Abstract
Background and Purpose
Treating patients with malignant cerebral infarctions remains a major unsolved problem in medicine. Decompressive craniectomy (DC) improves the bleak outlook, but is suboptimal. Here, using a rat model of severe ischemia / reperfusion (I/R) with very high mortality due to malignant cerebral edema, we tested the hypothesis that block of SUR1-regulated NC(Ca-ATP) channels using glibenclamide would compare favorably to DC when reperfusion and treatment were begun 6 h after onset of ischemia.
Methods
Male Wistar rats underwent filament occlusion of the middle cerebral artery to reduce LDF-perfusion signals >75%, with filament removal plus treatment 6 h later. In rats treated with vehicle vs. glibenclamide (10 μg/kg i.p. plus 200 ng/h subcutaneous), we compared mortality, neurological function and brain swelling at 24 h. In rats treated with DC vs. glibenclamide, we compared neurological function over 2 weeks and histological outcomes.
Results
Compared to vehicle, glibenclamide-treatment reduced 24-h mortality from 67% to 5%, and reduced hemispheric swelling at 24 h from 21% to 8%. DC eliminated 24-h mortality, but neurological function over 2 weeks was significantly better with glibenclamide compared to DC. Watershed cortex and deep white matter were significantly better preserved with glibenclamide compared to DC.
Conclusions
In a model of severe I/R, with reperfusion and treatment beginning 6 h following onset of ischemia, glibenclamide is as effective as DC in preventing death from malignant cerebral edema, but is superior to DC in preserving neurological function and the integrity of watershed cortex and deep white matter.
Keywords: cerebral ischemia, malignant cerebral edema, white matter, glibenclamide, decompressive craniectomy, SUR1, SUR1-regulated NCCa-ATP channel
Introduction
The treatment of patients with malignant cerebral infarctions remains one of the major unsolved problems in medicine. Malignant infarction occurs in 10–15% of stroke victims and is characterized by formation of malignant cerebral edema. It is the syndrome of a large stroke causing progressive edema that results in tissue swelling that compromises arterial inflow to surrounding tissues, culminating in further ischemic damage, enlargement of the infarct, herniation and death. Despite best available medical management, the prognosis for these patients is poor, with case fatality rates as high as 60–80%.1,2 Until recently, a large cerebral infarction was regarded chiefly as an untreatable condition with fatal outcome.
Decompressive craniectomy (DC) has improved the bleak outlook for malignant infarctions. In a meta-analysis of three prospective trials, DC was found to significantly reduce poor outcome and case fatality in patients who were randomized within 48 h of stroke onset.3 However, factors such as limited eligibility for surgery among patients who are gravely ill and have important co-morbidities, and reduced efficacy of DC in patients >60 years of age,4 continue to drive the search for non-surgical treatments for malignant cerebral edema.
Emerging evidence indicates that, following cerebral ischemia, newly upregulated sulfonylurea receptor 1 (SUR1)-regulated NCCa-ATP channels play a leading role in oncotic swelling (cytotoxic edema) and oncotic death of neurons and astrocytes, and in formation of space-occupying ionic and vasogenic edema caused by microvascular dysfunction.5,6 In several rat models of stroke, block of the channel by the sulfonylurea inhibitor, glibenclamide, confirmed the critical role of brain swelling in determining both infarct volume and mortality.
Here, we studied a rat model of severe ischemia with delayed reperfusion that mimicked the late presentation and high mortality often encountered in patients with stroke. Low-dose glibenclamide, administered 6 h after onset of ischemia at the time of filament withdrawal, reduced brain swelling, essentially eliminated mortality and was associated with surprisingly good neurological function that was durable out to two weeks. DC performed at the same time also eliminated mortality,7-9 but yielded less favorable neurological scores and worse damage to watershed cortex and deep white matter. Our findings suggest that glibenclamide may be preferable to DC in the treatment of malignant cerebral edema.
Methods
General
Surgical procedures were approved by the Institutional Animal Care and Use Committee of the University of Maryland. We studied 3 series of rats (male Wistar, 225–325 gm), including 2 for preclinical stroke trials. Series 1, treatments: vehicle vs. glibenclamide; all included rats (21 vehicle- and 22 glibenclamide-treated) were used for measurements of neurological function at 24 h or at death, if sooner; a subgroup of rats (12 vehicle- and 10 glibenclamide-treated) was used for measurements of hemispheric swelling at 24 h or at death, if sooner; another subgroup of rats (8 vehicle- and 10 glibenclamide-treated) was used for repeated measurements of neurological function over the course of 2 weeks or at death, if sooner, with 5 of the glibenclamide-treated rats used for histology at 2 weeks. Series 2, treatment: DC; all 10 included rats were used for repeated measurements of neurological function over the course of 2 weeks, with 5 rats used for histology at 2 weeks. Animals in Series 3 were untreated, and were used for measurements of laser Doppler flowmetry (LDF)-perfusion signals during reperfusion, or were sacrificed for pathology, immunohistochemistry, or in situ hybridization. Details consistent with good laboratory practice, including inclusion/exclusion criteria, methods for allocation to treatment group, etcetera are provided in the Online Supplement.
Rat model of malignant cerebral edema
Procedures for 6-h long MCAo via the external carotid artery (ECA) and for monitoring LDF-perfusion signals for >75% reduction have been described,6 with additional details provided in the Online Supplement.
Treatments
A loading dose of glibenclamide (10 μg/kg i.p.) was given 15 min before removing the occluder at 6 h. After the occluder was removed, a mini-osmotic pump (model 2001; 1.0 μl/hr, Durect Corp, Cupertino, CA) was implanted subcutaneously for continuous infusion of glibenclamide (200 ng/hr subcutaneous for 24 h or 7 days). Controls were administered vehicle (NS plus DMSO) in the same way. Solutions of glibenclamide (Sigma, St. Louis) were prepared as described.6
DC was performed immediately after removing the occluder at 6 h. The craniectomy plus dural opening extended from coronal to lambdoidal sutures, and from the sagittal suture to the zygoma (∼7×9 mm).
Outcome measures
Neurological function was assessed using the neuroscore10 and the modified Garcia score.11 For hemispheric swelling, area measurements of involved vs. non-involved hemispheres were made on 5 consecutive 2-mm coronal brain sections.
Analysis of preclinical data
Parametric data (swelling at 24 h, serial weights) were analyzed using Student's t-test or a repeated measures one-way ANOVA with Bonferroni comparisons. Mortality was analyzed using a 2×2 contingency table and Fisher's exact test. Non-parametric data (neuroscores and Garcia scores) were analyzed using the Mann-Witney U-test for the 24-h data or, for repeated measurements, by first rank-transforming the combined data, with tied ranks replaced by the average rank for the ties, and using a repeated measures two-way ANOVA with Bonferroni comparisons.
Histology, immunohistochemistry, in situ hybridization 5,6
To stain myelin, we used eriochrome cyanin-R (Sigma, St. Louis). To perform terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), we used the “In situ cell death detection kit, Fluorescein” (#1684795, Roche Molecular Biochemicals, Germany). For immunolabeling, we used primary antibodies directed against SUR1 (SC-5789; Santa Cruz Biotechnology); vonWillebrand factor (F3520; Sigma); oligodendrocyte-specific protein (OSP; ab7474; Abcam); oligodendrocyte transcription factor 2 (Olig2; IBL, Japan); specificity protein 1 (Sp1; clone PEP2; Santa Cruz). For in situ hybridization of mRNA for SUR1, we used digoxigenin-labeled probes (sense: ‘5-GCCCGGGCACCCTGCTGGCTCTGTGTGTCCTTCCGCGCCTGGGCATCG-3’) designed and supplied by GeneDetect.
Results
Temporary thrombosis of the MCA
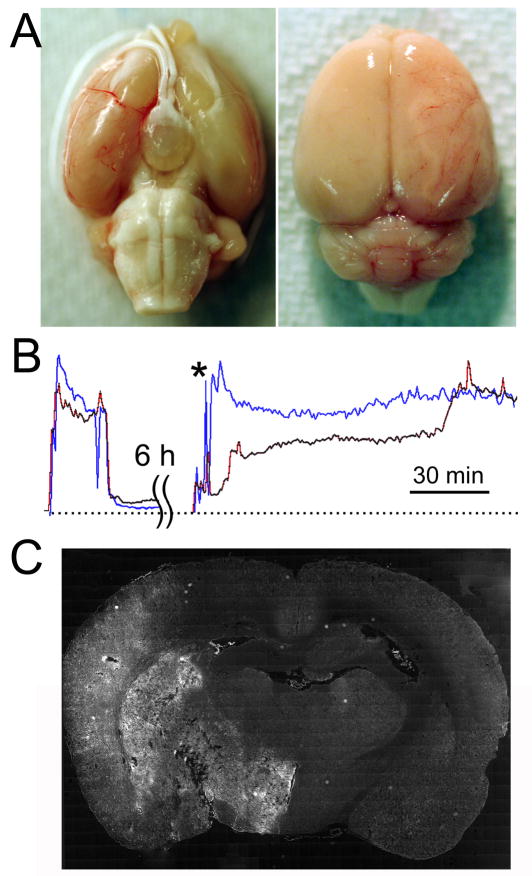
Occlusion of the origin of the MCA for 6 h that yielded a reduction in LDF-perfusion signal >75% was associated with thrombosis of the trunk and distal branches of the MCA (Fig. 1A).12 However, within 3 h of removing the occluder, MCA vessels had cleared by spontaneous thrombolysis (not shown).
Figure 1. Occlusion of the middle cerebral artery (MCA) for 6 h does not prevent reperfusion.
A: Images of a brain following 6 h ischemia, showing thrombosis of MCA trunk and distal MCA branches. B: LDF-perfusion signals at the onset of ischemia, and during and following filament withdrawal 6 h later, showing 2 different temporal patterns of reperfusion; the data shown are representative of reperfusion patterns in 6 rats. C: Coronal section following 6 h ischemia plus 3 h reperfusion, processed for terminal deoxynucleotidyl transferase dUTP nick end labeling, showing pathological involvement of the entire MCA territory; the data shown are representative of findings in 3 rats.
Thrombolysis of major MCA branches does not necessarily imply reperfusion, since microemboli could still occlude more distal branches. We therefore measured the time course of re-perfusion of the MCA territory in 6 rats using LDF during and following removal of the occluder. In some cases, reperfusion occurred rapidly, whereas in others, reperfusion occurred in a stepwise fashion over the course of 1–2 h (Fig. 1B). In the 6 rats examined by LDF, there was never failure to reperfuse, as judged by LDF-perfusion signals, making this model truly one of reperfusion and not of permanent occlusion. This insult of 6-h I/R resulted in massive infarcts that characteristically involved the entire MCA territory, with minimal mass effect from swelling at early times (Fig. 1C).13
SUR1 upregulation
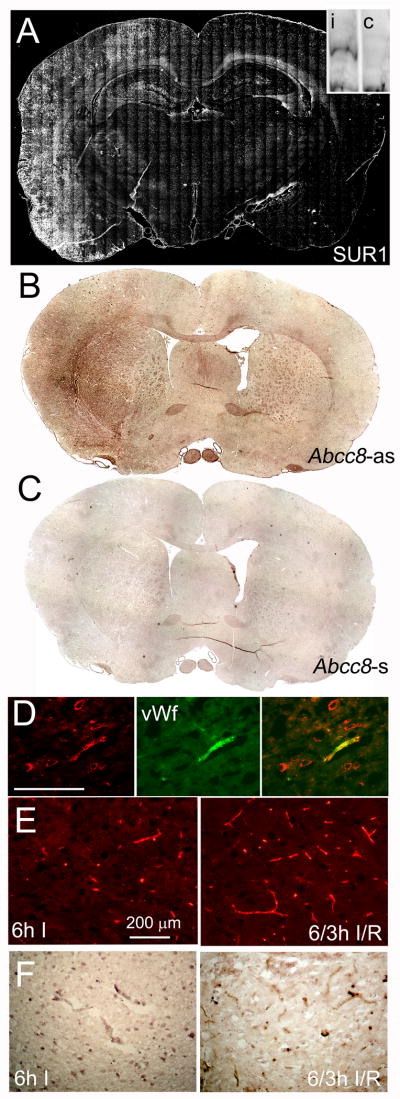
Brains were examined for SUR1 expression after 6 h of ischemia, without or with 3 h reperfusion. Immunolabeling showed prominent upregulation of SUR1 throughout the MCA territory, which was confirmed by immunoblot (Fig. 2A), consistent with previous detailed observations of progressive upregulation of SUR1 with time in a similar model of malignant cerebral edema.5 In situ hybridization for mRNA for Abcc8, the gene that encodes SUR1, confirmed transcriptional upregulation of SUR1 (Fig. 2B,C).
Figure 2. Stroke model with 6-h ischemia/reperfusion (I/R) is associated with upregulation of SUR1.
A–C: Montages of images of coronal brain sections immunolabeled for sulfonylurea receptor-1 (SUR1) (A) or processed for in situ hybridization for Abcc8 (B,C), following 6-h ischemia without (A) or with 3-h reperfusion (B,C); sense probe (Abcc8-s) was used as a control for antisense probe (Abcc8-as); the insert in A shows an immunoblot for SUR1 from ipsilateral (i) and contralateral (c) brain. D: High-magnification views of sections immunolabeled for SUR1 (red) and co-labeled for vonWillebrand factor (green), confirming SUR1 upregulation in capillaries; superimposed images are also shown. E,F: High-magnification views of sections immunolabeled for SUR1 (E) or processed for in situ hybridization (F), showing capillaries after 6-h ischemia (6h I) vs. 6-h ischemia plus 3-h reperfusion (6/3h I/R); the data shown are representative of findings in 3–6 rats.
At higher magnification, SUR1 immunolabeling was identified in large neuron-like cells as well as in capillaries (Fig. 2D), corroborating previous observations made in other models of stroke.5,6 In addition, here we observed that SUR1 upregulation in capillaries after 6 h of ischemia continued to increase during 3 h of reperfusion, in terms of both protein and mRNA (Fig. 2E,F).
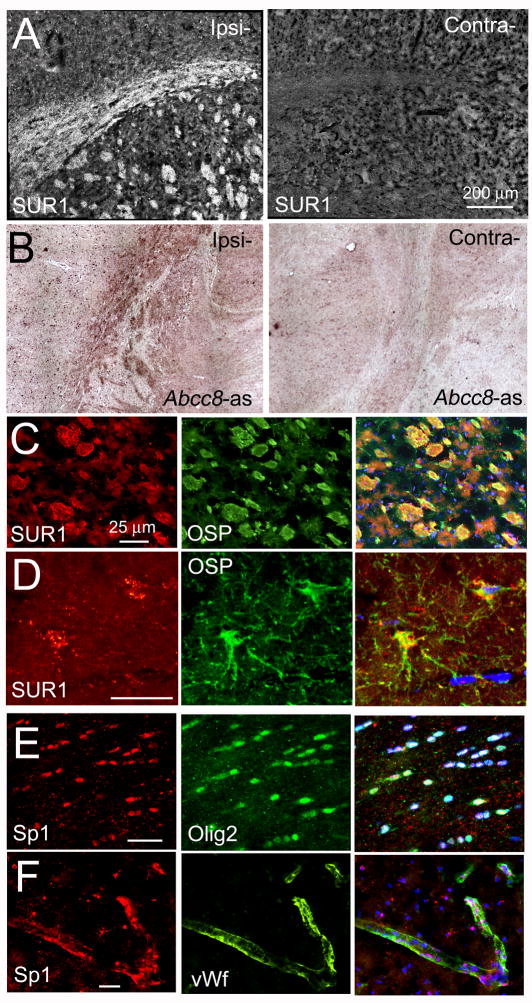
SUR1 was also upregulated in white matter, which has not been previously reported. SUR1 expression was prominent in the corpus callosum and in en face white matter bundles in the putamen (Fig. 3A). In situ hybridization for mRNA for Abcc8 confirmed transcriptional upregulation of SUR1 (Fig. 3B). Co-labeling for oligodendrocyte-specific protein (OSP) showed that SUR1 was upregulated in oligodendrocytes, not in axons (Fig. 3C,D).
Figure 3. Sulfonylurea receptor-1 (SUR1) is transcriptionally upregulated in white matter.
A,B: Images of sections following 6-h of ischemia plus 3-h reperfusion, immunolabeled for SUR1 (A) or processed for in situ hybridization for Abcc8 using antisense probe (Abcc8-as) (B), showing corpus callosum and en-face white matter bundles in the putamen; tissues ipsilateral (Ipsi-) and contralateral (Contra-) to the ischemic injury are shown. C,D: High-magnification views of sections immunolabeled for SUR1 (red) and co-labeled for oligodendrocyte-specific protein (OSP; green), confirming SUR1 upregulation in oligodendrocytes in the putamen (C) and in the cortex (D); superimposed images are also shown; nuclei are labeled with DAPI (blue); the data shown are representative of findings in 5 rats; bars, 25 μm. E,F: High-magnification views of sections of corpus callosum (E) and cortex (F) immunolabeled for Sp1 (red) and co-labeled for nuclear oligodendrocyte-specific transcription factor (Olig2; green), or von Willebrand factor (vWf; green); superimposed images are also shown, confirming Sp1 nuclear localization in oligodendrocytes (white nuclei) and in microvascular endothelial cells (pink nuclei); nuclei labeled with DAPI (blue); the data shown are representative of findings in 3 rats; bars, 25 μm.
Sp1 transcription factor
The promoter region Abcc8, the gene that encodes SUR1, contains multiple consensus sequences for binding Sp1, and Sp1 has been established as a key transcriptional regulator of SUR1 expression.14,15 The association between Abcc8 and Sp1 has been validated for neurons and other CNS cells in vivo following ischemic injury.5 This finding was confirmed here, showing that oligodendrocytes as well as microvascular endothelial cells exhibit prominent upregulation and nuclear translocation of Sp1 following severe I/R (Fig. 3E,F). Together, these findings reaffirm that Sp1 forms part of the transcriptional program responsible for upregulating SUR1/Abcc8 in multiple cell types following cerebral I/R.
Effect of glibenclamide at 24 h
We tested the effect of glibenclamide, the potent, highly selective blocker of SUR1.16 Rats were subjected to 6 h I/R and at 6 h received either vehicle or glibenclamide using the same formulation, dose and route of administration as in our previous study.6 Low-dose glibenclamide was previously reported to have no significant effect on systolic blood pressure,5 and to be minimally hypoglycemogenic.5,6 Here, we found that glibenclamide had no effect on LDF-perfusion signals, blood gases, or serum chemistry, with only a small effect on glucose at 1 h, but not at 24 h (see Online Supplement).
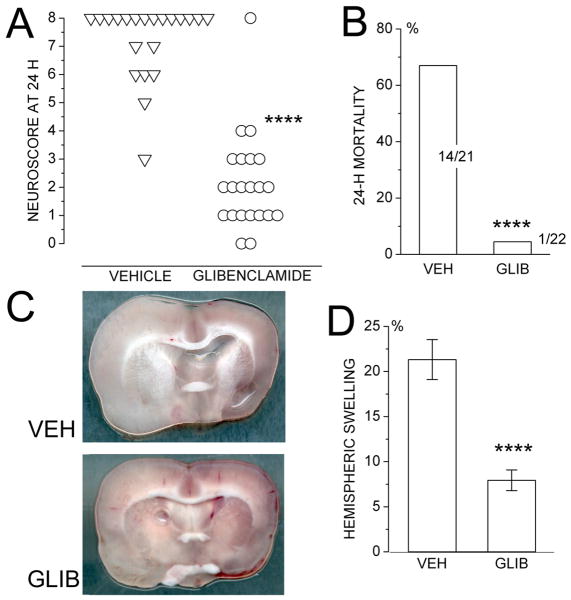
Functional outcome was evaluated at 24 h using the 9-point neuroscore (0 for no apparent deficit; 8 for death). Neuroscores at 24 h were significantly different between groups (P<0.0001), with median scores of 8 vs. 2 for vehicle-vs. glibenclamide-treated rats, respectively (Fig. 4A). In the same rats, 24-h mortality (neuroscore 8) was 14/21 (67%) in the control group vs. 1/22 (5%) in the glibenclamide-treated group (P<0.0001) (Fig. 4B).
Figure 4. Glibenclamide improves 24-h endpoints in a stroke model with 6-h ischemia/reperfusion (I/R).
A: Neuroscores at 24 h in rats subjected to 6-h I/R and treated at 6 h with either vehicle (21 rats) or glibenclamide (22 rats). B: Mortality at 24 h in the same rats. C,D: Images of coronal sections through the middle cerebral artery territory (C) and values of hemispheric swelling (D) at 24 h in a subset of the same rats; 12 and 10 rats per group; ****, P<0.0001.
A subset of rats from this series was examined for hemispheric swelling at 24 h or at death, if earlier. The brains of glibenclamide-treated rats showed significantly less swelling of the infarcted hemisphere compared to those of vehicle-treated controls (21.3±2.2 vs. 7.9±1.1%; P<0.0001) (Fig. 4C,D).
Effect of glibenclamide vs. decompressive craniectomy during 2 weeks
Other rats from this series were followed until death or up to 2 weeks. Only 1/8 vehicle-treated rats not euthanized at 24 h survived 2 weeks, making the overall mortality in this model ∼90%. By contrast, 10/10 glibenclamide-treated rats survived the entire 2-weeks.
Due to high mortality in vehicle-treated rats, further comparisons with glibenclamide-treated rats beyond 24 h were not feasible. An additional group of 10 rats was subjected to the same ischemic insult (6 h I/R), and were treated with DC at 6 h. The outcome of these rats over the course of 2 weeks was compared to the outcome of glibenclamide-treated rats that were followed for 2 weeks.
Individual neuroscores for the 2 treated groups showed that DC and glibenclamide were equally effective in essentially eliminating mortality – no rat in either group died (Fig. 5A). The fact that DC eliminated mortality confirmed that the cause of death in this model was brain swelling. The fact that glibenclamide was as effective as DC in eliminating mortality confirmed that a critical mechanism of action of glibenclamide was to reduce brain swelling, consistent with the 24-h measurements of hemispheric swelling given above.
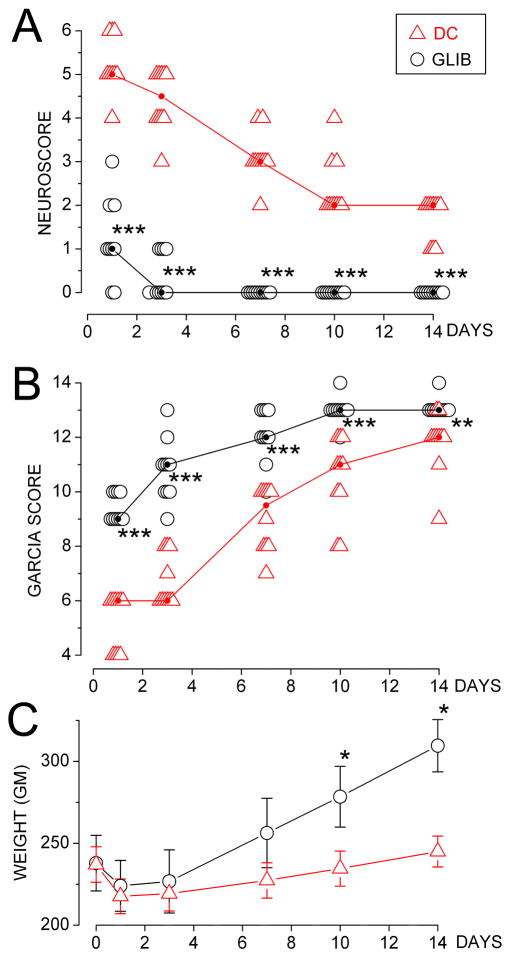
Figure 5. Neurological outcome following 6-h ischemia/reperfusion is better with glibenclamide than with decompressive craniectomy (DC).
A,B: Neuroscores (A) and Garcia scores (B) for individual rats treated with glibenclamide (empty circle) or DC (empty triangle), measured on the days indicated after the ischemic insult; median scores for each day are also shown (small filled circles joined by lines); 10 rats per group; rank-transformed scores were analyzed using a 2-way repeated measures ANOVA with Bonferroni comparisons between groups on each day; ***, P<0.001; **, P<0.01. C: Weights (mean±S.E.) for rats treated with glibenclamide (empty circle) or DC (empty triangle), measured on the days indicated after the ischemic insult; data were analyzed for each group separately, using a 1-way repeated measures ANOVA with Bonferroni comparisons between pre-injury and post-injury weights; *, P<0.05.
Neuroscores were better for glibenclamide-treated rats, compared to DC-treated rats (Fig. 5A). All animals treated with glibenclamide reached a neuroscore of 0 by the end of 1 week, whereas none of the DC-treated rats achieved this score after 2 weeks. By the end of 2-weeks, DC-treated rats exhibited upper motor neuron deficits not evident in the glibenclamide-treated group.
The difference between treatment groups was borne out by the Garcia scores (Fig. 5B). After 7–10 days of recovery, glibenclamide-treated rats all showed stable hemisensory neglect to contralateral whisker stimulation, but otherwise appeared remarkably unaffected, with no overt motor deficit. DC-treated rats all exhibited the same hemisensory deficit but in addition showed difficulty with beam walk or did not achieve symmetry of locomotion.
We recorded body weight as an objective measure of well-being. Glibenclamide-treated rats showed a brief interruption in weight gain after the ischemic insult, then recovered progressive weight gain at the expected pace (Fig. 5C). DC-treated rats showed no weight gain during the 2-week period of observation (Fig. 5C).
Effect of glibenclamide vs. decompressive craniectomy on tissue sparing
Histological examination showed that all animals regardless of treatment exhibited large areas of necrosis involving the MCA territory.13 However, glibenclamide-treated rats showed significantly better preservation of the medial cortex (anterior cerebral artery territory and watershed cortex), which comprise hindlimb and forelimb areas of cortex13 (Fig. 6A,B). Measurements of these cortical areas in coronal sections showed significantly larger values in glibenclamide-treated rats compared to DC-treated rats (P<0.01) (Fig. 6C).
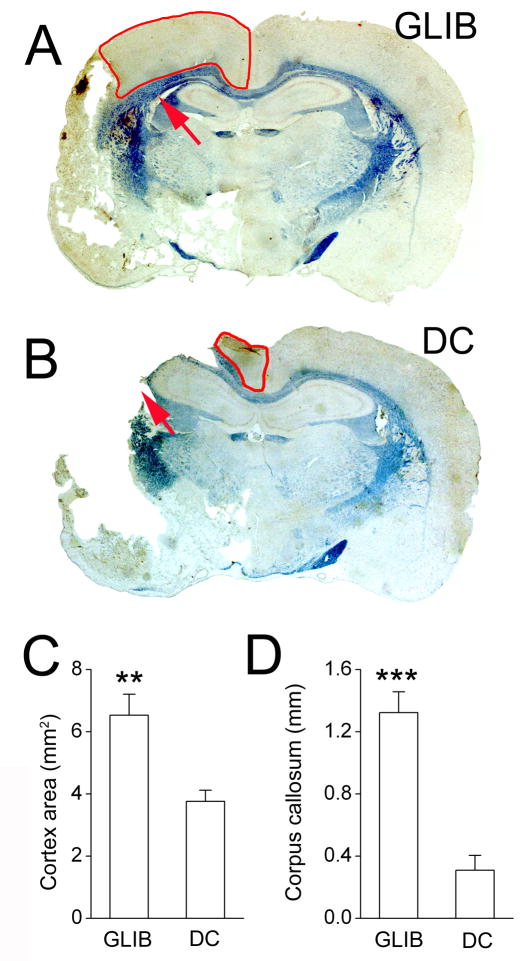
Figure 6. Histological outcome following 6-h ischemia/reperfusion is better with glibenclamide than with decompressive craniectomy (DC).
A,B: Eriochrome-stained coronal sections of brains 2 weeks following the ischemic insult and treatment with glibenclamide (A) or DC (B); anterior cerebral plus watershed territory outlined in red; arrows point to the deep white matter of the lateral corpus callosum lateral to the lateral ventricle. C,D: Measurements of cortical areas (C) and of the thickness of deep white matter (D) in glibenclamide-vs. DC-treated rats; 5 rats per group; **, P<0.01; ***, P<0.001.
Eriochrome staining for white matter revealed a striking difference between glibenclamide- and DC-treated rats. In DC-treated rats, deep white matter of the lateral corpus callosum, lateral to the lateral ventricle, was usually destroyed, whereas in glibenclamide-treated rats, this deep white matter was spared (Fig. 6A,B, arrows). Measurements of the thickness of the deep white matter in this region showed that it was significantly greater in glibenclamide-treated rats than in DC-treated rats (P<0.001) (Fig. 6D).
Discussion
Most rodent models of stroke focus on the outcome measure of lesion size and strive to achieve low mortality.17 This narrow focus does not reflect the wide rage of severity of stroke that can afflict humans, since it deliberately omits study of the most severe strokes that result in death. In humans, DC is used exclusively to save life, but surprisingly, studies of DC in rodent models have usually used relatively modest ischemia associated with mortality rates far below the 60-80% rates encountered in humans with malignant infarctions who are candidates for DC.7-9 Here, rats were subjected to 6 h ischemia with reduction in LDF-perfusion signals >75%. The occluder was withdrawn at 6 h, but actual reperfusion occurred as much as 1–2 h later. This model of severe I/R was associated with 67–90% mortality,18 closely approximating the high case fatality rates associated with malignant infarcts in humans.1,2 DC performed at 6 h in this model was highly effective in reducing mortality, reflecting the experience with DC in humans. As noted by the Stroke Therapy Academic Industry Roundtable (STAIR), the closer that animal studies link directly to actual clinical conditions, the likelier it will be that promising animal studies will be reproduced in humans.19
Although beneficial, DC is an imperfect solution to the problem of malignant cerebral edema. Not all patients are candidates for surgery, beneficial effects of DC are limited by age,4 and decompression may adversely affect penumbral tissues. The sharp decrease in tissue pressure that follows DC likely accelerates edema formation and may facilitate hemorrhagic transformation.20 From a physiological standpoint, preventing swelling is preferable to decompressing the already swollen brain.
To our knowledge, this is the first report to demonstrate that pharmacological treatment can lead to a reduction of brain swelling sufficient to prevent mortality as effectively as DC. We have studied death as an endpoint in 2 models of malignant cerebral edema in male Wistar rats, the one reported here and another, where we also showed good penetration of glibenclamide into ischemic tissues.5 Here, low-dose glibenclamide was administered at 6 h when reperfusion was started, a time chosen to realistically simulate delayed presentation of patients with stroke. Glibenclamide not only eliminated death, but it also reduced brain swelling at 24 h by half or more,5 and was associated with significantly better neurological outcomes compared to DC, with this effect being durable out to 2 weeks.
To our knowledge, this is the first report to demonstrate that a pharmacological treatment can aid in preserving white matter following severe ischemic injury. SUR1, the regulatory subunit of the SUR1-regulated NCCa-ATP channel, was upregulated in oligodendrocytes. This channel has previously been implicated in cell death of neurons, astrocytes and endothelial cells,5 and may also be involved in death of oligodendrocytes. The human brain has a higher proportion of white matter than the rodent brain, making it important that stroke treatments protect white matter as well as neurons. As noted by STAIR, it is unlikely that any treatment that targets only neurons and that does not salvage white matter would have widespread clinical relevance.19
In summary, we show that, in a model of severe I/R with reperfusion and treatment started 6 h following onset of ischemia, glibenclamide is as effective as DC in preventing death from malignant cerebral edema, but is superior to DC in preserving neurological function and in maintaining the integrity of watershed cortex and deep white matter.
Supplementary Material
Acknowledgments
This work was supported by grants to JMS from the National Heart, Lung and Blood Institute (HL082517) and the National Institute of Neurological Disorders and Stroke (NS061808, NS060801).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest. JMS holds a US patent (# 7,285,574), “A novel non-selective cation channel in neural cells and methods for treating brain swelling”, and is a member of the scientific advisory board and holds shares in Remedy Pharmaceuticals. No support, direct or indirect, was provided to JMS, or for this project, by Remedy Pharmaceuticals.
Reference List
- 1.Hacke W, Schwab S, Horn M, Spranger M, De GM, von KR. ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53:309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 2.Berrouschot J, Sterker M, Bettin S, Koster J, Schneider D. Mortality of space-occupying (‘malignant’) middle cerebral artery infarction under conservative intensive care. Intensive Care Med. 1998;24:620–623. doi: 10.1007/s001340050625. [DOI] [PubMed] [Google Scholar]
- 3.Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van GJ, van der Worp HB. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 2009;8:326–333. doi: 10.1016/S1474-4422(09)70047-X. [DOI] [PubMed] [Google Scholar]
- 4.Arac A, Blanchard V, Lee M, Steinberg GK. Assessment of outcome following decompressive craniectomy for malignant middle cerebral artery infarction in patients older than 60 years of age. Neurosurg Focus. 2009;26:E3. doi: 10.3171/2009.3.FOCUS0958. [DOI] [PubMed] [Google Scholar]
- 5.Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, Tsymbalyuk N, West GA, Gerzanich V. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12:433–440. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simard JM, Yurovsky V, Tsymbalyuk N, Melnichenko L, Ivanova S, Gerzanich V. Protective effect of delayed treatment with low-dose glibenclamide in three models of ischemic stroke. Stroke. 2009;40:604–609. doi: 10.1161/STROKEAHA.108.522409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doerfler A, Forsting M, Reith W, Staff C, Heiland S, Schabitz WR, von KR, Hacke W, Sartor K. Decompressive craniectomy in a rat model of “malignant” cerebral hemispheric stroke: experimental support for an aggressive therapeutic approach. J Neurosurg. 1996;85:853–859. doi: 10.3171/jns.1996.85.5.0853. [DOI] [PubMed] [Google Scholar]
- 8.Engelhorn T, von KR, Reith W, Forsting M, Doerfler A. What is effective in malignant middle cerebral artery infarction: reperfusion, craniectomy, or both? An experimental study in rats. Stroke. 2002;33:617–622. doi: 10.1161/hs0202.102374. [DOI] [PubMed] [Google Scholar]
- 9.Forsting M, Reith W, Schabitz WR, Heiland S, von KR, Hacke W, Sartor K. Decompressive craniectomy for cerebral infarction. An experimental study in rats. Stroke. 1995;26:259–264. doi: 10.1161/01.str.26.2.259. [DOI] [PubMed] [Google Scholar]
- 10.Xia CF, Smith RS, Jr, Shen B, Yang ZR, Borlongan CV, Chao L, Chao J. Postischemic brain injury is exacerbated in mice lacking the kinin B2 receptor. Hypertension. 2006;47:752–761. doi: 10.1161/01.HYP.0000214867.35632.0e. [DOI] [PubMed] [Google Scholar]
- 11.Shimamura N, Matchett G, Tsubokawa T, Ohkuma H, Zhang J. Comparison of silicon-coated nylon suture to plain nylon suture in the rat middle cerebral artery occlusion model. J Neurosci Methods. 2006;156:161–165. doi: 10.1016/j.jneumeth.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Takenaka K, Yamagishi S, Matsui T, Nakamura K, Jinnouchi Y, Yoshida Y, Ueda S, Katsuki Y, Katsuda Y, Imaizumi T. Pigment epithelium-derived factor (PEDF) administration inhibits occlusive thrombus formation in rats: a possible participation of reduced intraplatelet PEDF in thrombosis of acute coronary syndromes. Atherosclerosis. 2008;197:25–33. doi: 10.1016/j.atherosclerosis.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 13.Garcia JH, Yoshida Y, Chen H, Li Y, Zhang ZG, Lian J, Chen S, Chopp M. Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am J Pathol. 1993;142:623–635. [PMC free article] [PubMed] [Google Scholar]
- 14.Ashfield R, Ashcroft SJ. Cloning of the promoters for the beta-cell ATP-sensitive K-channel subunits Kir6.2 and SUR1. Diabetes. 1998;47:1274–1280. doi: 10.2337/diab.47.8.1274. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez-Sanchez C, Ito Y, Ferrer J, Reitman M, LeRoith D. Characterization of the mouse sulfonylurea receptor 1 promoter and its regulation. J Biol Chem. 1999;274:18261–18270. doi: 10.1074/jbc.274.26.18261. [DOI] [PubMed] [Google Scholar]
- 16.Gribble FM, Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46:875–891. doi: 10.1007/s00125-003-1143-3. [DOI] [PubMed] [Google Scholar]
- 17.Aspey BS, Taylor FL, Terruli M, Harrison MJ. Temporary middle cerebral artery occlusion in the rat: consistent protocol for a model of stroke and reperfusion. Neuropathol Appl Neurobiol. 2000;26:232–242. doi: 10.1046/j.1365-2990.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 18.Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34:2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]
- 19.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.