Abstract
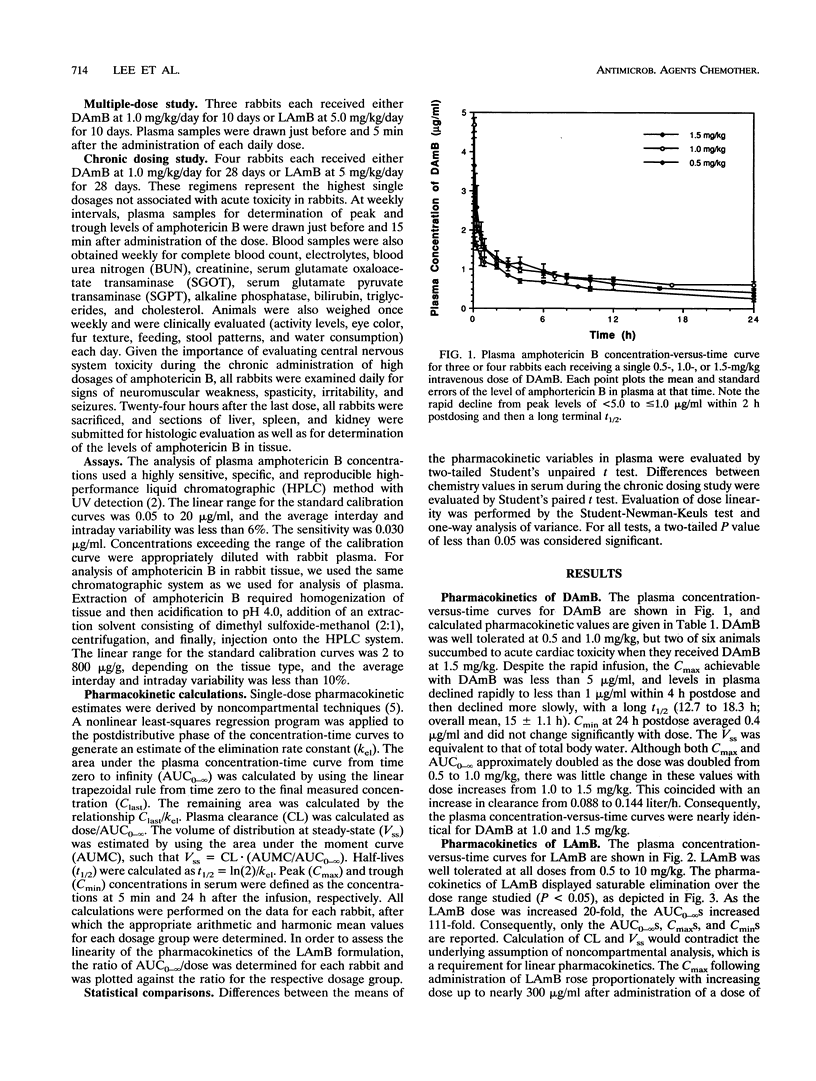
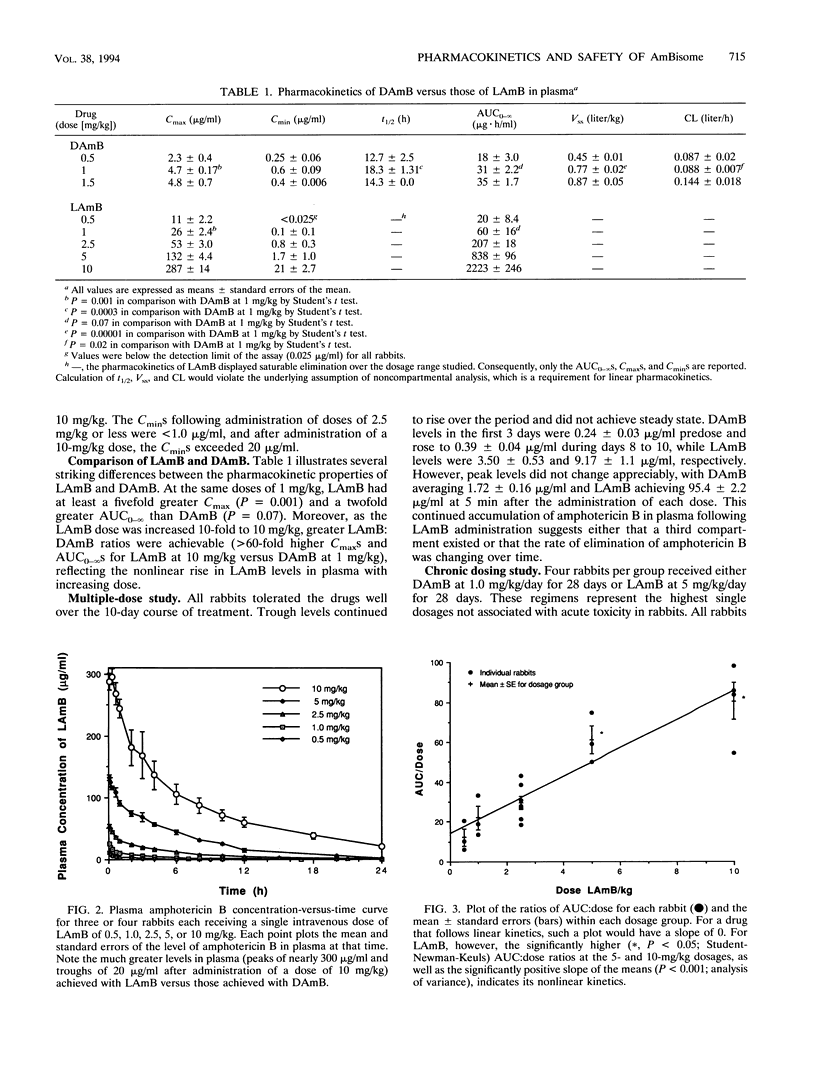
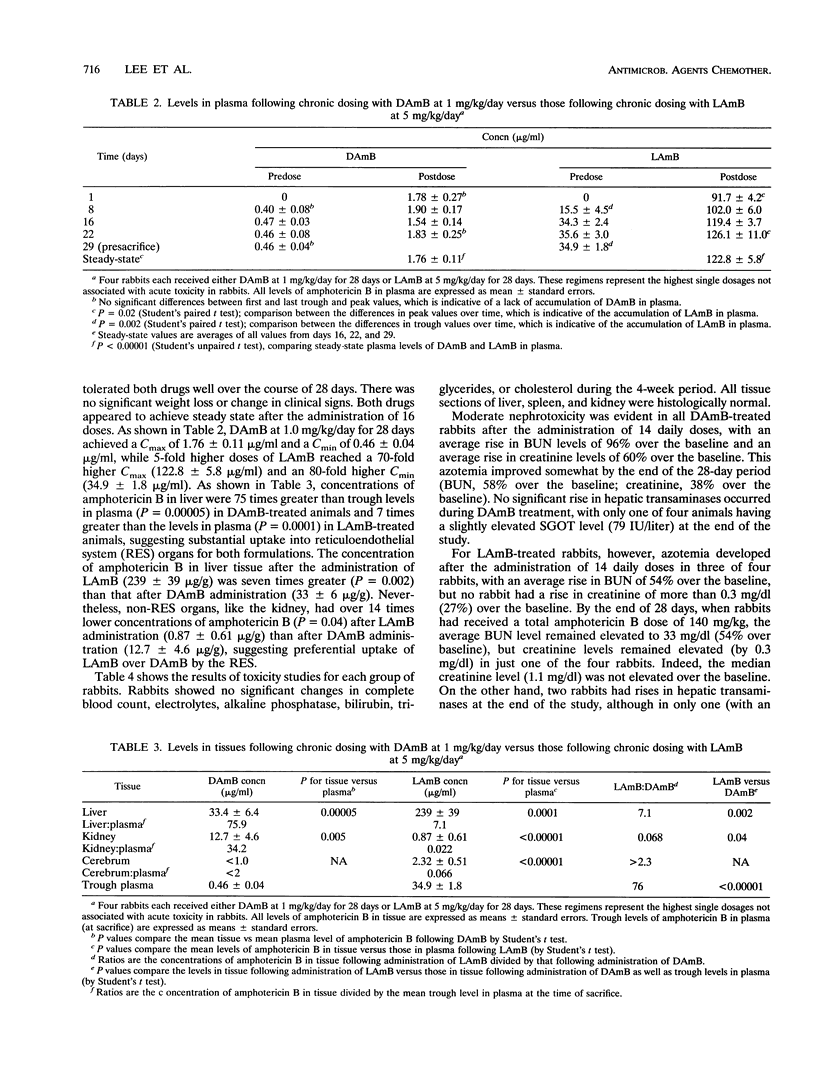
A unilamellar liposomal formulation of amphotericin B (LAmB) known as AmBisome was safely administered intravenously to 20 rabbits at 0.5, 1.0, 2.5, 5, or 10 mg/kg of body weight, whereas of 12 rabbits given desoxycholate amphotericin B (DAmB) intravenously at 0.5, 1.0, or 1.5 mg/kg, 2 died of acute cardiac toxicity when DAmB was administered at the highest dose. Single-dose LAmB (1 mg/kg) achieved a maximum concentration in serum (Cmax) of 26 +/- 2.4 micrograms/ml and an area under the curve to infinity (AUC0-infinity) of 60 +/- 16 micrograms.h/ml, while single-dose DAmB (1.0 mg/kg), by comparison, achieved a lower Cmax (4.7 +/- 0.2 micrograms/ml; P = 0.001) and a lower AUC0-infinity (30.6 +/- 2.2 micrograms.h/ml; P = 0.07). Following administration of a single dose of LAmB (10 mg/kg), a disproportionately higher Cmax (287 +/- 14 micrograms/ml) and AUC0-infinity (2,223 +/- 246 micrograms.h/ml) occurred, indicating saturable elimination. After chronic dosing (n = 4) with LAmB at 5.0 mg/kg/day for 28 days or DAmB at 1.0 mg/kg/day for 28 days, LAmB achieved daily peak levels of 122.8 +/- 5.8 micrograms/ml and trough levels of 34.9 +/- 1.8 micrograms/ml, while DAmB reached a peak of only 1.76 +/- 0.11 microgram/ml and a trough of 0.46 +/- 0.04 microgram/ml (P < or = 0.001). Significant accumulations of amphotericin B into reticuloendothelial organs were observed, with 239 +/- 39 micrograms/g found in the liver after chronic LAmB dosing (5 mg/kg/day), which was seven times higher than the 33 +/- 6 micrograms/g after DAmB dosing (1 mg/kg/day) (P = 0.002). Accumulation in kidneys, however, remained 14-fold lower (P =0.04) following LAmB dosing (0.87 +/- 0.61 microgram/g) than after DAmB dosing (12.7 +/- 4.6 microgram/g). Nephrotoxicity occurred in only one of four LAmB treated animals, while it occurred in all four chronically DAmB-treated animals: mild hepatozicity with transaminase elevations was seen in one LAmB-treated rabbit. We conclude that LAmB safely achieved higher Cmax(s) and AUC0-infinity(s) and demonstrated saturable, nonlinear elimination from plasma via reticuloendothelial organ uptake. Take reduced nephrotoxicity of LAmB correlated with diminished levels of amphotericin B in the kidneys.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abra R. M., Hunt C. A. Liposome disposition in vivo. III. Dose and vesicle-size effects. Biochim Biophys Acta. 1981 Dec 23;666(3):493–503. doi: 10.1016/0005-2760(81)90311-8. [DOI] [PubMed] [Google Scholar]
- Brassinne C., Laduron C., Coune A., Sculier J. P., Hollaert C., Collette N., Meunier F. High-performance liquid chromatographic determination of amphotericin B in human serum. J Chromatogr. 1987 Aug 7;419:401–407. doi: 10.1016/0378-4347(87)80307-9. [DOI] [PubMed] [Google Scholar]
- Chopra R., Blair S., Strang J., Cervi P., Patterson K. G., Goldstone A. H. Liposomal amphotericin B (AmBisome) in the treatment of fungal infections in neutropenic patients. J Antimicrob Chemother. 1991 Oct;28 (Suppl B):93–104. doi: 10.1093/jac/28.suppl_b.93. [DOI] [PubMed] [Google Scholar]
- Coker R. J., Murphy S. M., Harris J. R. Experience with liposomal amphotericin B (AmBisome) in cryptococcal meningitis in AIDS. J Antimicrob Chemother. 1991 Oct;28 (Suppl B):105–109. doi: 10.1093/jac/28.suppl_b.105. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G. Overview of liposomes. J Antimicrob Chemother. 1991 Oct;28 (Suppl B):39–48. doi: 10.1093/jac/28.suppl_b.39. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G., Kasi L., Rosenblum M. G., Haynie T., Jahns M., Glenn H., Mehta R., Mavligit G. M., Hersh E. M. Clinical pharmacology of 99mTc-labeled liposomes in patients with cancer. Cancer Res. 1984 Jan;44(1):375–378. [PubMed] [Google Scholar]
- Meunier F., Prentice H. G., Ringdén O. Liposomal amphotericin B (AmBisome): safety data from a phase II/III clinical trial. J Antimicrob Chemother. 1991 Oct;28 (Suppl B):83–91. doi: 10.1093/jac/28.suppl_b.83. [DOI] [PubMed] [Google Scholar]
- Miller M. A. Reversible hepatotoxicity related to amphotericin B. Can Med Assoc J. 1984 Nov 15;131(10):1245–1247. [PMC free article] [PubMed] [Google Scholar]
- Proffitt R. T., Satorius A., Chiang S. M., Sullivan L., Adler-Moore J. P. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J Antimicrob Chemother. 1991 Oct;28 (Suppl B):49–61. doi: 10.1093/jac/28.suppl_b.49. [DOI] [PubMed] [Google Scholar]
- Proffitt R. T., Williams L. E., Presant C. A., Tin G. W., Uliana J. A., Gamble R. C., Baldeschwieler J. D. Liposomal blockade of the reticuloendothelial system: improved tumor imaging with small unilamellar vesicles. Science. 1983 Apr 29;220(4596):502–505. doi: 10.1126/science.6836294. [DOI] [PubMed] [Google Scholar]
- Ringdén O., Meunier F., Tollemar J., Ricci P., Tura S., Kuse E., Viviani M. A., Gorin N. C., Klastersky J., Fenaux P. Efficacy of amphotericin B encapsulated in liposomes (AmBisome) in the treatment of invasive fungal infections in immunocompromised patients. J Antimicrob Chemother. 1991 Oct;28 (Suppl B):73–82. doi: 10.1093/jac/28.suppl_b.73. [DOI] [PubMed] [Google Scholar]
- Walsh T. J., Bacher J., Pizzo P. A. Chronic silastic central venous catheterization for induction, maintenance and support of persistent granulocytopenia in rabbits. Lab Anim Sci. 1988 Aug;38(4):467–471. [PubMed] [Google Scholar]


