Abstract
Inhalation of allergens can result in mast cell degranulation and release of granule contents, including tryptase, in the lung. Injury to human pulmonary microvascular endothelial cells (HMVEC-L) can also result in activation of the coagulation cascade and thrombin generation. We hypothesize that these proteases activate calcium-independent phospholipase A2 (iPLA2), in HMVEC-L, leading to the production of membrane phospholipids-derived inflammatory mediators. Both thrombin and tryptase stimulation of HMVEC-L increased iPLA2 activity that was inhibited by pretreatment with the iPLA2 selective inhibitor bromoenol lactone (BEL). Arachidonic acid and prostaglandin I2 (PGI2) release were also increased in tryptase and thrombin stimulated cells and inhibited by BEL pretreatment. Pretreating the endothelial cells with AACOCF3 a cytosolic PLA2 inhibitor did not inhibit tryptase or thrombin induced arachidonic acid and PGI2 release. In addition thrombin and tryptase also increased HMVEC-L platelet activating factor (PAF) production that significantly contributes to the recruitment and initial adherence of polymorphonuclear neutrophils (PMN) to the endothelium. Tryptase or thrombin stimulated increase in PMN adherence to the endothelium was inhibited by pretreatment of HMVEC-L with BEL or pretreatment of PMN with CV3988, a PAF receptor specific antagonist. Collectively, these data support our hypothesis that iPLA2 activity is responsible for membrane phospholipid hydrolysis in response to tryptase or thrombin stimulation in HMVEC-L. Therefore selective inhibition of iPLA2 may be a pharmacological target to inhibit the early inflammation in pulmonary vasculature that occurs as a consequence of mast cell degranulation or acute lung injury.
Keywords: Tryptase, Thrombin, Calcium independent phospholipase A2 (iPLA2), Platelet activating factor (PAF), Bromoenol lactone (BEL)
2. INTRODUCTION
Human pulmonary microvascular endothelial cells are subject to injurious stimuli both from the environment and the circulation. A breach in the integrity of the endothelial barrier can result in compromised vascular permeability and acute lung injury (Ware et al., 2000). The increased morbidity and mortality associated with lung injury is due to widespread destruction of the capillary endothelium, extravasation of protein rich fluid and interstitial edema. In addition, the alveolar basement membrane is damaged, and fluid seeps into the airspaces, stiffening the lungs and causing ventilation-perfusion mismatch (Ware et al., 2000). Neutrophils are central to the microvascular and tissue injury during acute phase of lung injury (Doerschuk, 2001, Tate et al., 1983, Wagner et al., 1988) and infiltration of neutrophils into the lung is facilitated by endothelial cell barrier dysfunction (Dull et al., 2002, Garcia et al., 1998, Razavi et al., 2002).
Mast cells are found in the interstitium underlying the endothelial cells. Once the endothelial barrier is compromised, activation of the interstitial mast cells can result in degranulation and release of mediators that can cause stimulation of airway smooth muscle and increased mucous gland secretion. If inflammation proceeds unchecked, these changes collectively result in airway remodeling thereby compromising gas exchange (Carroll et al., 2002, Liu et al., 1990).
Serine proteases such as thrombin and tryptase can activate protease activated receptors type 1 and 2 (PAR-1 and PAR-2) respectively on the endothelial cell surface. This leads to activation of endothelial cell phospholipase A2. Increased platelet activating factor (PAF) production following iPLA2 activation (Rastogi et al., 2007, Rickard et al., 2005)contribute to the progression of inflammation by increasing vascular permeability and the expression of endothelial cell surface adhesion molecules, which facilitate the adhesion and transmigration of inflammatory cells across the endothelial cell monolayer.
The inhibition of specific PLA2 isoforms is potentially an effective therapy for several inflammatory conditions and it has been actively explored for several years. Development of a nebulized form of selective PLA2 inhibitors could therefore be beneficial in pulmonary inflammation and have minimal systemic side effects. Use of these agents would inhibit the production of several membrane phospholipids-derived metabolites that have a role in inflammation, including prostaglandins and PAF.
3. MATERIALS AND METHODS
3.1 Materials
Human lung microvascular endothelial cells (HMVEC-L) were obtained from Lonza (Walkersville, MD). Bromoenol lactone (BEL) was from Cayman Chemicals (Ann Arbor, MI), Thrombin was obtained from Sigma (Saint Louis, MO) and rh skin β-tryptase was obtained from Promega (Madison, WI). All other reagents were purchased from Sigma Chemical Company (St. Louis, MO).
3.2 Culture of endothelial cells
HMVEC-L were grown to confluence in EGM-2MV media (Lonza) and incubated at 37°C, with an atmosphere of 95% O2, 5% CO2. Cells were passaged using the subculture pack (Lonza) in a 1:3 ratio. Cells from passage 3–4 were used for experiments.
3.3 Thrombin and tryptase stimulation
Human recombinant skin β-tryptase and thrombin were diluted with medium (iPLA2 assay, arachidonic acid release, and neutrophil adhesion assay), or Hanks’ balanced salt solution (PAF production) to the working concentration. Tryptase or thrombin was added to the cell culture plate, and the plate was gently rotated to ensure thorough mixing and even distribution of tryptase or thrombin on the HMVEC-L monolayer.
3.4 Phospholipase A2 activity
Cells were grown to confluence in 100 mm culture dishes. At the end of each incubation period, media was removed and immediately replaced with ice cold buffer containing (mmol/l): 250 sucrose, 10 KCl, 10 imidazole, 5 EDTA, 2 dithiothreitol, with 10% glycerol (pH=7.8). The cells were removed from the tissue culture plate by scraping and the suspension was sonicated on ice for 6 bursts of 10 seconds. For separating the cells into membrane and cytosolic fractions, the sonicate was centrifuged at 14,000 g for 10 minutes. The supernatant was centrifuged at 100,000 g for 60 minutes to separate the membrane fraction (pellet) from the cytosolic fraction (supernatant). PLA2 activity was assessed by incubating enzyme (200 µg cellular protein or 8µg membrane protein) with 100 µM (16:0; [3H] 18:1) plasmenylcholine substrate (specific activity approximately 150 dpm/pmol) in assay buffer containing 10mM Tris, 10% glycerol, 4mM EGTA, pH = 7.0 at 37°C for 5 minutes in a total volume of 200µl. Reactions were initiated by adding the radiolabeled phospholipid substrate as a concentrated stock solution in ethanol. Reactions were terminated by the addition of 100µl butanol. The radiolabeled fatty acid released in the above reaction was isolated by application of 25µl of the butanol phase to channeled Silica Gel G plates and then developed in petroleum ether/diethyl ether/acetic acid (70/30/1,v/v/v). Results were quantified by liquid scintillation spectrometry and normalized for protein content in each sample.
3.5 Arachidonic acid release
HMVEC-L were grown to confluence in 35 mm tissue culture dishes. Arachidonic acid release was determined by measuring [3H] arachidonic acid released into the surrounding medium from HMVEC-L prelabeled with 1 µCi of [3H] arachidonic acid (specific activity 100 Ci/mmol; Perkin-Elmer Life Sciences, Boston, MA) per culture dish for 18 h. HMVEC-L were washed three times with HEPES buffer containing (in mmol/l) 133.5 NaCl, 4.8 KCl, 1.2 CaCl2, 1.2 MgCl2, 1.2 KH2PO4, 10 HEPES (pH 7.4), 10 glucose, and 0.36% bovine serum albumin and incubated at 37°C for 15 min before experimental conditions. At the end of the stimulation period, the surrounding medium was transferred to a scintillation vial and the remaining cells were lysed in 10% SDS and the lysate then transferred to a separate vial. Radioactivity in the medium and cells was quantified by liquid scintillation spectrometry. Arachidonic acid mobilized from cellular phospholipids was expressed as the percentage of total incorporated radioactivity.
3.6 PGI2 release
HMVEC-L were grown to confluence in 16 mm tissue culture dishes. Cells were washed twice with Hanks balanced salt solution (HBSS) containing (in mmol/l) 135 NaCl, 0.8 MgSO4, 10 HEPES (pH=7.6), 1.2 CaCl2, 5.4 KCl, 0.4 KH2PO4, 0.3 Na2HPO4, and 6.6 glucose. After washing, 0.5 ml of HBSS with 0.36% bovine serum albumin was added to each culture well. HMVEC-L were then stimulated with the appropriate tryptase and thrombin concentrations. The surrounding buffer was removed from the HMVEC-L after selected time intervals and PGI2 release was measured immediately using an immunoassay kit (R&D Systems, Minneapolis, MN). The protein content for the HMVEC-L confluent monolayers was determined in 3 representative cell culture wells and was assumed to be constant between wells for each experiment.
3.7 Measurement of PAF production
HMVEC-L grown in 34-mm culture dishes (Corning) were washed twice with Hanks’ balanced salt solution containing (in mM) 135 NaCl, 0.8 MgSO4, 10 HEPES (pH 7.4), 1.2 CaCl2, 5.4 KCl, 0.4 KH2PO4, 0.3 Na2HPO4, and 6.6 glucose. Cells were incubated with 10µCi [3H] acetic acid/well for 20 min. After stimulation with tryptase or thrombin, lipids were extracted from the cells using the method of Bligh and Dyer. The chloroform layer was concentrated by evaporation under nitrogen, resuspended in 9:1 CHCl3/ CH3OH, applied to a silica gel 60 TLC plate, and developed in chloroform-methanol-acetic acid-water (50:25:8:4 vol/vol/vol/vol). The region corresponding to [3H]PAF was scraped, and radioactivity was quantified by liquid scintillation spectrometry. Loss of PAF during extraction and chromatography was corrected by adding a known amount of [14C]PAF as an internal standard.
3.8 P-selectin and E-selectin cell surface expression assay
HMVEC- L were grown to confluence in 16 mm culture dishes. Cells were incubated with tryptase (20ng/ml) in Hanks’ buffer for 10 and 30 minutes at 37°C in 95% O2, 5% CO2. At the end of incubations, buffer was quickly removed and cells were immediately fixed with 1% paraformaldehyde and incubated overnight at 4°C. Cells were then washed 3 times with PBS and then blocked with TBST supplemented with 0.8% BSA (wt/vol) and 0.5% fish gelatin (wt/vol) for 1 hour at room temperature. Appropriate primary antibody ( 1:50, Santa Cruz Biotech, CA) was used before treatment with horseradish peroxidase-conjugated rabbit anti-goat secondary antibody (1: 5000, Santa Cruz Biotech, CA). Subsequently, each well was incubated in the dark with the 3,3’, 5,5’-tetramethyl-benzidine liquid substrate system for 30 min. Reactions were stopped by the addition of sulfuric acid, and color development was measured with a microtiter plate spectrophotometer at 450 nm.
3.9 Neutrophil adherence assay
Adult peripheral blood was collected (approved by Saint Louis University School of Medicine-Institutional review board) in vials containing 3.8% sodium citrate, layered over Polymorphprep (Axis-Shield, Oslo, Norway) and centrifuged at 500 × g for 30 min. The top band at the sample/medium interface consisting of mononuclear cells and the lower band of polymorphonuclear cells were removed and washed with Hanks buffer. Supernatant was discarded and the cell pellet re-suspended with 3 ml of 0.2% NaCl and incubated for 3 minutes at room temperature to lyse the red cells. Cells were again resuspended in Hanks, centrifuged at 175 × g for 10 minutes at 4°C. Supernatant was removed and cells resuspended in Hanks buffer. An aliquot was taken for a cell count utilizing a hemacytometer. HMVEC-L were grown to confluence on a 12 -well plate, washed with Hanks buffer and respective stimulants /inhibitors were added. CV3988 was added directly to the neutrophils when used. 500 µl of neutrophils in suspension were added to each of the wells and incubated for 10 minutes at room temperature. Media and unbound neutrophils were removed and discarded. Plates were washed twice with Dulbecco’s Phosphate Buffered Saline (PBS). 1 ml of 0.2% Triton X-100 was added to each well to lyse adherent neutrophils and HMVEC-L. Cell lysates were scraped from the plate and transferred to an Eppendorf tube. A 500 µl aliquot of neutrophil suspension was added to 500 µl of 0.2% Triton X-100 and used as the theoretical maximal binding sample. Samples were sonicated (550 Sonic Dismembrator, Fisher Scientific, Pittsburgh, PA) for 10 seconds. To measure neutrophil peroxidase activity, 400 µl of cell lysate was transferred to a glass tube and 1 ml of PBS, 1.2 ml Hanks Buffer + BSA, 200 µl 3,3'-dimethyoxybenzidine, and 200 µl of 0.05% H2O2 added and mix incubated for 15 min at room temperature. 200 µl of 1% sodium azide (NaN3) was added to stop the reaction. The absorbance was then measured using a 4050 UV/Visible Spectrophotometer (Biochrom, Cambridge, England) at 450 nm.
3.10 Statistical analysis
Serial changes in within-group variables were analyzed by a repeated – measure analysis of variance. For all of the studies in which the experimental protocol involved three or more groups, data were analyzed using one-way ANOVA. Values are presented as means± SE. P-values of <0.05 were considered statistically significant (shown as * or +); p-values of <0.01 were considered highly statistically significant (shown as ** or ++). StatMost (Dataxiom Software Inc. Los Angeles, CA) software was used for all calculations.
4. RESULTS
4.1 Effect of tryptase and thrombin stimulation on PLA2 activity
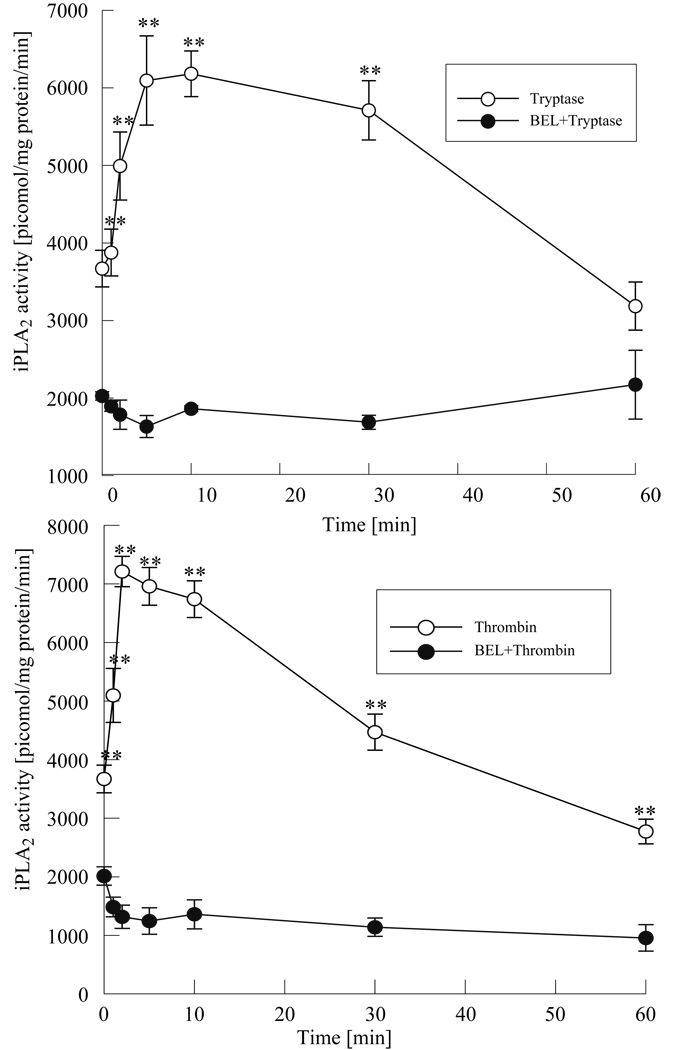
Our studies have shown that thrombin and tryptase activate endothelial cell calcium independent phospholipase A2 (Rastogi et al., 2007, Rickard et al., 2005). Since HMVEC-L has several unique features distinguishing it from other vascular endothelial beds (Horgan et al., 1991, Six et al., 2000) we wanted to examine if tryptase and thrombin would also activate iPLA2 in these cells. We treated HMVEC-L with tryptase (20ng/ml) or thrombin (0.1 IU/ml) and measured PLA2 activity using (16:0; [3H] 18:1) plasmenylcholine as a substrate. EDTA was added to the assay medium to chelate calcium and selectively measure the activity of calcium independent PLA2 (iPLA2). As shown in Figure 1a incubation with tryptase resulted in a rapid increase in iPLA2 activity that was maximal at 10 minutes and was followed by a gradual decline to basal levels by 60 min. Stimulation of HMVEC-L with thrombin also showed a rapid increase in iPLA2 activity by 2 minutes, which returned to unstimulated levels by 60 minutes (Figure 1b). Pretreatment of the cells with BEL (2µM, 10 min.) a selective iPLA2 inhibitor attenuated both tryptase and thrombin induced increase in iPLA2 activity. A slight delay in iPLA2 activation with tryptase compared with thrombin stimulation may reflect a difference in coupling of the enzyme to the receptors for thrombin and tryptase in HMVEC-L.
Figure 1 a and b. Tryptase and thrombin induced iPLA2 activity in HMVEC-L.
Calcium independent PLA2 activity was measured in HMVEC-L after stimulation with tryptase (20ng/ml, Figure 1 a) or thrombin (0.1 IU/ml, Figure 1b) with or without pretreatment with BEL (2µM, 10 min.). Data shown represent the mean ±SE for 4 independent experiments.**p< 0.01 when comparing tryptase or thrombin stimulated values vs. corresponding BEL pretreated samples.
4.2 Effect of tryptase and thrombin stimulation on arachidonic acid release
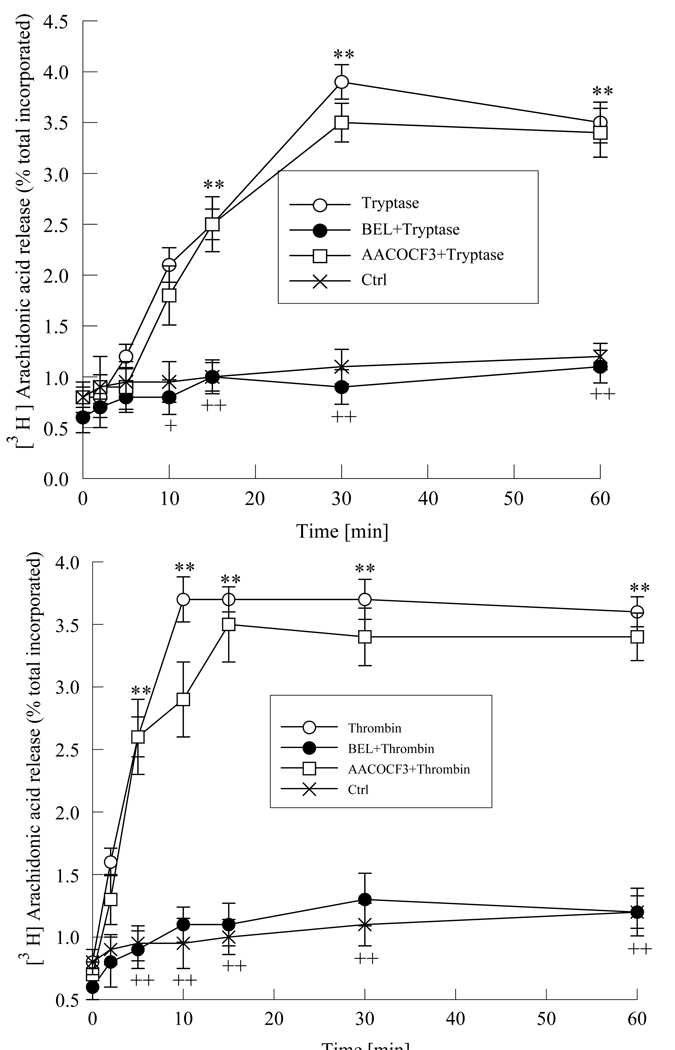
Activation of iPLA2 results in hydrolysis of cell membrane phospholipids, leading to increased arachidonic acid release. We stimulated HMVEC-L with tryptase (20ng/ml) or with thrombin (0.1 IU/ml) and measured arachidonic acid release over 60 minutes. Maximal increase in arachidonic acid release from tryptase stimulated cells was seen at 30 minutes (Figure 2a) in contrast to thrombin (0.1 IU/ml, Figure 2b) stimulated HMVEC-L where a maximal response was seen by 10 minutes. The increase in arachidonic acid release from HMVEC-L stimulated with thrombin or tryptase was completely inhibited by pretreatment with BEL (2µM, 10 min.), further supporting the involvement of iPLA2 (Figure 2). Pretreatment of the cells with AACOCF3 (2µM, 10 min.) a cytosolic PLA2 inhibitor does not cause any significant change in both tryptase or thrombin stimulated arachidonic acid release. This suggests that in the early phase of response to thrombin or tryptase stimulation endothelial cell iPLA2 plays a major role.
Figure 2 a and b. Tryptase and thrombin increased arachidonic acid release.
Arachidonic acid release was measured in cells after stimulating with tryptase (20ng/ml, Figure 2a) or thrombin (0.1 IU/ml, Figure 2b) with or without pre-treatment with BEL (2µM,10min.), for increasing time intervals. Cells were also pretreated with AACOCF3 (5µM, 10min.) prior to tryptase stimulation. Results represent mean ± SE of 4 independent experiments. * and ** P< 0.05 and 0.01 respectively when compared to control cells. + p<0.05 and ++ p<0.01 when compared to tryptase or thrombin treated cells respectively
4.3 Effect of tryptase and thrombin stimulation on prostaglandin I2 production
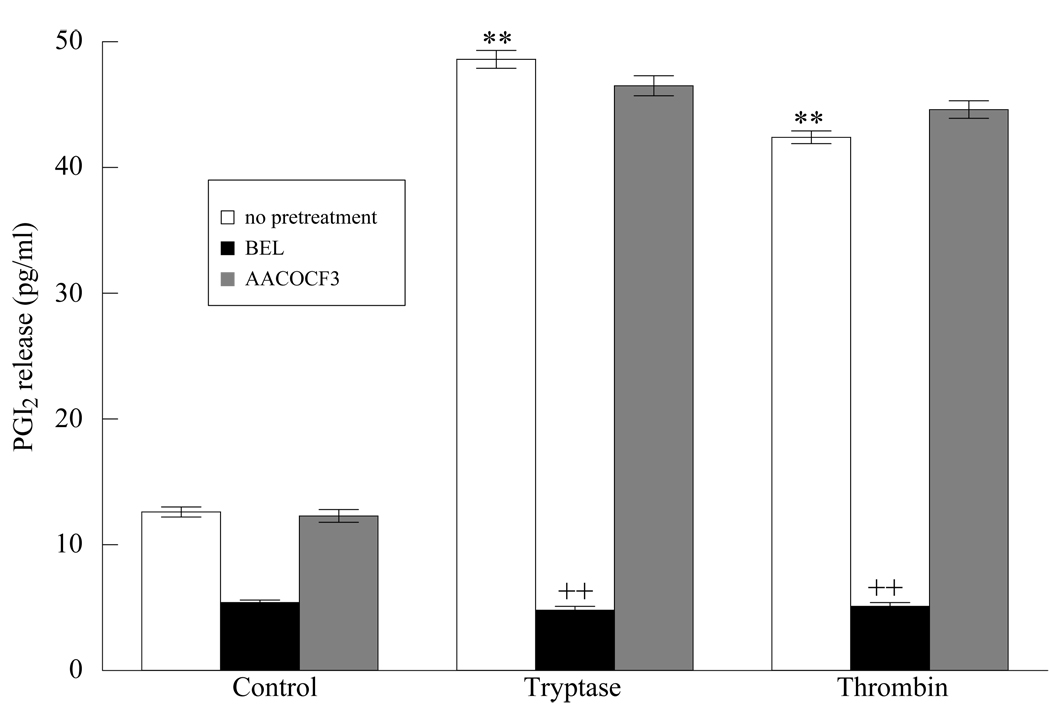
To determine whether arachidonic acid liberated from membrane phospholipids is further metabolized by cyclooxygenase enzymes, resulting in release of prostaglandin I2 from HMVEC-L monolayers, we measured PGI2 production in response to tryptase and thrombin stimulation. We observed a dose and time dependent increase in PGI2 release with both thrombin and tryptase (Figure 3). Pretreatment with BEL completely inhibited PGI2 release in response to thrombin and tryptase stimulation (Figure 4). Incubation of the cells with AACOCF3 (cytosolic PLA2 inhibitor) did not cause a significant change in the levels of PGI2 release further supporting our findings that iPLA2 plays a major role in the early phase of membrane phospholipid hydrolysis.
Figure 3. Dose dependent changes in PGI2 production in HMVEC-L.
HMVEC-L were treated with tryptase (2, 20, 200 ng/ml) or thrombin (0.1, 0.5, 1.0 IU/ml ) for upto 60 min. and PGI2 production was measured. Values represent mean ± SE for 4 separate experiments. ** P< 0.01 when compared to control cells.
Figure 4. PGI2 production increased in HMVEC-L.
HMVEC-L were treated with tryptase (20 ng/ml, 10 min.) or thrombin (0.1 IU/ml, 10 min.). PGI2 production was measured with or without pretreatment BEL (2µM, 10 min.), or AACOCF3. (2µM, 10 min.) for 10 minutes. ** P< 0.01 when compared to control cells, ++ P<0.01 compared to tryptase or thrombin stimulated cells. Results represent mean ± SE of 4 separate experiments.
4.4 Effect of tryptase and thrombin stimulation on platelet activating factor production
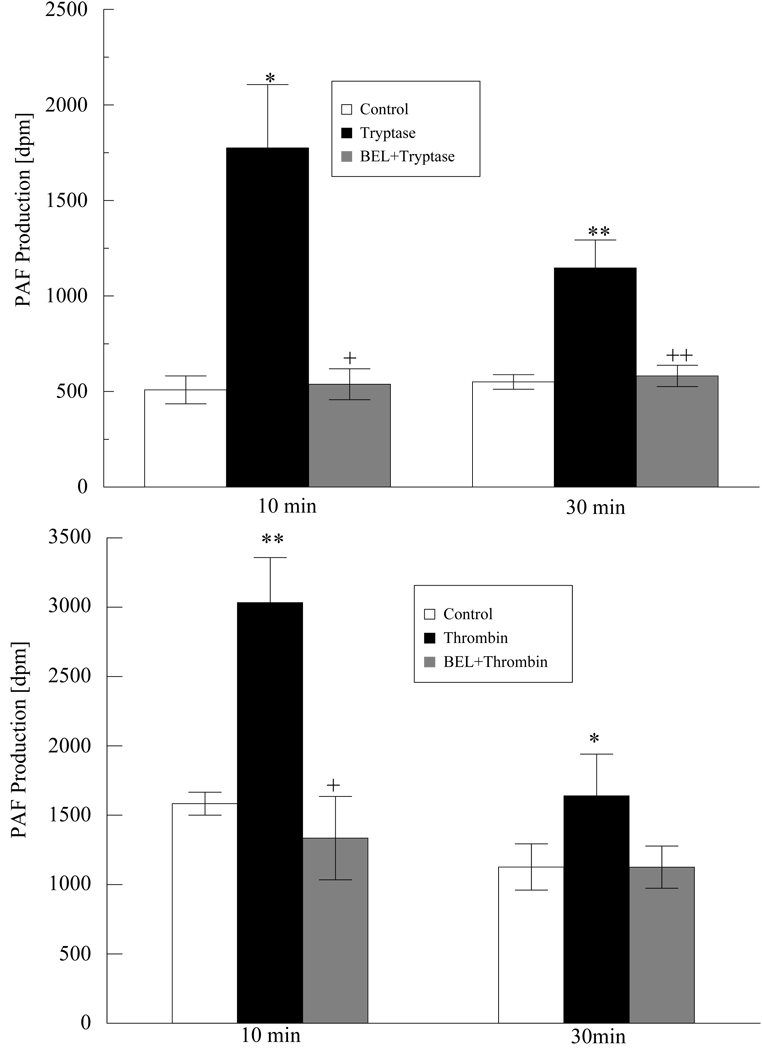
PLA2 catalyzed release of arachidonic acid from cell membrane phospholipids is accompanied by the stoichiometric production of lysophospholipids including lyso-PAF, which can be rapidly acetylated to PAF. To determine whether PAF production in HMVEC-L is dependent on iPLA2 activation, we measured PAF production at 10 and 30 min. after tryptase (Figure 5) or thrombin (Figure 5) stimulation. Both agents resulted in a significant increase in PAF production at 10 minutes. The PAF production remained elevated significantly even at 30 minutes. The tryptase or thrombin induced PAF production was completely inhibited by BEL pretreatment indicating that iPLA2 is also involved in PAF production.
Figure 5 a and b. PAF production increased in tryptase and thrombin stimulated HMVEC-L.
Cells were incubated with tryptase (20ng/ml, Figure 4a) or thrombin (0.1IU/ml, Figure 4b) for 10 and 30 min. or left untreated. PAF production was measured with or without pretreatment with BEL ( 2µM, 10 min). Data represent means ± SE for 4 separate experiments. * P < 0.05 and **P<0.01 compared with control. + P<0.05 and ++ P<0.01 compared to tryptase or thrombin stimulated cells.
4.5 Effect of tryptase and thrombin stimulation on neutrophil adherence
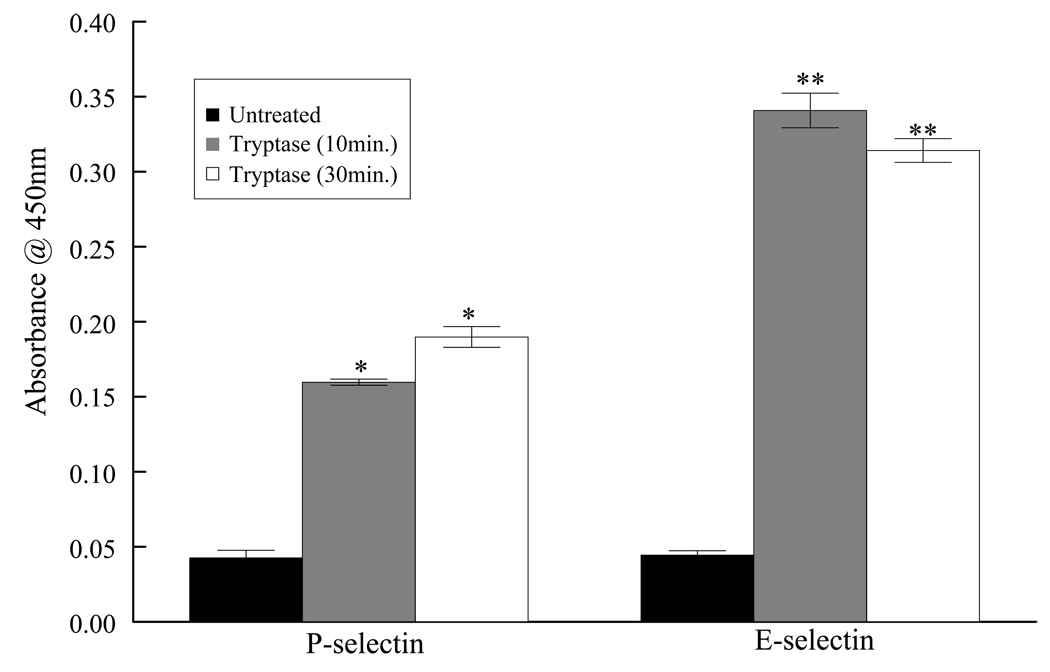
PAF expressed by endothelial cells binds to its cognate receptors on circulating inflammatory cells, leading to neutrophil adherence to the endothelium (Ostrovsky et al., 1998). Because tryptase and thrombin stimulation of HMVEC-L both lead to increased PAF production, we determined whether these proteases promote adhesion of circulating neutrophils to the endothelium. In addition, glycoproteins such as P and E selectins also participate in the initial rolling and adhesion of neutrophils to the activated endothelium. Indeed stimulation of HMVEC-L with tryptase resulted in 4.5 fold increase in P-selectin and 7.75 fold increase in E-selectin expression by 30 min. on the endothelial cell surface allowing for the possibility of increased adherence of inflammatory cells (Figure 6).
Figure 6a. Tryptase increased cell surface expression of selectins.
HMVEC-L were stimulated with tryptase (20ng/ml) and cell surface expressiob of P- and E-selectin was measured at 10 and 30 min. Results represent mean ± SE from four separate experiments. * P <0.05 and **P<0.01 when compared with control cells.
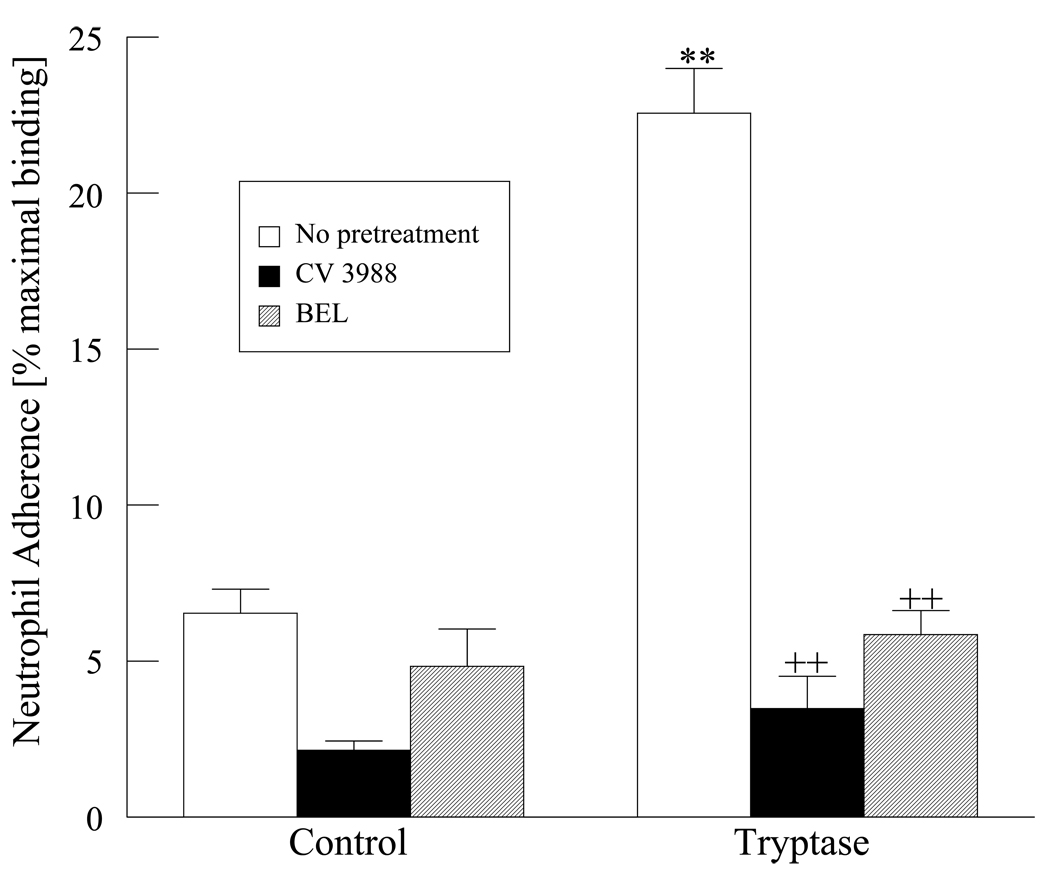
Neutrophils were isolated from peripheral human blood and incubated with a confluent monolayer of HMVEC-L stimulated with either tryptase or thrombin. As shown in Figure 7, tryptase stimulation of HMVEC-L monolayers results in a 3 fold increase in neutrophil adherence over unstimulated HMVEC-L. Thrombin stimulation of the endothelial cell monolayers also results in a 3.5 fold increase in neutrophil adherence. Pretreatment of the HMVEC-L with BEL significantly inhibited neutrophil adherence after either tryptase or thrombin stimulation. In addition, pretreatment of the neutrophils with CV3988 a PAF receptor specific antagonist also inhibited neutrophil adherence to thrombin or tryptase stimulated endothelium (Figure 7).
Figure 7. Tryptase and thrombin induced neutrophil adherence.
Neutrophil adherence was measured in tryptase (20ng/ml, 10 min.) or thrombin (0.1 IU/ml, 10 min.) treated HMVEC-L. Endothelial cells were pretreated with BEL (2µM,10min.). CV 3988 (10µM,10min.) was incubated directly with neutrophils to inhibit PAF receptor. ** P <0.01 compared to control cells, ++ P <0.01 when compared to tryptase or thrombin treated samples. Results represent mean ± SEM of 3 separate experiments.
Taken together these data demonstrate that tryptase and thrombin can activate iPLA2 in HMVEC-L leading to increased arachidonic acid and PGI2 release in conjunction with PAF production and neutrophil adherence. All of these factors can collectively contribute to pulmonary inflammation.
5. DISCUSSION
The pulmonary vascular bed has several properties distinguishing it from other systems. The normal pulmonary circulation is a low pressure, high-flow vascular bed, accommodating the entire cardiac output (Kuwano et al., 1993, Weibel, 1963). In response to an increase in cardiac output, there is recruitment of underperfused microvessels and distension of patent vessels. In addition, tone of the smooth muscle in the media of pulmonary arterioles is lower and the smooth muscle coat of pulmonary resistance vessels is thinner than that of most systemic vascular beds. As a result of obstructive pathological changes in the pulmonary vasculature, such as thrombotic lesions, intimal fibrosis, there is an increase in pulmonary vascular resistance and artery pressure (Vonk-Noordegraaf et al., 2005). However, due to the distensibility and the large recruitment capacity of the pulmonary vascular bed the pulmonary arterial pressures will rise much later in the course of obstruction. This outlines the necessity of exploring other markers as potential predictors of injury to pulmonary microcirculation.
Inhalation of allergens in the airways leads to activation of mast cells via cross linking of IgE receptors and their rapid degranulation. Inflammation is the initial response to lung injury occurring secondary to mast cell degranulation or activation of the coagulation cascade. It is characterized by release of numerous plasma membrane–derived mediators, including metabolites of arachidonic acid, sphingomyelin, lysophospholipid, ceramide, and platelet-activating factor (PAF) which contribute to the recruitment of macrophages, neutrophils, lymphocytes, and eosinophils within the alveolar and interstitial compartment of the lung (Liu et al., 1996).
Thrombin is present in the plasma exudate during inflammation and activates the G-protein coupled protease receptor receptor-1(PAR-1). It acts on aortic smooth muscle causing vasoconstriction and increases pulmonary microvascular endothelial permeability (Garcia et al., 1996, Horgan et al., 1991 a and b). It causes lung injury by increasing permeability of alveolar epithelium and vascular endothelium resulting in extravasation of plasma proteins, activation of the coagulation system, and deposition of fibrin clots in the alveolar spaces that impairs gas exchange (Miller et al., 2002). Additionally thrombin can also affect cytokine release and adhesion molecule expression (Senden et al., 1998). Thrombin serves as both a pro- and anticoagulant molecule. Additionally it to play multifunctional roles related to inflammation, allergy, tumor growth and metastasis, and wound healing (Coughlin SR, 2000, Cirino et al., 2000). While many actions of thrombin can be attributed to activation of PAR1 (and possibly PAR-3 and PAR-4) it should be pointed out, however, that PAR1 does not represent the only target for action of thrombin. Other non-PAR high-affinity binding sites, such as those found on platelets, macrophages (Kudahl et al., 1991) and other conserved thrombin sequences apart from the catalytic domain (Bar-Shavit et al., 1986, Steinhoff et al., 2005) may also play a role in the cellular actions of thrombin in a variety of target tissues.
Tryptase, a serine protease released from mast cell granules in response to an allergen challenge can activate protease activated receptor -2 (PAR-2) in a manner similar to thrombin activation of PAR-1. Tryptase levels are elevated in the bronchoalveolar lavage (BAL) fluid from atopic asthmatics compared with nonasthmatic controls at baseline and are significantly increased shortly after antigen challenge in patients with allergic asthma (Jarjour et al., 1991). Tryptase promotes human mast cell degranulation (He et al., 1998), induces eosinophil and neutrophil migration, amplifies the bronchoconstrictor effects of histamine on lung tissue (Tam et al., 1990) and stimulates the growth of airway fibroblasts, smooth muscle cells, and epithelial cells (Cairns et al., 1996).
In our experiments, thrombin and tryptase stimulation of HMVEC-L increases iPLA2 activity and release of membrane phospholipid metabolites including arachidonic acid that is rapidly metabolized to prostagland I2. There are 3 classes of PLA2 namely secretory (sPLA2), cytosolic (cPLA2) and calcium independent (iPLA2) that represent an expanding family of distinct enzymes which coexist in mammalian cells and interact with each other (Meyer et al., 2005, Six et al., 2000). The 2 crucial rate-limiting steps for PG synthesis are activation of PLA2 to release arachidonic acid from membrane phospholipids and subsequent conversion of arachidonic acid to the intermediate PG precursor PGH2. It remains unclear to date whether distinct PLA2 isoforms are used specifically with each COX isoform in eicosanoid generation. cPLA2α has been proposed to have a central role in immediate and delayed eicosanoid production, primarily since cPLA2α knockout mice produce minimal eicosanoids in response to stimuli (Fujishima et al., 1999).
The difference in the time course between iPLA2 activation and PAR-1 or PAR-2 stimulation as observed in this study suggests that there may be a difference in receptor density or time of receptor activation at the endothelial cell surface, or that there are differences in the intracellular signaling pathways between activation of iPLA2 and receptor activation. Previous experiments by our laboratory examining the signaling events linking PAR-1 to activation of iPLA2 have demonstrated that a member of the novel protein kinase C (PKC) isoenzyme family (calcium-independent, phorbol 12-myristate, 13-acetate dependent) mediates iPLA2 activation following thrombin stimulation of the isolated membrane fraction. Activation of iPLA2 following thrombin stimulation could be the result of any combination of the following three events: 1) direct phosphorylation of iPLA2 by PKC resulting in enhanced catalytic activity 2) modulation of enzyme activity by protein-protein interactions mediated by PKC phosphorylation of an iPLA2 regulatory protein or 3) targeted delivery of iPLA2 to membrane domains enriched in arachidonylated plasmalogen phospholipids, the preferred substrate for HCAEC iPLA2 (Meyer et al., 2005). Such events have yet to be explored for tryptase mediated activation of PAR-2.
In our study we noted that pretreatment with BEL completely inhibited PGI2 production. Bromoenol lactone (BEL) appears to be the most isoform-specific inhibitor developed to date. It demonstrates 100-fold selectivity for iPLA2 when compared to cPLA2 and sPLA2 isoforms (Hazen et al., 1991). Our data using BEL pretreatment of HMVEC-L suggest that iPLA2 is responsible for the production of arachidonic acid in the immediate response to thrombin and tryptase. Pre-treatment of the endothelial cells with AACOCF3 did not have any effect on PGI2 release. AACOCF3 is a tight-binding, reversible inhibitor that forms a stable hemiketal with the active site serine residues in both cPLA2 and iPLA2 enzymes (Nagase et al., 2003). Therefore it appears that iPLA2 and not cPLA2 is primarily responsible for the early phase of prostaglandin generation in endothelial cells. Thus, it is possible that the PLA2 isoform responsible for immediate PG production can depend on the cell type and the species studied (Rastogi et al., 2007, Rickard et al., 2005).
HMVEC-L upon stimulation with either tryptase or thrombin produce platelet-activating factor (PAF), a biologically active phospholipid which is then translocated to the plasma membranes of endothelial cells where it mediates PMN adhesion by binding to its receptor on the PMN (Ostrovsky et al., 1998, Riches et al., 1990). PAF also causes increased permeability and pulmonary edema by inducing ceramide synthesis and cyclooxygenase activity (Göggel et al., 2004). After production, PAF remains cell associated and assists in the tethering and migration of circulating cells through the endothelium. The resultant injury is characterized by increased pulmonary microvascular permeability, and sequestration of neutrophils, platelets, and fibrin (Cooper, et al., 1984).
Additionally, treatment of HMVEC-L with tryptase leads to an upregulation of cell surface adhesion molecules including P and E-selectin (data not shown). Selectins also play an important role in the initial adherence of circulating neutrophils to the endothelium. P-selectin is stored in Weibel-Palade bodies and rapidly expressed on the surface in response to tryptase and thrombin stimulation (Epperson et al., 2000). It primarily binds to P-selectin glycoprotein ligand-1 (PSGL-1) expressed on the neutrophil surface where it mediates their tethering and adhesion (Xia et al., 2002). Additionally P-selectin can itself activate the endothelium and set up a positive feedback loop. It is reported that P- selectin primes monocytes for increased phagocytosis and induces the formation of tissue factor thus having a direct role in inflammtion. P-selectin knockout mice demonstrate a prolonged bleeding time (Berger et al., 2003, Elstad et al., 1995) and hearts from P-selectin KO mice when transplanted into wt mice showed decreased graft neutrophil infiltration and increased graft survival (Xia et al., 2002). E-selectin participates in the process of firm adhesion of neutrophils to activated endothelial layer (Smith et al., 2004). E-selectin is expressed in the skin where it supports the recruitment of skin specifc T-lymphocytes under baseline conditions (Keelan et al., 1994).
Subsequent to tryptase or thrombin induced PAF production we observed increase in neutrophil adherence to the HMVEC-L. Neutrophils infiltrate the pulmonary interstitium in response to an inflammatory stimulus and can additionally contribute to the generation of reactive oxygen species and proteolytic enzymes. They serve to amplify and perpetuate the recruitment of other leukocytes to the airspaces in certain diseases (Doherty et al., 1994). Neutrophils can also generate leukotrienes from arachidonic acid and add to lung injury (Van Antwerpen et al., 1993). In response to noxious stimuli neutrophils are induced to leave the pulmonary vasculature, migrate through the lung interstitium, and ultimately accumulate in the airways. This process involves a complex series of events including sequential changes in cell size and stiffness, up-regulation of adhesion molecules, cytoskeletal rearrangement, and chemotaxis (Rosengren et al., 1994, Worthen et al., 1994). Superoxide production induced by activation of neutrophils adherent to the vessel wall can cause endothelial injury leading to edema formation. Pretreatment of the neutrophils with PAF receptor antagonist CV3988 significantly reduced neutrophil adherence indicating the importance of PAF-PAF receptor interaction in this process.
The data presented here demonstrate that thrombin and tryptase stimulation of HMVEC-L activates iPLA2, resulting in increased production of arachidonic acid, PAF, and PGI2. that may play a role in inflammation. Using both BEL and CV3988 a platelet activating factor receptor (PTAFR) selective antagonist inhibits the neutrophil adherence to the endothelium. This indicates that iPLA2 inhibition and/or platelet activating factor receptor antagonism may be effective targets to limit pulmonary microvascular endothelial inflammation.
Table No. 1.
Phospholipase A2 (PLA2) activity (nmol.mg protein−1.min−1) in membrane and cytosolic subcellular fractions from human lung microvascular endothelial cells under control conditions or stimulated with thrombin (0.1 IU/ml, 5 mins) or tryptase (20 ng/ml, 5 mins). Where appropriate, cells were pretreated with bromoenol lactone (BEL, 2 µM, 10 mins) or arachidonyl trifluoromethyl ketone (AACOCF3, 5 µM, 10 mins) prior to thrombin or tryptase stimulation. PLA2 activity was measured using (16:0, [3H]18:1) plasmenylcholine substrate in the absence (4 mM EGTA) or presence (1 mM Ca2+) of calcium. Values represent mean ± SEM for separate measurements from 3 different cell isolations.
| Cell Fraction | EGTA | Ca2+ | EGTA | Ca2+ | EGTA | Ca2+ | |
|---|---|---|---|---|---|---|---|
| BEL | BEL | AACOCF3 | AACOCF3 | ||||
| Membrane | Control | 4.22 ± 0.16 | 4.56 ± 0.21 | 1.81 ± 0.12 | 1.72 ± 0.21 | 4.19 ± 0.21 | 4.33 ± 0.31 |
| Thrombin | 8.78 ± 0.34 | 9.13 ± 0.57 | 3.11 ± 0.31 | 2.95 ± 0.37 | 7.94 ± 0.52 | 8.03 ± 0.41 | |
| Tryptase | 8.19 ± 0.48 | 8.56 ± 0.41 | 2.78 ± 0.42 | 2.56 ± 0.18 | 8.31 ± 0.47 | 8.55 ± 0.18 | |
| Cytosol | Control | 0.42 ± 0.05 | 0.51 ± 0.06 | 0.19 ± 0.04 | 0.21 ± 0.04 | 0.29 ± 0.05 | 0.31 ± 0.08 |
| Thrombin | 0.48 ± 0.12 | 0.51 ± 0.12 | 0.22 ± 0.02 | 0.16 ± 0.03 | 0.32 ± 0.04 | 0.31 ± 0.09 | |
| Tryptase | 0.45 ± 0.09 | 0.53 ± 0.12 | 0.17 ± 0.02 | 0.23 ± 0.02 | 0.29 ± 0.03 | 0.27 ± 0.05 |
ACKNOWLEDGEMENTS
This work was supported by NHLBI Grant HL-68588 (JM) and AHA-Heartland Affiliate pre doctoral fellowship 0610118Z (PR).
REFERENCES
- Bar-Shavit R, Wilner GD. Biologic activities of nonenzymatic thrombin: elucidation of a macrophage interactive domain. Semin. Thromb. Hemost. 1986;12:244–249. doi: 10.1055/s-2007-1003561. [DOI] [PubMed] [Google Scholar]
- Burger PC, Wagner DD. Platelet P-selectin facilitates atherosclerotic lesion development. Blood. 2003;101(7):2661–2666. doi: 10.1182/blood-2002-07-2209. [DOI] [PubMed] [Google Scholar]
- Cairns JA, Walls AF. Mast cell tryptase is a mitogen for epithelial cells stimulation of IL-8 production and intercellular adhesion molecule-1 expression. J Immunol. 1996;156(1):275–283. [PubMed] [Google Scholar]
- Carroll NG, Mutavdzic S, James AL. Distribution and degranulation of airway mast cells in normal and asthmatic subjects. Eur Respir J. 2002;19:879–885. doi: 10.1183/09031936.02.00275802. [DOI] [PubMed] [Google Scholar]
- Cirino G, Napoli C, Bucci M, Cicala C. Inflammation-coagulation network: are serine protease receptors the knot? Trends Pharmacol Sci. 2000;21:170–172. doi: 10.1016/s0165-6147(00)01469-3. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Solano SJ, Bizios R, Kaplan JE, Malik AB. Pulmonary neutrophil kinetics after thrombin-induced intravascular coagulation. J Appl Physiol. 1984;57(3):826–832. doi: 10.1152/jappl.1984.57.3.826. [DOI] [PubMed] [Google Scholar]
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- Doerschuk CM. Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation. 2001;8:71–88. [PubMed] [Google Scholar]
- Doherty DE, Downey GP, Schwab BI, Elson E, Worthen GS. Lipopolysaccharide-induced monocyte retention in the lung: role of monocyte stiffness, actin assembly, and CD18-dependent adherence. J. Immunol. 1994;153:241. [PubMed] [Google Scholar]
- Dull RO, Garcia JGN. Leukocyte-induced microvascular permeability: how contractile tweaks lead to leaks. Circ Res. 2002;90:1143–1144. doi: 10.1161/01.res.0000023047.87638.76. [DOI] [PubMed] [Google Scholar]
- Elstad MR, La Pine TR, Cowley FS, McEver RP, McIntyre TM, Prescott SM, Zimmerman GA. P-selectin regulates platelet-activating factor synthesis and phagocytosis by monocytes. J Immunol. 1995;155(4):2109–2122. [PubMed] [Google Scholar]
- Epperson TK, Patel KD, McEver RP, Cummings RD. Noncovalent association of P-selectin glycoprotein ligand-1 and minimal determinants for binding to P-selectin. J Biol Chem. 2002;275(11):7839–7853. doi: 10.1074/jbc.275.11.7839. [DOI] [PubMed] [Google Scholar]
- Fujishima H, Sanchez Mejia RO, Bingham CO, 3rd, Lam BK, Sapirstein A, Bonventre JV, Austen KF, Arm JP. Cytosolic phospholipase A2 is essential for both the immediate and the delayed phases of eicosanoid generation in mouse bone marrow-derived mast cells. Proc Natl Acad Sci U S A. 1999;96(9):4803–4807. doi: 10.1073/pnas.96.9.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JG, Verin AD, Herenyiova M, English D. Adherent neutrophils activate endothelial myosin light chain kinase: role in transendothelial migration. J Appl Physiol. 1998;84:1817–1821. doi: 10.1152/jappl.1998.84.5.1817. [DOI] [PubMed] [Google Scholar]
- Göggel R, Winoto-Morbach S, Vielhaber G, Imai Y, Lindner K, Brade L, Brade H, Ehlers S, Slutsky AS, Schütze S, Gulbins E, Uhlig S. PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med. 2004;10(2):155–160. doi: 10.1038/nm977. [DOI] [PubMed] [Google Scholar]
- Hazen SL, Zupan LA, Weiss RH, Getman DP, Gross RW. Suicide inhibition of canine myocardial cytosolic calcium-independent phospholipase A2. Mechanism-based discrimination between calcium-dependent and -independent phospholipases A2. J Biol Chem. 1991;266(11):7227–7732. [PubMed] [Google Scholar]
- He S, Gaça MD, Walls AF. A role for tryptase in the activation of human mast cells: modulation of histamine release by tryptase and inhibitors of tryptase. J Pharmacol Exp Ther. 1998;286(1):289–297. [PubMed] [Google Scholar]
- Horgan MJ, Pinheiro JM, Malik AB. Mechanism of endothelin-1-induced pulmonary vasoconstriction. Circ Res. 1991a;69(1):157–164. doi: 10.1161/01.res.69.1.157. [DOI] [PubMed] [Google Scholar]
- Horgan MJ, Ge M, Gu J, Rothlein R, Malik AB. Role of ICAM-1 in neutrophil-mediated lung vascular injury after occlusion and reperfusion. Am J Physiol. 1991b;261(5 Pt 2):H1578–H1584. doi: 10.1152/ajpheart.1991.261.5.H1578. [DOI] [PubMed] [Google Scholar]
- Jarjour NN, Calhoun WJ, Schwartz LB, Busse WW. Elevated bronchoalveolar lavage fluid histamine levels in allergic asthmatics are associated with increased airway obstruction. Am Rev Respir Dis. 1991;144(1):83–87. doi: 10.1164/ajrccm/144.1.83. [DOI] [PubMed] [Google Scholar]
- Keelan ET, Licence ST, Peters AM, Binns RM, Haskard DO. Characterization of E-selectin expression in vivo with use of a radiolabeled monoclonal antibody. Am J Physiol. 1994;266(1 Pt 2):H278–H290. doi: 10.1152/ajpheart.1994.266.1.H279. [DOI] [PubMed] [Google Scholar]
- Kudahl K, Fisker S, Sonne O. A thrombin receptor in resident rat peritoneal macrophages. Exp Cell Res. 1991;193:45–53. doi: 10.1016/0014-4827(91)90536-4. [DOI] [PubMed] [Google Scholar]
- Kuwano K, Bosken CH, Paré PD, Bai TR, Wiggs BR, Hogg JC. Small airways dimensions in asthma and in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1993;148(5):1220–1225. doi: 10.1164/ajrccm/148.5.1220. [DOI] [PubMed] [Google Scholar]
- Liu L, Mul FP, Kuijpers TW, Lutter R, Roos D, Knol EF. Neutrophil transmigration across monolayers of endothelial cells and airway epithelial cells is regulated by different mechanisms. Ann N Y Acad Sci. 1996;796:21–29. doi: 10.1111/j.1749-6632.1996.tb32563.x. [DOI] [PubMed] [Google Scholar]
- Liu MC, Bleecker ER, Lichtenstein LM, Kagey-Sobotka A, Niv Y, McLemore TL, Permutt S, Proud D, Hubbard WC. Evidence for elevated levels of histamine, prostaglandin D2, and other bronchoconstricting prostaglandins in the airways of subjects with mild asthma. Am Rev Respir Dis. 1990;142(1):126–132. doi: 10.1164/ajrccm/142.1.126. [DOI] [PubMed] [Google Scholar]
- Meyer MC, Rastogi P, Beckett CS, McHowat J. Phospholipase A2 inhibitors as potential anti-inflammatory agents. Curr Pharm Des. 2005;11(10):1301–1312. doi: 10.2174/1381612053507521. [DOI] [PubMed] [Google Scholar]
- Meyer MC, Kell PJ, Creer MH, McHowat J. Calcium-independent phospholipase A2 is regulated by a novel protein kinase C in human coronary artery endothelial cells. Am. J. Physiol. Cell Physiol. 2005;288(2):C475–C482. doi: 10.1152/ajpcell.00306.2004. [DOI] [PubMed] [Google Scholar]
- Miller DL, Welty-Wolf K, Carraway MS, Ezban M, Ghio A, Suliman H, Piantadosi CA. Extrinsic coagulation blockade attenuates lung injury and proinflammatory cytokine release after intratracheal lipopolysaccharide. Am J Respir Cell Mol Biol. 2002;26(6):650–658. doi: 10.1165/ajrcmb.26.6.4688. [DOI] [PubMed] [Google Scholar]
- Nagase T, Uozumi N, Aoki-Nagase T, Terawaki K, Ishii S, Tomita T, Yamamoto H, Hashizume K, Ouchi Y, Shimizu T. A potent inhibitor of cytosolic phospholipase A2, arachidonyl trifluoromethyl ketone, attenuates LPS-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2003;284(5):L720–L726. doi: 10.1152/ajplung.00396.2002. [DOI] [PubMed] [Google Scholar]
- Ostrovsky L, King AJ, Bond S, Mitchell D, Lorant DE, Zimmerman GA, Larsen R, Niu XF, Kubes P. A juxtacrine mechanism for neutrophil adhesion on platelets involves platelet-activating factor and a selectin-dependent activation process. Blood. 1998;91(8):3028–3036. [PubMed] [Google Scholar]
- Rastogi P, Beckett CS, McHowat J. Prostaglandin production in Human coronary artery endothelial cells is modulated differentially by selective Phospholipase A2 inhibitors. Prostaglandins Leukot Essent Fatty Acids. 2007;76(4):205–212. doi: 10.1016/j.plefa.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Razavi HM, Werhun R, Scott JA, Weicker S, Wang LF, McCormack DG, Mehta S. Effects of inhaled nitric oxide in a mouse model of sepsis-induced acute lung injury. Crit Care Med. 2002;30:868–873. doi: 10.1097/00003246-200204000-00026. [DOI] [PubMed] [Google Scholar]
- Riches DW, Young SK, Seccombe JF, Henson JE, Clay KL, Henson PM. The subcellular distribution of platelet-activating factor in stimulated human neutrophils. J Immunol. 1990;145(9):3062–3070. [PubMed] [Google Scholar]
- Rickard A, Portell C, Kell PJ, Vinson SM, McHowat J. Protease-activated receptor stimulation activates a Ca2_-independent phospholipase A2 in bladder microvascular endothelial cells. Am J Physiol Renal Physiol. 2005;288:F714–F721. doi: 10.1152/ajprenal.00288.2004. [DOI] [PubMed] [Google Scholar]
- Rosengren S, Henson PM, Worthen GS. Migration-associated volume changes in neutrophils facilitate the migratory process in vitro. Am J Physiol. 1994;267(6 Pt 1):C1623–C1632. doi: 10.1152/ajpcell.1994.267.6.C1623. [DOI] [PubMed] [Google Scholar]
- Senden NH, Jeunhomme TM, Heemskerk JW, Wagenvoord R, van't Veer C, Hemker HC, Buurman WA. Factor Xa induces cytokine production and expression of adhesion molecules by human umbilical vein endothelial cells. J Immunol. 1998;161(8):4318–4324. [PubMed] [Google Scholar]
- Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488(1–2):1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- Smith ML, Olson TS, Ley K. CXCR2- and E-selectin-induced neutrophil arrest during inflammation in vivo. J Exp Med. 2004;200(7):935–939. doi: 10.1084/jem.20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergonlle N, Luger TA, Hollenberg MD. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005;26(1):1–43. doi: 10.1210/er.2003-0025. [DOI] [PubMed] [Google Scholar]
- Tam EK, Caughey GH. Degradation of airway neuropeptides by human lung tryptase. Am J Respir Cell Mol Biol. 1990;3:27–32. doi: 10.1165/ajrcmb/3.1.27. [DOI] [PubMed] [Google Scholar]
- Tate RM, Repine JE. Neutrophils and the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1983;128:552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- Van Antwerpen L, Theron AJ, Myer MS, Richards LA, Wolmarans L, Booysen U, van der Merwe CA, Sluis-Cremer GK, Anderson R. Cigarette smoke-mediated oxidant stress, phagocytes, vitamin C, vitamin E, and tissue injury. Ann. NY Acad. Sci. 1993;686:53–65. doi: 10.1111/j.1749-6632.1993.tb39153.x. [DOI] [PubMed] [Google Scholar]
- Vonk-Noordegraaf A, van Wolferen SA, Marcus JT, Boonstra A, Postmus PE, Peeters JW, Peacock AJ. Noninvasive assessment and monitoring of the pulmonary circulation. Eur Respir J. 2005;25:758–766. doi: 10.1183/09031936.05.00122104. [DOI] [PubMed] [Google Scholar]
- Wagner EM, Bleecker ER, Permutt S, Liu MC. Direct assessment of small airways reactivity in human subjects. Am J Respir Crit Care Med. 1988;157(2):447–452. doi: 10.1164/ajrccm.157.2.9611043. [DOI] [PubMed] [Google Scholar]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Weibel ER. Principles and methods for the morphometric study of the lung and other organs. Lab Invest. 1963;12:131–155. [PubMed] [Google Scholar]
- Worthen GS, Henson PM, Rosengren S, Downey GP, Hyde DM. Neutrophils increase volume during migration in vivo and in vitro. Am J Respir Cell Mol Biol. 1994;10(1):1–7. doi: 10.1165/ajrcmb.10.1.8292373. [DOI] [PubMed] [Google Scholar]
- Xia L, Sperandio M, Yago T, McDaniel JM, Cummings RD, Pearson-White S, Ley K, McEver RP. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Invest. 2002;109(7):939–950. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]