto A, Marrazzo A, Prezzavento O, Ronsisvalle G, et al. Intrastriatal administration of sigma ligands inhibits basal dopamine release in vivo. Neuropharmacology. 2003;45:945–953. doi: 10.1016/s0028-3908(03)00253-3. [DOI] [PubMed] [Google Scholar]
- 25.Weiser SD, Patrick SL, Mascarella SW, Downing-Park J, Bai X, Carroll FI, et al. Stimulation of rat striatal tyrosine hydroxylase activity following intranigral administration of sigma receptor ligands. Eur J Pharmacol. 1995;275:1–7. doi: 10.1016/0014-2999(94)00718-m. [DOI] [PubMed] [Google Scholar]