Abstract
In many organisms, female and male meiosis display extensive sexual dimorphism in the temporal meiotic program, the number and location of recombination events, sex chromosome segregation, and checkpoint function. We show here that both meiotic prophase timing and germ-line apoptosis, one output of checkpoint signaling, are dictated by the sex of the germ line (oogenesis vs. spermatogenesis) in Caenorhabditis elegans. During oogenesis in feminized animals (fem-3), a single pair of asynapsed autosomes elicits a checkpoint response, yet an unpaired X chromosome fails to induce checkpoint activation. The single X in males and fem-3 worms is a substrate for the meiotic recombination machinery and repair of the resulting double strand breaks appears to be delayed compared with worms carrying paired X chromosomes. Synaptonemal complex axial HORMA domain proteins, implicated in repair of meiotic double strand breaks (DSBs) and checkpoint function, are assembled and disassembled on the single X similarly to paired chromosomes, but the central region component, SYP-1, is not loaded on the X chromosome in males. In fem-3 worms some X chromosomes achieve nonhomologous self-synapsis; however, germ cells with SYP-1-positive X chromosomes are not preferentially protected from apoptosis. Analyses of chromatin and X-linked gene expression indicate that a single X, unlike asynapsed X chromosomes or autosomes, maintains repressive chromatin marks and remains transcriptionally silenced and suggests that this state locally precludes checkpoint signaling.
IN metazoans, sexual dimorphisms manifest not only striking morphological somatic forms but also distinct differences in female and male germ-line biology. In the germ line, meiosis is coupled to cellular differentiation programs that result in the production of two very distinct sex-specific haploid gamete types, oocytes and sperm. The regulation of meiosis can differ considerably between the sexes as reflected in both the phenotypic manifestations of mutants as well as the temporal program of events (Hunt and Hassold 2002; Morelli and Cohen 2005). For example, female mammals enter prophase in utero whereas males initiate prophase postnatally (Handel and Eppig 1998). In the nematode Caenorhabditis elegans prophase I for oogenesis in hermaphrodites (functionally female) takes twice as long as prophase I for spermatogenesis in males (Jaramillo-Lambert et al. 2007). Furthermore, female germ cells of many species initiate a meiotic arrest during prophase I that does not occur in male germ cells (Masui and Clarke 1979; Eppig et al. 1996; McCarter et al. 1999). In late meiotic prophase, chromosome morphology (Shakes et al. 2009), chromatin compaction (Wu and Chu 2008), and the presence of centriole-containing centrosomes (Manandhar et al. 2005; Shakes et al. 2009; Wignall and Villeneuve 2009) can also exhibit sex-specific differences.
With some exceptions, early events in meiotic prophase such as chromosome pairing and synapsis, the close alignment of homologous chromosomes through the elaboration of the synaptonemal complex (SC), are largely conserved between the sexes; however, the extent of genetic exchange can differ significantly. Meiotic recombination rates are higher in females compared to males in humans (Donis-Keller et al. 1987), mice (Blank et al. 1988), zebrafish (Singer et al. 2002), Drosophila (Morgan 1912), and C. elegans (Zetka and Rose 1990; Meneely et al. 2002). In virtually all of these organisms, however, sex not only affects the rate of recombination but also influences the placement of exchange events along the length of the chromosome. Too few, or inappropriately placed recombination events can lead to chromosome nondisjunction (Koehler et al. 1996; Lamb et al. 1996; Ross et al. 1996). In humans meiotic failure rates as measured by aneuploidy are higher in oocytes (up to 25%) than in sperm (2%) (Hassold and Hunt 2001). It has been proposed that checkpoints monitoring meiotic events such as recombination are less stringent in females compared to males. C. elegans also appears to have sex-specific differences in checkpoint function; hermaphrodites respond to errors in synapsis, recombination, or DNA damage by culling germ cells through apoptosis (Gartner et al. 2000; Bhalla and Dernburg 2005; Stergiou et al. 2007). However, the male germ line does not induce apoptosis in response to DNA damage (Gartner et al. 2000).
The mechanism by which sex chromosomes (e.g., XX female vs. XY male) segregate at meiosis I is of necessity different between the sexes. While the homogametic chromosome pair segregates similarly to autosomes, multiple strategies have evolved to segregate the heterogametic sex chromosomes. For example, in marsupial males, the X and Y chromosomes never synapse but remain associated through the formation of a dense plate (Page et al. 2006). In most male placental mammals the X and Y sex chromosomes pair, synapse, and undergo exchange at the small pseudoautosomal region, leaving the bulk of the sex chromosomes unpaired (Perry et al. 2001; Handel 2004). In a number of organisms, unpaired DNA in the germ line is transcriptionally silenced in a process referred to as meiotic silencing of unpaired chromatin (MSUC); meiotic sex chromosome inactivation (MSCI) of the unpaired regions of the X and Y has been proposed to be a related process and is important for completion of meiosis in male mice (Mahadevaiah et al. 2008). In chickens, the W and Z chromosomes of females achieve nonhomologous synapsis but still undergo MSCI, suggesting that transcriptional silencing is important for the heterogametic sex regardless if male or female (Schoenmakers et al. 2009).
Double strand breaks (DSBs) and their repair via the homologous chromosome are essential for chromosome segregation during the first meiotic division (Kleckner 1996; Roeder 1997; Zickler and Kleckner 1999). Surprisingly, asynapsed regions of the mammalian XY bivalent incur DSBs (Ashley et al. 1995; Moens et al. 1997), yet lack a homologous chromosome for repair and must use the sister chromatid as template, a situation that normally triggers a checkpoint response. An outstanding question remains, namely, what prevents constitutive checkpoint activation in the germ line of the heterogametic sex?
Here, we analyzed several aspects of female and male meiosis in the C. elegans germ line. C. elegans exists predominantly as a self-fertilizing hermaphrodite; during development, hermaphrodites initially produce sperm and then switch to making oocytes, and thus as adults are female. However, males arise spontaneously in the population at a low frequency due to meiotic chromosome nondisjunction and can be maintained through genetic crosses. C. elegans has six chromosomes: five autosomes and one sex chromosome, the X. The ratio of the number of X chromosomes to the number of autosomes determines sex (XX/AA = 1 is hermaphrodite; X0/AA = 0.5 is male) (Madl and Herman 1979). We show that meiotic prophase kinetics and germ-line apoptosis are controlled in a sex-specific manner in C. elegans. Taking advantage of the availability of sex determination mutants, we discovered that a single X has unique properties that prevent the checkpoint machinery from recognizing the X as asynapsed. We also found that a single X chromosome incurs meiotic DSBs, and repair of these breaks appears to be delayed. SC axial components that play roles in interhomolog bias during meiotic recombination and checkpoint signaling are assembled on the single X, indicating that failure in checkpoint activation is not due to the absence of these proteins. Our studies suggest that detection of asynapsis by checkpoints is regulated by the chromatin/transcriptional status of the chromosome.
MATERIALS AND METHODS
Genetics:
Except where noted all C. elegans strains (Bristol N2 background) were propagated under standard procedures at 20° (Brenner 1974). The following strains were used: CB2754: tra-2(e1095)/dpy-10(e128) unc-4(e120) II; JK816: fem-3(q20) IV; JK551: unc-5(e53) fem-3(q22) IV; JK2878: fog-1(q325) I/hT2 (I;III); CB1489: him-8(e1489) IV; CA151: him-8(me4) IV; CB678 lon-2(e678) X; AV106: spo-11(ok79) IV/nT1 (IV;V); fem-3(e1996)/nT1-GFP IV (a generous gift from Tim Schedl), and CA258: zim-2(tm574) IV. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources (NCRR).
To generate homozygous fem-3(e1996) X0 animals, homozygous fem-3(e1996); lon-2(e678) XX females were mated to fem-3(e1996)/nT1-GFP X0 males and nongreen, long animals were selected for analysis. To generate homozygous fog-1(q325) X0 worms, fog-1(q325)/hT2-GFP XX hermaphrodites were crossed to N2 X0 males. Nongreen male cross progeny were then mated to homozygous fog-1(q325) XX females and the male cross progeny carrying oocytes were selected. tra-2(e1095) was maintained over dpy-10unc-4(e120) alleles by picking heterozygous hermaphrodites (wild-type phenotype). Homozygous tra-2(e1095) XX males were a product of the heterozygous self-mating. To elicit the temperature-sensitive phenotype of fem-3(q20) and fem-3(q22), L3 progeny were shifted to 25° 26 hr prior to injection or 48 hr prior to examining germ lines for apoptosis (this early shift to 25° prevents the formation of any oocytes).
Meiotic prophase progression kinetics:
Microinjection of Cy3-dUTP (Amersham Biosciences; Piscataway, NJ) and meiotic progression assays were carried out as in (Jaramillo-Lambert et al. 2007).
RNA interference:
RNAi was performed by the feeding method of (Timmons et al. 2001). L4 worms [L3 for the fem-3(q22) strain] were fed bacteria expressing double stranded RNA to indicated gene (syp-1, him-8, zim-1, zim-2, pch-2, and chk-1) from RNAi feeding library (Kamath et al. 2003). RNAi efficacy was monitored by counting DAPI staining bodies in hermaphrodite diakinesis nuclei (him-8, zim-1, and zim-2) and by monitoring plates for dead embryos and incidence of male progeny (syp-1). chk-1(RNAi) efficiency was determined by monitoring progeny for sterility. Bacteria expressing feeding vector (L4440) were used as controls. Cells were seeded onto NGM plates that contained 25 μg/ml carbenicillin and 1 mm IPTG and allowed to grow at room temperature for 24 hr. Seeded plates were stored at 4° and used within ∼2 weeks.
Quantification of germ-line apoptosis:
Acridine orange (AO) staining of apoptotic germ cells in tra-2(e1095) XX, fog-1(q325) X0, fem-3(e1996) X0, N2 XX, and N2 X0 was carried out using modifications of procedure in (Gartner et al. 2004). tra-2(e1095) XX and wild-type control animals were synchronized by picking L4 larvae to new plates and holding at 20° for 48 hr. fog-1(q325) X0, fem-3(e1996) X0, and wild-type controls were synchronized by picking L4 larvae to fresh plates and incubating at 20° for 24 hr. A total of 0.5 ml of 50 μg/ml AO (Molecular Probes, Invitrogen; Carlsbad, CA) in M9 was added to 60-mm plates containing adult worms and incubated at room temperature for 1 hr. Worms were replated to new 60-mm plates, allowed to recover, and then mounted under cover slips in M9 on 3% agarose pads containing 0.2 mm tetramisole (Sigma; St. Louis). Apoptotic bodies were scored by fluorescence microscopy and DIC.
fem-3(q22) XX and wild-type controls were synchronized by picking L3 larvae and holding at either the permissive (15°) or restrictive (25°) temperatures for 48 hr. Apoptosis was scored by CED-1-GFP fluorescence (Bhalla and Dernburg 2005); CED-1 is a transmembrane protein on phagocytic cells that is important for engulfment of apoptotic cells (Zhou et al. 2001).
Germ-line apoptosis in N2 XX hermaphrodites and fem-3(e1996) X0 animals subjected to RNAi was quantified by CED-1-GFP 48 hr post-L4 larvae.
Immunostaining:
Rabbit anti-RAD-51 (1:50) (Colaiácovo et al. 2003), rabbit anti-SYP-1 (1:200), and guinea pig anti-SYP-1 (1:800) (MacQueen et al. 2002) were generous gifts from A. Villeneuve. Guinea pig anti-HIM-8 (1:500) (Phillips et al. 2005), guinea pig anti-HTP-3 (1:500) (MacQueen et al. 2005), and rabbit anti-HTP-1/2 (1:500) (Martinez-Perez et al. 2008) were generously donated by A. Dernburg. Rabbit anti-HIM-3 (1:200) (Zetka et al. 1999) was generously provided by M. Zetka. Rabbit anti-GFP (1:500) was purchased from Novus Biologicals (Littleton, CO). Rabbit anti-histone H3 dimethyl-lysine 9 (H3dimethylK9) (1:500) (Kelly et al. 2002) was purchased from Upstate USA (Charlottesville, VA). Rabbit anti-histone H3 dimethyl-lysine 4 (H3dimethylK4) (1:500) (Kelly et al. 2002) was purchased from Cell Signaling Technology (Danvers, MA). The secondary antibodies Alexa Fluor-488 donkey anti-rabbit (1:200), Alexa Fluor-555 donkey anti-rabbit (1:200), and Alexa Fluor-488 goat anti-guinea pig (1:200) were purchased from Molecular Probes, Invitrogen. Antibody staining of gonads was performed as in Jaramillo-Lambert et al. (2007). The slides were imaged using an API Delta Vision deconvolution microscope. Images were deconvolved using Applied Precision SoftWoRx image analysis software.
RAD-51 foci were quantified in three germ lines of age-matched hermaphrodites and males (20 hr post-L4) of each genotype (Colaiácovo et al. 2003). Germ lines were divided by meiotic prophase substage and number of foci per nucleus was scored for each stage. Quantification of RAD-51 foci specific to the X chromosome(s) was scored as foci that localized to the chromosome that stained with the X-specific marker, HIM-8. Data were analyzed using Applied Precision SoftWoRx image analysis deconvolution software.
RNA in situ hybridization:
Gonads were dissected and processed on slides as described for batch processing (Lee and Schedl 2006). Both sense and antisense probes were synthesized for the X-linked, oocyte-enriched genes, K08A8.1 and F52D2.2 (Reinke et al. 2000; Kelly et al. 2002). The control sense probes gave little or no signal (data not shown). Images were captured with a Zeiss Axioplan 2 microscope equipped with a color Axiocam.
RESULTS
Meiotic prophase progression is dependent on the sex of the germ line:
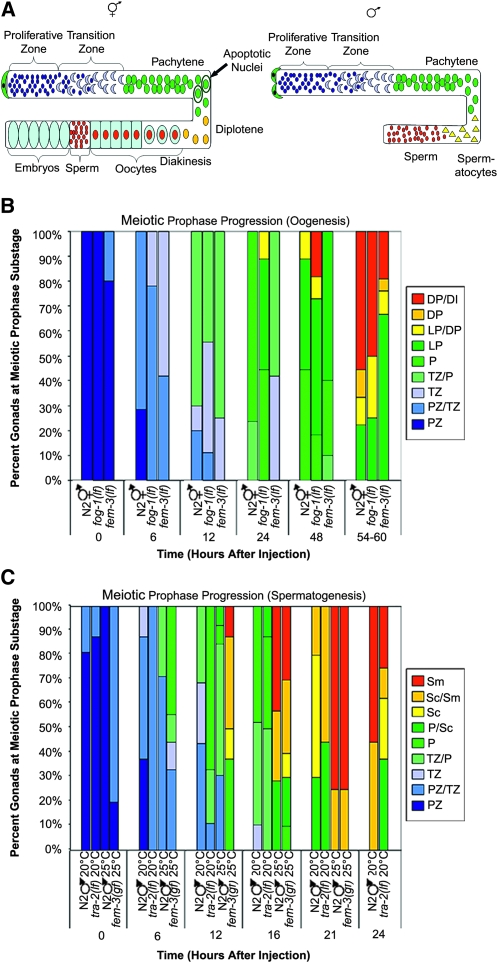
The C. elegans germ line is housed in two (hermaphrodites) or one (male) U-shaped gonads; syncytial germ cells within the gonad are arranged in a temporal/spatial gradient with classical stages of meiotic prophase easily distinguishable (Figure 1A) (Hubbard and Greenstein 2005). We previously developed an S phase labeling assay and found that meiotic prophase progression for oogenesis in adult hermaphrodites takes twice as long as meiotic prophase progression for spermatogenesis in adult males (54–60 hr vs. 20–24 hr) (Jaramillo-Lambert et al. 2007). To determine whether the difference in timing is dependent on chromosome sex (XX vs. X0), somatic sex (female vs. male body), or germ-line sex (oogenesis vs. spermatogenesis) we used S phase labeling (Jaramillo-Lambert et al. 2007) to monitor progression of nuclei through the meiotic prophase substages over time in several sex determination mutants that uncoupled chromosome, somatic, and germ-line sex.
Figure 1.—
Meiotic prophase progression is dependent on germ-line sex. (A) Cartoon of hermaphrodite (left) and male (right) gonad arms. Distal end is capped by somatic distal tip cell(s) (green) and contains a population of proliferating germ cells (dark blue). As cells move proximally they enter meiotic prophase (transition zone-leptotene/zygotene; light blue crescents) and then progress through pachytene (green), diplotene (yellow), and diakinesis (red). Initially, hermaphrodites form sperm (small red circles) and then switch to oocyte production (light blue with red centers) beginning at L4 stage. After switch to oocyte production, cells in the pachytene region of the germ line undergo physiological apoptosis (circled green nuclei). In males, germ-line cells differentiate into spermactocytes (yellow triangles) and then sperm (red circles); no apoptosis occurs. (B) Meiotic prophase timing in sex determination mutants undergoing oogenesis. Percentage of gonads displaying labeled nuclei at indicated stages is shown. Proliferative zone (PZ), transition zone (TZ), pachytene (P), late pachytene (LP), diplotene (DP), and diakinesis (DI). Number of gonads examined at each time point were N2 XX: 0 hr = 9, 6 hr = 14, 12 hr = 10, 24 hr = 17, 48 hr = 9, and 54–60 hr = 9; fog-1(q325) X0: 0 hr = 7, 6 hr = 9, 12 hr = 9, 24 hr = 9, 48 hr = 11, and 54–60 hr = 8; and fem-3(e1996) X0: 0 hr = 10, 6 hr = 12, 12 hr = 8, 24 hr = 12, 48 hr = 10, and 54–60 hr = 18. (C) Meiotic prophase timing in sex determination mutants undergoing spermatogenesis. Percentage of gonads with labeled nuclei at indicated stages is shown. Proliferative zone (PZ), transition zone (TZ), pachytene (P), spermatocytes (Sc), and sperm (Sm). Number of gonads examined at each time point were N2 X0 20°: 0 hr = 16, 6 hr = 8, 12 hr = 16, 16 hr = 19, 21 hr = 10, and 24 hr = 9; tra-2(e1095) XX 20°: 0 hr = 8, 6 hr = 8, 12 hr = 9, 16 hr = 8, 21 hr = 9, and 24 hr = 8; N2 X0 25°: 0 hr = 7, 6 hr = 7, 12 hr = 13, 16 hr = 7, and 21 hr = 4 (15/19 gonads no label); and fem-3(q20) XX 25°: 0 hr = 10, 6 hr = 9, 12 hr = 8, 16 hr = 10, and 21 hr = 8.
fem-3(e1996lf) X0 animals have a female body and undergo oogenesis (Hodgkin 1986), while fog-1(q325lf) X0 animals have a male body, but an oogenic germ line (Barton and Kimble 1990). Meiotic prophase timing in these mutants was slow (54–60 hr), similar to N2 XX hermaphrodites. Progression of labeled nuclei through each prophase substage in fem-3(lf) X0 and fog-1(lf) X0 similarly paralleled N2 XX hermaphrodites (Figure 1B). These experiments reveal that irrespective of the number of X chromosomes or whether there is a male or female body, mutants undergoing oogenesis have slow meiotic prophase kinetics.
We also examined prophase kinetics of tra-2(e1095lf) XX and fem-3(q20gf) XX mutants. tra-2(lf) XX animals have a male body and spermatogenic germ line (Hodgkin and Brenner 1977). Prophase progression timing of tra-2(lf) XX males was similar to N2 X0 males with prophase completed by 20–24 hr; progression of nuclei through each substage was similar to N2 X0 males, although there were fewer labeled nuclei in the transition zone in tra-2(lf) XX compared to N2 X0 males (Figure 1C). fem-3(q20gf) XX animals have a female soma, but the germ line only produces sperm at 25°, the nonpermissive temperature (Barton et al. 1987). Analysis of meiotic prophase progression in N2 X0 males revealed that prophase took slightly less time at 25° compared to standard temperature of 20° (completed between 16–21 hr; Figure 1C). Similarly, meiotic prophase kinetics of fem-3(gf) XX animals (25°) was completed by 21 hr (Figure 1C). Thus, worms undergoing spermatogenesis have fast meiotic prophase kinetics. Taken together, these results indicate that meiotic prophase timing is dictated by the sex of the germ line.
Physiological and checkpoint-activated germ-line apoptosis are dependent on germ-line sex:
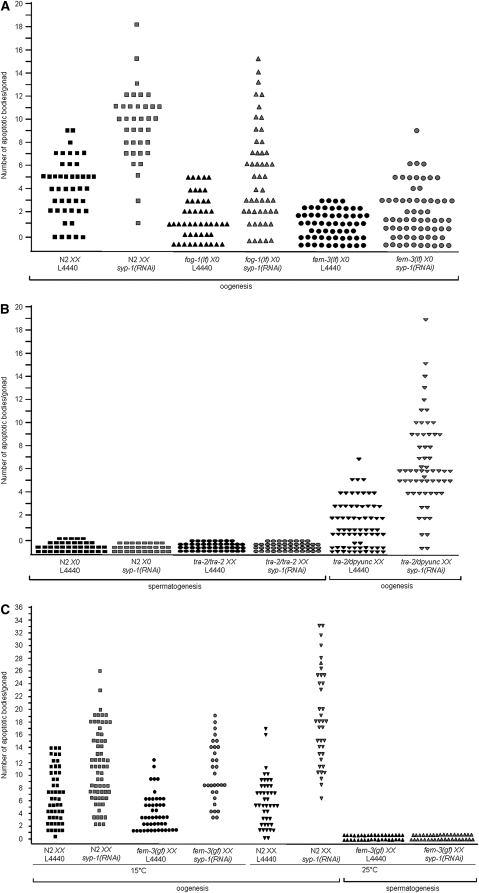
Another striking germ-line difference between hermaphrodites and males is apoptosis. Nuclei in the pachytene region of the hermaphrodite germ line undergo both physiological and checkpoint-activated apoptosis (Figure 1A) (Gumienny et al. 1999; Gartner et al. 2000; Bhalla and Dernburg 2005). Contrastingly, the male germ line does not undergo physiological apoptosis (Gumienny et al. 1999) or apoptosis in response to exogenous DNA damage (Gartner et al. 2000). To determine whether nuclei in the male germ line undergo checkpoint-activated apoptosis in response to chromosome asynapsis, and/or failure to repair meiotic DSBs, we examined apoptosis in germ lines where syp-1 was depleted by RNAi. SYP-1 is a central region component of the SC; knockdown of syp-1 results in increased levels of germ-line apoptosis in hermaphrodites because all chromosome pairs are asynapsed and chiasmata are not formed (Figure 2A) (MacQueen et al. 2002). In contrast to hermaphrodites, no apoptosis was observed in the male germ line when chromosome asynapsis was induced by depletion of syp-1 (Figure 2B).
Figure 2.—
Physiological and checkpoint-activated apoptosis are dependent on germ-line sex. (A) Scatterplot depicting number of apoptotic bodies detected in germ lines of N2 XX, fog-1(q325) X0, and fem-3(e1996) X0 animals (by AO staining and DIC). Apoptosis was scored in adult animals 24 hr post-L4. Y-axis value for each point represents number of apoptotic bodies/gonad. Total number of gonads examined for each: N2 XX L4440 N = 50, N2 XX syp-1(RNAi) N = 52, fog-1(q325) X0 L4440 N = 48, fog-1(q325) X0 syp-1(RNAi) N = 50, fem-3(e1996) X0 L4440 N = 60, and fem-3(e1996) X0 syp-1(RNAi) N = 66. (B) Scatterplot depicting number of apoptotic bodies detected in germ lines of N2 X0, tra-2 XX, or tra-2/dpyunc XX worms (by AO staining and DIC). Apoptosis was scored in adult animals 48 hr post-L4. Y-axis value for each point represents number of apoptotic bodies/gonad. Total number of gonads examined for each: N2 X0 L4440 N = 37, N2 X0 syp-1(RNAi) N = 28, tra-2(e1095) XX L4440 N = 44, tra-2(e1095) XX syp-1(RNAi) N = 44, tra-2(e1095)/dpyunc XX L4440 N = 70, and tra-2(e1095)/dpyunc XX syp-1(RNAi) N = 57. (C) Scatterplot depicting number of apoptotic bodies detected in gonads of N2 XX and fem-3(q22) XX worms by CED-1∷GFP fluorescence, for both control (L4440) and syp-1(RNAi). fem-3(q22) is temperature sensitive and each experiment was performed at both 15° and 25°. Apoptotic bodies were scored in adult animals 48 hr post-L3. Y-axis values represent number of apoptotic bodies/gonad. Total number of gonads examined for 15°: N2 XX L4440 N = 50, N2 XX syp-1(RNAi) N = 65, fem-3(q22) XX L4440 N = 48, and fem-3(q22) XX syp-1(RNAi) N = 31. Total number of gonads examined for 25°: N2 XX L4440 N = 44, fem-3(q22) X0 L4440 N = 36, N2 XX syp-1(RNAi) N = 38, and fem-3(q22) XX syp-1(RNAi) N = 37.
To determine whether checkpoint-activated apoptosis is under the same genetic control as meiotic prophase progression, we examined the sex determination mutants analyzed above (Figure 1) for both physiological (L4440; feeding vector) and checkpoint-activated apoptosis [syp-1(RNAi)]. Apoptosis in oogenic germ lines was quantified for N2 XX, fem-3(lf) X0, and fog-1(lf) X0 using the vital dye, AO. Both fem-3(lf) X0 and fog-1(lf) X0 germ lines are competent for physiological apoptosis as shown by the low levels of apoptotic bodies seen in control worms (Figure 2A). This is consistent with previous analysis that indicated that oogenesis dictates physiological apoptosis (Gumienny et al. 1999). Additionally, when SYP-1 was depleted, both fem-3(lf) X0 and fog-1(lf) X0 worms had increased levels of apoptosis (Figure 2A). Thus oogenic germ lines undergo both physiological and checkpoint-activated apoptosis.
Apoptosis in N2 X0 males, tra-2/dpyunc XX hermaphrodites, and tra-2/tra-2 XX mutants was also quantified using AO. tra-2/dypunc XX hermaphrodites were competent for both physiological and checkpoint-activated apoptosis, but no apoptosis was observed in N2 X0 and tra-2/tra-2 XX males under either condition (Figure 2B). Apoptosis in N2 XX hermaphrodites and the fem-3(gf) XX mutant was scored at both the permissive (15°) and restrictive (25°) temperature by CED-1-GFP fluorescence (Boulton et al. 2004; Bhalla and Dernburg 2005). At 15° germ-line differentiation of fem-3(gf) XX behaves like N2 XX, first undergoing spermatogenesis and then switching to oogenesis as an adult. N2 XX and fem-3(gf) XX at 15° were competent for both physiological and checkpoint-activated apoptosis (Figure 2C). However, at 25° fem-3(gf) XX germ cells in the adult continued spermatogenesis and were not competent for either physiological or checkpoint-activated apoptosis (Figure 2C). Together these experiments indicate that regardless of X chromosome content or somatic sex, both physiological and checkpoint-activated apoptosis are dictated by the sex of the germ line.
The single X chromosome is not recognized to be partnerless:
Checkpoints operate in meiosis to monitor both the status of chromosome synapsis and meiotic DSB repair and can detect a single asynapsed chromosome pair (Bhalla and Dernburg 2005; Hochwagen and Amon 2006). Analysis of apoptosis in fem-3(lf) X0 and fog-1(lf) X0 germ lines revealed low levels of basal apoptosis (Figure 2A, L4440), even though they have an asynapsed X. To confirm that checkpoints were not activated in fem-3(lf) X0 worms, we monitored apoptosis in fem-3(lf) X0 worms depleted for either chk-1 or pch-2. CHK-1 is a conserved checkpoint kinase that functions in both the DNA damage and recombination checkpoint to activate p53 for apoptosis (Walworth and Bernards 1996; Levine 1997; Harrison and Haber 2006); PCH-2 is a AAA-ATPase that has been implicated in the chromosome synapsis checkpoint in C. elegans (Bhalla and Dernburg 2005), although the pathway and interaction with the recombination checkpoint has not been elucidated. Consistent with the absence of checkpoint activation, we observed no decrease in apoptosis when chk-1 or pch-2 was depleted in fem-3(lf) X0 (Table 1), suggesting that neither the recombination nor synapsis checkpoint pathways are induced in fem-3(lf) X0 animals.
TABLE 1.
fem-3(lf) X0 germ lines sense a single pair of asynapsed autosomes but not the unpaired X chromosome
| Asynapsed chromosomes | No. apoptotic bodies/gonads |
||
|---|---|---|---|
| RNAi | N2 XX | fem-3(lf) X0 | |
| L4440 | NA | 5.6 ± 0.3 | 1.8 ± 0.2 |
| zim-2 | V | 8.2 ± 0.6* | 4.9 ± 0.6* |
| zim-1 | II and III | 12.0 ± 1.5* | 3.9 ± 0.5* |
| syp-1 | All | 14.0 ± 0.8* | 5.2 ± 0.5* |
| chk-1 | NA | 5.1 ± 0.8 | 1.8 ± 0.7 |
| syp-1;chk-1 | All | 4.6 ± 0.8 | 2.8 ± 0.7 |
| pch-2 | NA | 7.5 ± 1.0 | 1.5 ± 0.3 |
| syp-1;pch-2 | All | 9.1 ± 1.9* | 2.4 ± 0.4 |
| him-8 | X | 9.9 ± 1.1* | 2.4 ± 0.3 |
Number of apoptotic nuclei/gonad arm was determined by CED-1-GFP 48 hr post-L4. Minimum of 15 gonad arms were scored for each genotype. Average number of bivalents/univalents in diakinesis oocytes by DAPI staining for L4440 = 5.7, zim-2(RNAi) = 6.5, fem-3(e1996); zim-2(RNAi) = 6.4, zim-1(RNAi) = 7.0, fem-3(e1996); zim-1(RNAi) = 7.4, and him-8(RNAi) = 6.4. syp-1(RNAi) efficiency was determined by monitoring plates for dead embryos and male progeny. The data shown are means ± SEM Statistical comparisons between the mutants and L4440 were conducted using the two-tailed Mann-Whitney test. *P < 0.001.
To determine whether failure to recognize the asynapsed X chromosome is a unique property of the X or due to the inability to detect low levels of checkpoint signaling, we examined the consequence of inducing asynapsis of different autosomal pairs in fem-3(lf) X0 worms. We observed increased levels of apoptosis in both N2 XX and fem-3(lf) X0 worms when asynapsis of a single autosome pair (Phillips and Dernburg 2006), two autosome pairs (Phillips and Dernburg 2006), or all chromosome pairs were induced (MacQueen et al. 2002) (Table 1). Further, the increase in apoptosis in syp-1(RNAi) worms was dependent on both chk-1 and pch-2 (Table 1). On the other hand, depletion of him-8, which is important for X chromosome pairing and synapsis (Phillips et al. 2005), resulted in increased levels of apoptosis in N2 XX but not fem-3(lf) X0 worms (Table 1). These results suggest that the lone X escapes detection by the checkpoint machinery.
Double strand break levels are elevated and perdure in X0 animals:
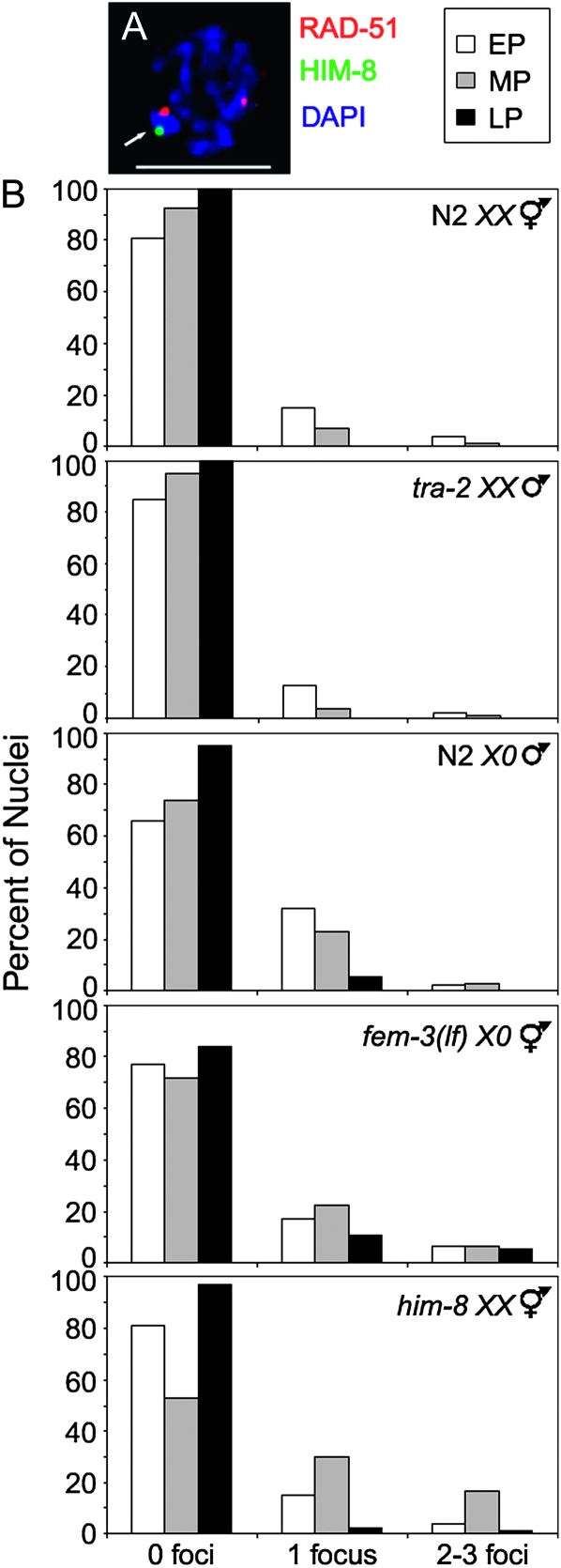
During meiosis the intentional formation of DSBs, and repair via the homologous chromosome, is needed to form chiasmata essential for chromosome segregation at the first meiotic division (Lee and Amon 2001). In hermaphrodites, failure in chromosome synapsis activates the recombination checkpoint pathway leading to increased levels of apoptosis due to the absence of a homologous partner for repair of DSBs (MacQueen et al. 2002; Colaiácovo et al. 2003; Smolikov et al. 2007, 2009). As the male X chromosome has no homologous chromosome to repair DSBs and fails to activate the apoptotic pathway even in an oogenic germ line [fem-3(lf) X0, Figure 2A, Table 1], we reasoned that the single X chromosome does not incur DSBs. To examine DSBs specifically on the X chromosome(s) in germ-line nuclei of wild-type and mutant animals we monitored the localization of the strand exchange protein RAD-51, which is transiently associated with DSBs (Alpi et al. 2003), as well as HIM-8, an X-specific transacting factor (Phillips et al. 2005) (Figure 3A). In germ lines of N2 XX hermaphrodites and tra-2 XX male mutants the majority of nuclei did not have RAD-51 foci on the X chromosome pair at the time of dissection, but 15 and 13% of early and 7 and 4% of midpachytene nuclei, respectively, had one RAD-51 focus, and <5% had two to three RAD-51 foci. By late pachytene there were no RAD-51 foci detected on the X chromosome pair in these germ lines (Figure 3B, top two histograms). In contrast, 32% of early pachytene and 23% of midpachytene nuclei of N2 X0 male germ lines had one focus while 2% in early and 3% in midpachytene had two to three RAD-51 foci. In fem-3(lf) X0 mutant germ lines 17% in early pachytene and 22% in midpachytene had one RAD-51 focus on the X chromosome while 6% in both early and midpachytene had two to three RAD-51 foci. In both N2 X0 male and fem-3(lf) X0 germ lines RAD-51 foci were also detected in late pachytene nuclei (Figure 3B, middle two histograms). The overall increase in the number of Xs with RAD-51 foci was similar to what was observed in him-8(me4) XX germ lines where RAD-51 foci were elevated on the asynapsed X chromosomes and persisted into late pachytene (Figure 3B, bottom histogram). This analysis was possible as the him-8(me4) is a missense mutation that encodes a protein that binds the X chromosome and is still recognized by HIM-8 antibodies (Phillips et al. 2005). The RAD-51 foci observed on the single X in N2 males were SPO-11 dependent, indicating that breaks were induced by the meiotic recombination machinery (data not shown). Taken together, these data indicate that the single X chromosome is a substrate for the meiotic recombination machinery and suggest that the dynamics of DSB repair on the X chromosome(s) is influenced by the lack of a homologous chromosome.
Figure 3.—
Abundance of RAD-51 foci on X chromosome(s). (A) Nucleus showing RAD-51 focus on X (arrow). Nucleus labeled with α-RAD-51 (red) and α-HIM-8 (green), counterstained with DAPI (blue). Scale bar, 5 μm. (B) Histograms representing quantification of RAD-51 foci on X chromosome(s) in N2 XX, tra-2 XX males, N2 X0, fem-3(e1996) X0, and him-8(me4) XX. Y-axis indicates percentage of nuclei that contain 0, 1, or 2–3 RAD-51 foci per X chromosome for early pachytene (white), midpachytene (gray), and late pachytene (black).
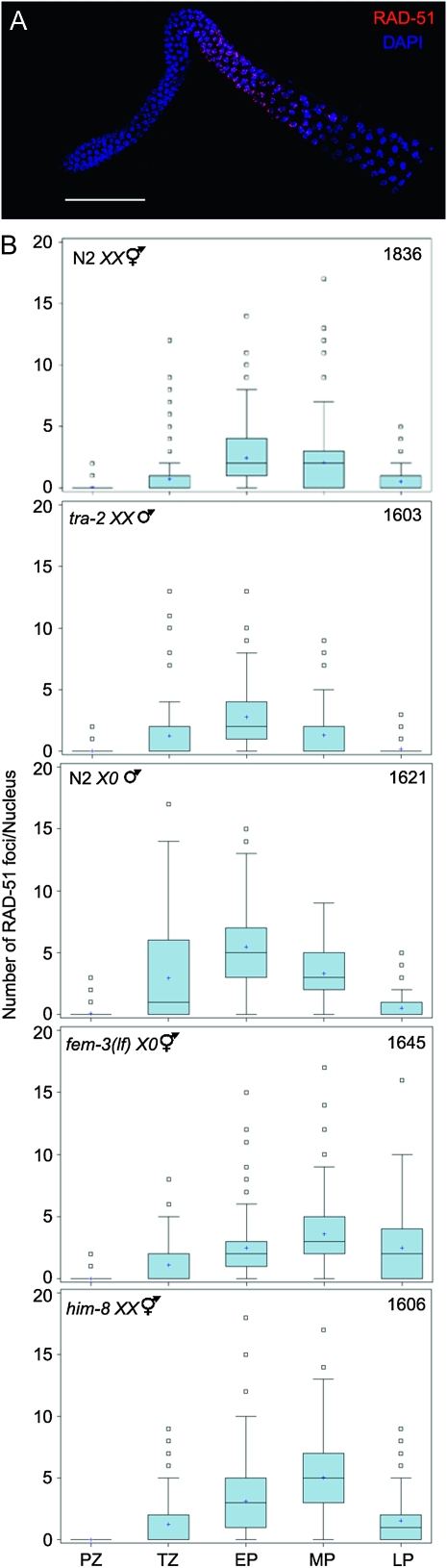
In mutant hermaphrodite germ lines that are unable to repair breaks due to unavailability of the homologous chromosome there is an apparent global increase in the number of DSBs as well as a persistence of breaks on all chromosomes into late stages of pachytene (Colaiácovo et al. 2003; Carlton et al. 2006; Smolikov et al. 2007, 2009). To determine whether DSBs on the single X chromosome in N2 males also had this effect, we examined the global appearance and removal of RAD-51 in male and hermaphrodite germ lines. RAD-51 foci in N2 XX germ lines appeared in the transition zone, peaked during early pachytene, and disappeared by late pachytene (Figure 4B) (Colaiácovo et al. 2003; Carlton et al. 2006). RAD-51 foci in N2 X0 male germ lines also first appeared in the transition zone, but quantification revealed higher overall levels of RAD-51 foci and a persistence of these foci into the late pachytene substage of meiotic prophase (Figure 4, A and B).
Figure 4.—
Assembly and removal of RAD-51 foci during meiotic prophase progression. (A) Image of adult N2 X0 gonad stained with DAPI (blue) and α-RAD-51 (red). Scale bar, 50 μm. (B) Quantification of RAD-51 focus formation in N2 XX, tra-2(e1095) XX, N2 X0, fem-3(e1996) X0, and him-8(me4) XX. Gonads were divided into prophase substages and nuclei assigned to each region on the basis of morphology and location. Graphs display box-whisker plots of focus numbers. X-axis indicates meiotic prophase stages: Proliferative zone (PZ), transition zone (TZ), early pachytene (EP), midpachytene (MP), and late pachytene (LP); y-axis indicates number of RAD-51 foci/nucleus. Center horizontal line of each box indicates the median measurements; lines extending above and below boxes indicate standard deviation and outliers indicate the entire range of measurements. Numbers of nuclei observed for each strain are indicated in upper right.
Progression of RAD-51 focus formation was also examined in tra-2 XX male and fem-3(lf) X0 female germ lines. In tra-2 XX mutant germ lines, RAD-51 foci progression and abundance mirrored N2 XX hermaphrodite germ lines (Figure 4B). In fem-3(lf) X0 germ lines, the progression of RAD-51 focus formation and removal was similar to N2 X0 males where RAD-51 foci were shifted to later meiotic prophase substages and overall levels were higher (Figure 4B). This resembled what was observed in him-8(me4) XX hermaphrodite germ lines (Figure 4B, bottom), where asynapsis of a single chromosome pair has global effects on RAD-51 progression (Carlton et al. 2006). We also observed an extension of the transition zone in N2 X0 and fem-3(lf) X0 germ lines compared to tra-2 XX male and N2 XX hermaphrodite germ lines, respectively, as in him-8 XX hermaphrodite germ lines (data not shown; Figure 1, B and C; Carlton et al. 2006). Thus, the single X chromosome of males and fem-3(lf) mutants has global effects on DSB repair and meiotic prophase progression in the germ line; however, this does not result in activation of checkpoints as it does in him-8 XX hermaphrodites (Table 1; Bhalla and Dernburg 2005).
HORMA domain proteins are loaded onto the single X chromosome:
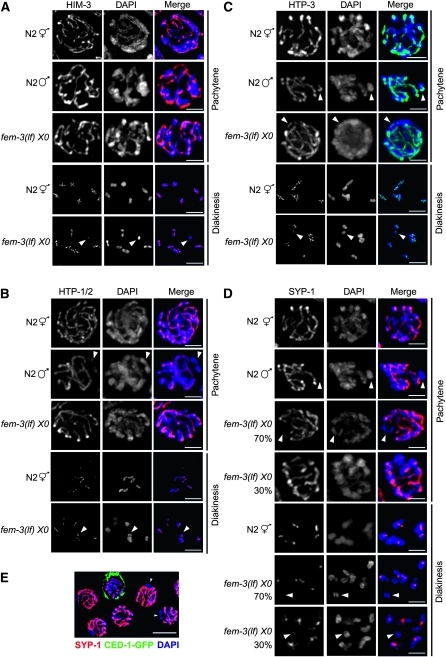
What is different about the single X chromosome that enables delayed repair of breaks using a sister chromatid as a template without eliciting a checkpoint response? In Sacccharomyces cerevisiae, Hop1, the meiosis-specific HORMA (Aravind and Koonin 1998) domain protein of the chromosomal axes (Hollingsworth et al. 1990), is essential for interhomolog bias during meiotic recombination (Niu et al. 2005). HIM-3, an ortholog of Hop1, associates with the chromosome core of both synapsed and asynapsed chromosomes in hermaphrodites (Zetka et al. 1999). In him-3 mutants, recombination is initiated and breaks are repaired efficiently, despite the failure in synapsis, suggesting that HIM-3 also functions in interhomolog bias during meiotic recombination (Couteau et al. 2004). Interestingly, in him-3 null mutant hermaphrodites there is no increase in apoptosis, suggesting that checkpoints are not activated even though there is chromosomal asynapsis and lack of a homologous chromosome for repair of DSBs (Couteau et al. 2004). During male meiosis, immunofluorescence studies revealed that the single X chromosome lacks HIM-3 staining at metaphase I (Zetka et al. 1999), suggesting that HIM-3's absence may explain the failure in checkpoint signaling. As metaphase I chromosomes have already completed recombination, we examined HIM-3 loading onto the X chromosome during meiotic prophase when recombination events are initiated and repaired. We observed HIM-3 on the single X chromosome in pachytene nuclei in the male germ line as well as in fem-3(lf) germ lines (Figure 5A, top three panels) (see also Shakes et al. 2009). Furthermore, in the fem-3(lf) X0 mutant HIM-3 was maintained on the single X in diakinesis as in N2 XX hermaphrodites (bottom two panels).
Figure 5.—
Loading of SC axial and central elements onto the X chromosome(s). Immunolocalization of (A) HIM-3 (red), (B) HTP-1/2 (red), (C) HTP-3 (green), and (D) SYP-1 (red) counterstained with DAPI (blue) in nuclei from N2 XX, N2 X0, and fem-3(e1996) X0 germ lines. Arrowheads point to X chromosome determined by lack of localization of SYP-1 or by size of DAPI staining body. Scale bar, 2 μm. (E) SYP-1 (red) and CED-1-GFP (green) localization in a fem-3(e1996) X0 germ line. Arrowheads indicate chromosome lacking SYP-1. Scale bar, 5 μm.
In addition to HIM-3, C. elegans has three paralogous HORMA domain proteins that are structural components of the meiotic chromosome axes and have also been implicated in checkpoints: HTP-1, HTP-2 (Couteau and Zetka 2005; Martinez-Perez and Villeneuve 2005; Martinez-Perez et al. 2008), and HTP-3 (Goodyer et al. 2008). We monitored the assembly and disassembly of these proteins in hermaphrodite XX, male X0, and fem-3(lf) X0 mutant germ lines and found that all of these proteins were loaded onto both the paired Xs and the single X (Figure 5, B and C). Hence it is not the absence of HORMA domain axial components on the single X that prevents a checkpoint response.
Some fem-3(lf) X chromosomes achieve self-synapsis:
We also monitored the loading of the SC central component, SYP-1, on the single X chromosome in pachytene (Figure 5D, top four panels) and diakinesis nuclei (Figure 5D, bottom three panels). SYP-1 was not loaded on the single X of N2 males; however, ∼30% of fem-3(lf) X chromosomes had SYP-1 staining, suggesting that these chromosomes had achieved nonhomologous self-synapsis (Figure 5D; arrowheads denote X chromosome). This is analogous to what has been observed in the X0 mouse, where 30% of the X chromosomes engage in nonhomologous self-synapsis (Speed 1986; Turner et al. 2005).
The observation that some X chromosomes engage in self-synapsis suggested that these nuclei may escape detection by the checkpoint machinery and that this may account for the failure in checkpoint signaling in the fem-3(lf) X0 mutant. To investigate this possibility, we simultaneously monitored apoptosis (using GFP antibodies) and SYP-1 by immunofluorescence in worms expressing CED-1-GFP (Figure 5E). We found that germ cell nuclei with SYP-1 and without SYP-1 on the X chromosome were equally likely to undergo apoptosis, indicating that self-synapsis of the X does not prevent checkpoint signaling.
Transcriptional silencing of the single X chromosome late in prophase correlates with lack of checkpoint signaling:
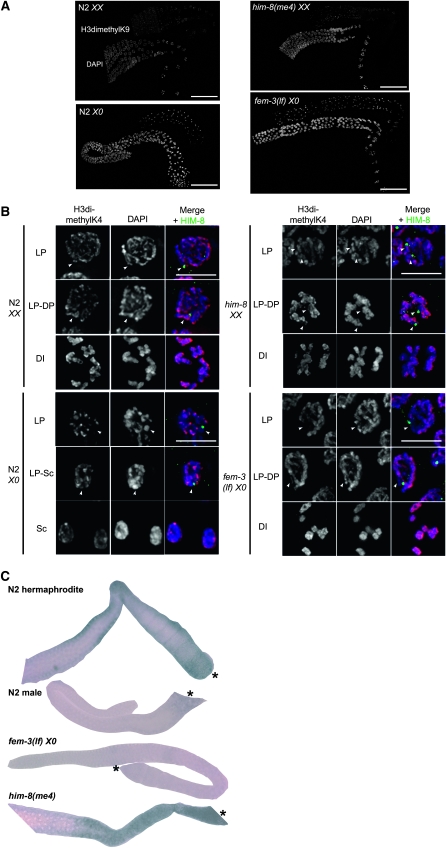
Asynapsed chromosomes, including the male X, accumulate the heterochromatin mark H3dimethylK9 to a greater extent than paired X chromosomes (Kelly et al. 2002; Bean et al. 2004). Further, the X chromosome pair becomes transcriptionally active for a set of oocyte-enriched genes late in pachytene while the single X remains silent in males (Reinke et al. 2000; Kelly et al. 2002). To investigate the relationship between chromatin/transcriptional state and checkpoint signaling, we monitored repressive and activating chromatin marks and X-linked gene transcription in N2 XX hermaphrodites, N2 X0 males, fem-3(lf) X0 females, and him-8 XX hermaphrodites. As previously reported, H3dimethylK9 is found predominantly on the paired X chromosomes in N2 hermaphrodite germ lines and accumulates on the single X in male and the asynapsed Xs in him-8 hermaphrodite germ lines (Kelly et al. 2002; Bean et al. 2004). A single intense focus is also observed in the fem-3(lf) X0 germ line, which presumably represents the unpaired X (Figure 6A). In N2 and him-8 hermaphrodites, this mark is redistributed throughout the genome in the transition from pachytene to diplotene (Kelly et al. 2002; Bean et al. 2004). In N2 male germ lines, H3dimethylK9 persisted on the single X chromosome until the transition to spermatocytes (Figure 6A). In fem-3(lf) X0 germ lines, the H3dimethylK9 foci remained intense until diakinesis, although there was some redistribution of the mark in diplotene (Figure 6A).
Figure 6.—
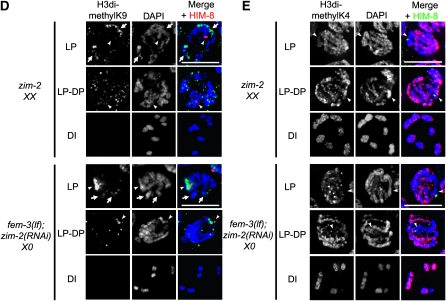
Chromatin/transcriptional state of X chromosome(s). Immunolocalization of (A) H3dimethylK9; scale bar, 50 μm and (B) H3methylK4 (red) counterstained with DAPI (blue); scale bar, 5 μm in N2 XX, N2 X0, fem-3(e1996) X0, and him-8(me4) XX germ lines. Images were captured with same exposure time. Arrowheads indicate X chromosome(s) as determined by HIM-8 staining (green); HIM-8 is not present on diakinesis or spermatocyte nuclei. (C) In situ hybridization of oocyte-enriched X-linked F52D2.2 in N2 XX, N2 X0, fem-3(e1996) X0, and him-8(me4) XX germ lines. fem-3(e1996) X0 worms were mated with N2 males. Asterisk denotes proximal gonad. (D) Immunolocalization of H3dimethylK9 (green) counterstained with DAPI (blue); scale bar, 5 μm in zim-2(tm574) XX and fem-3(e1996); zim-2(RNAi) X0 germ lines. Arrowheads indicate X chromosome(s) (HIM-8; red); arrows indicate chromosomes with H3dimethlyK9 that presumably represent chromosome V. (E) H3methylK4 (red) counterstained with DAPI (blue); scale bar, 5 μm in zim-2(tm574) XX and fem-3(e1996); zim-2(RNAi) X0 germ lines. Arrowheads indicate X chromosome(s) (HIM-8; green). Late pachytene (LP), diplotene (DP), diakinesis (DI), and spermatocyte (Sc).
The release of H3dimethylK9 in hermaphrodites, but not in males, is accompanied by an accumulation of activating marks and the onset of transcription of a set of X-linked oocyte-enriched genes (Reinke et al. 2000; Kelly et al. 2002). It was noted that him-8(e1489) also loads activating marks on the asynapsed Xs late in prophase (Bean et al. 2004). Analysis of H3dimethylK4 showed that indeed this activating mark accumulated on the X chromosomes in both N2 and him-8(me4) hermaphrodites late in pachytene and was maintained through diakinesis (Figure 6B). In N2 X0 and fem-3(lf) X0 worms H3dimethylK4 was not found on the X in late pachytene but began to weakly stain the X at the transition to spermatocytes (N2 males) and at the transition to diplotene [fem-3(lf) X0] (Figure 6B). H3dimethylK4 labels all six DAPI-staining bodies of fem-3(lf) X0 at diakinesis (Figure 6B).
To determine whether the late acquisition of activating marks correlated with lack of transcription, we performed in situ hybridization with two X-linked oocyte-enriched genes, which are activated in late pachytene in hermaphrodites (Kelly et al. 2002). As expected, N2 XX and him-8(me4) XX hermaphrodites exhibited expression of these genes in late pachytene extending to diakinesis (Figure 6C and data not shown). Expression was also observed in him-8(e1489) XX hermaphrodites (data not shown). In contrast, in the germ lines of both N2 X0 males and fem-3(lf) X0 females transcription of these genes was not activated (Figure 6C and data not shown). These results indicate that the single X in fem-3(lf) has chromatin/transcriptional properties similar to the single X in N2 males.
To further examine the relationship between chromatin state and checkpoint signaling, we monitored repressive H3dimethylK9 and activating H3dimethylK4 in zim-2(tm574) XX and fem-3(lf); zim-2(RNAi) X0 worms, in which the recombination checkpoint is activated because of chromosome V asynapsis (Table 1). In zim-2 XX hermaphrodite germ lines, H3dimethylK9 was modestly enriched on two chromosomes, which presumably represent the unpaired Vs, in pachytene, and was redistributed throughout the nucleus in late pachytene-diplotene (Figure 6D). In fem-3(lf); zim-2(RNAi) X0 germ lines, H3dimethylK9 was enriched on the X (marked with HIM-8) and also found on two chromosomes, presumably the unpaired Vs; H3dimethylK9 was retained only on the X into late pachytene-diplotene (Figure 6D). In contrast, H3dimethylK4 was observed on all chromosomes by late pachytene-diplotene in zim-2 XX mutants, but its acquisition was delayed on the single X in fem-3(lf); zim-2(RNAi) worms (Figure 6E). Taken together, these results suggest that it is the unique chromatin/transcriptional state of the X that enables heterogametic sex chromosomes to be hidden from the checkpoint machinery.
DISCUSSION
In this study, we show that both meiotic prophase kinetics and apoptosis are sexually dimorphic in C. elegans: germ lines undergoing oogenesis have slow prophase kinetics and both physiological and checkpoint-activated apoptosis. Contrastingly, germ lines undergoing spermatogenesis have fast prophase kinetics and lack germ-line apoptosis. Additionally, we found that meiotic DSBs are induced on the single X chromosome of wild-type males and the lack of a homologous chromosome to repair these breaks is sensed by the germ line but fails to induce apoptosis even in a background competent for checkpoint signaling. We also provide evidence that the chromatin/transcriptional state of a single X chromosome is distinct from unpaired X chromosomes or autosomes and suggest that this helps mask the X from the checkpoint machinery thereby preventing constitutive checkpoint activation.
Sex-dependent coordination of meiotic prophase kinetics and apoptosis:
Slow meiotic prophase kinetics and apoptosis were observed in germ lines of worms undergoing oogenesis regardless of the soma or X chromosome constitution, suggesting that these processes are important for formation of functional oocytes, but not sperm, and are coordinately regulated. As in many organisms, C. elegans oocytes are significantly larger than sperm as oocytes must incorporate yolk lipoproteins, maternal mRNAs, and other cellular components required for early embryogenesis (Hall et al. 1999). In contrast, during spermatogenesis the developing spermatids bud from a central residual body where most of the cellular components are left behind (L'hernault 2006). Thus, it is not surprising that the most striking difference in meiotic prophase timing in the sexes is observed at pachytene (Jaramillo-Lambert et al. 2007). Apoptosis also occurs at pachytene and has been proposed to serve a nurse cell function by contributing to the pool of cellular components ultimately packaged into oocytes (Gumienny et al. 1999; Wolke et al. 2007; Andux and Ellis 2008). Furthermore, physiological apoptosis plays a maternal age-dependent role in oocyte quality, while the quality of sperm is not affected by paternal age (Andux and Ellis 2008).
MAP kinase pathways play integral roles in controlling and coordinating several aspects of female and male germ-line biology including meiotic prophase progression, apoptosis, and sexual fate (Lee et al. 2007). In C. elegans hermaphrodites, sustained activation of MAP kinase, MPK-1, is needed for germ cells to transition through pachytene (Greenstein 2005; Lee et al. 2007). MPK-1 was originally reported to also function in pachytene progression in the male germ line as loss-of-function alleles of mpk-1 resulted in a male pachytene arrest phenotype (Church et al. 1995). However, genetic and cellular analyses revealed that MPK-1 functions to promote male germ cell fate and the observed pachytene arrest in mpk-1 males was due to feminization of the germ line (Lee et al. 2007). Thus, it is unlikely that MPK-1 is required in the male germ line for cells to transit pachytene as MPK-1 is not present in this region of the germ line. The presence of MPK-1 in the pachytene region of hermaphrodite but not male germ lines may also explain the lack of apoptosis in the male germ line. If so, then one prediction is that expression of MPK-1 in the pachytene region of the male germ line would both slow meiotic prophase progression and promote germ-line apoptosis.
Sex-specific differences in meiotic prophase timing and germ-line apoptosis may also influence the phenotypic outcome of meiotic mutants. Mutations of essential meiotic components result in sexually dimorphic phenotypes in mammals. For example, mutations of SC components and proteins involved in DSB repair result in male sterility with meiosis unable to proceed beyond zygonema and a subsequent culling of germ cells by apoptosis, while female mutants show reduced fertility with oocytes that progress beyond pachynema (Morelli and Cohen 2005). C. elegans meiotic mutants also display sexually dimorphic phenotypes. Disruption of the sister chromatid cohesin REC-8 results in precocious separation of sister chromatids (Pasierbek et al. 2001). Surprisingly, progeny from rec-8 hermaphrodites mated to wild-type males have a high hatch rate (89%) while only 16% of embryos hatch when the sperm come from rec-8 males (Severson et al. 2009). Mutations that disrupt chromosome synapsis or recombinational repair in either hermaphrodites or males result in extensive embryonic lethality, but only the adult hermaphrodite germ line responds by inducing apoptosis (MacQueen et al. 2002; Alpi et al. 2003; Colaiácovo et al. 2003; Smolikov et al. 2007, 2009). Perhaps, a lengthier prophase in hermaphrodites gives the germ line time to launch a checkpoint response, which allows for DNA damage repair, or to remove defective germ cells. Interestingly, more progeny survive when sperm are spo-11 deficient (20% hatching) than when oocytes come from spo-11 mothers (11%) (Severson et al. 2009). SPO-11 is a conserved topisomerase responsible for initiating meiotic DSBs; in spo-11 mutants there are no breaks and consequently checkpoints are not activated (Dernburg et al. 1998; MacQueen et al. 2002; Bhalla and Dernburg 2005). That spo-11 produce sperm with the correct ploidy more often than oocytes suggest that achiasmatic chromosomes are segregated more effectively in males than in hermaphrodites and may explain why hermaphrodites, but not males, have active checkpoint pathways.
Asynapsed chromosomes, sex, and meiotic checkpoints:
Errors in chromosomal synapsis often lead to removal of germ cells by apoptosis (Bhalla and Dernburg 2005; Morelli and Cohen 2005; Burgoyne et al. 2009). The X chromosome of C. elegans males lacks a pairing partner, a situation that triggers checkpoint-activated apoptosis in hermaphrodites. However, N2 X0 males do not have physiological apoptosis (Gumienny et al. 1999) nor do they respond to chromosomal asynapsis by inducing apoptosis (Figure 2B; Table 1), thereby preventing elimination of all germ cells. On the other hand, fem-3(lf) X0 and fog-1(lf) X0 germ lines have elevated levels of apoptosis in response to autosomal asynapsis but have low levels of apoptosis under physiological conditions (Figure 2A and Table 1), suggesting that they do not recognize the X as unpaired. Consistent with this, depletion of either pch-2, required for the synapsis checkpoint (Bhalla and Dernburg 2005), or chk-1, which functions in the recombination and DNA damage checkpoints (Rhind and Russell 2000), had no affect on apoptotic levels, suggesting that neither the synapsis nor the recombination checkpoint pathways is activated in these worms. That the levels of basal apoptosis were lower than wild type is most likely a consequence of a reduction in meiotic maturation as fem-3(e1996) X0 females do not produce sperm (Hodgkin 1986). Major sperm protein (MSP) promotes meiotic maturation and the absence of sperm causes oocytes to arrest at diakinesis (McCarter et al. 1999). Although we mated fem-3(lf) X0 females with N2 males prior to quantification of apoptosis to provide a source of MSP, these females tended to have more oocytes than N2 hermaphrodites, suggesting that meiotic maturation had been delayed.
In the C. elegans germ line, unrepaired recombination intermediates are detected by the 9-1-1 (Rad9-Rad1-Hus1) complex and the P-I-3-kinase-related protein kinases, ATM and ATR (Hofmann et al. 2002; Garcia-Muse and Boulton 2005). CHK-1 functions downstream of ATR/ATM to transduce the checkpoint signal to the apoptotic machinery via phosphorylation of the p53 homolog, CEP-1 (Rhind and Russell 2000). Checkpoint-activated apoptosis due to activation of the synapsis checkpoint is mediated through the AAA-ATPase, PCH-2, independently of CEP-1 (Bhalla and Dernburg 2005; Phillips and Dernburg 2006); however, how PCH-2 relays the signal has not been elucidated. Consistent with a previous study, our analysis of apoptosis in N2 XX hermaphrodites and fem-3(lf) X0 germ lines revealed that depletion of PCH-2 when chromosomal asynapsis is induced results in intermediate levels of apoptosis (Table 1) (Bhalla and Dernburg 2005), as the recombination checkpoint is also activated when chromosome synapsis is impaired (Bhalla and Dernburg 2005). Surprisingly, knockdown of CHK-1 in the presence of chromosomal asynapsis reduced the number of apoptotic nuclei to physiological levels (Table 1), suggesting that the PCH-2 synapsis checkpoint signal is transmitted to the apoptotic machinery through CHK-1. The interconnection between the recombination and synapsis checkpoint signaling pathways awaits further characterization.
Heterogametic sex chromosomes and checkpoint signaling:
We have shown that the single X chromosome of males and fem-3(lf) animals incur DSBs and that the absence of a homologous chromosome is detected by the germ line as evidenced by increased levels of RAD-51 foci on all chromosomes and a delay in the disappearance in these foci (Figures 3 and 4). Male genetic recombination frequencies have been reported to be reduced (Zetka and Rose 1990; Meneely et al. 2002) or the same as hermaphrodites (Lim et al. 2008), yet we observed an increase in the levels of RAD-51 foci in the male compared to hermaphrodite germ line. This apparent discrepancy is most likely because we are only looking at a single point in time and the levels of RAD-51 foci reflect both the number of breaks and repair kinetics. Furthermore, some of the breaks may be repaired as noncrossovers, which would not influence the genetic map distance. Nevertheless, it appears that germ lines with a single X chromosome sense the absence of a homologous chromosome but do not activate checkpoints. Early in meiosis, a bias is established to promote the use of the homolog to repair DSBs. The interhomolog bias is mediated through axial components of the SC, which have also been implicated in checkpoint function (Zetka et al. 1999; Couteau and Zetka 2005; Martinez-Perez and Villeneuve 2005; Niu et al. 2005;Goodyer et al. 2008; Martinez-Perez et al. 2008). One possibility is that the single X chromosome evades checkpoint detection through an early release in the bias to allow repair of DSBs via the sister chromatid. Our examination of HIM-3, HTP-1/2, and HTP-3 localization revealed that these proteins are retained on the X chromosome core throughout prophase consistent with the delay in removal of RAD-51 foci, and thus early release of these axial components is unlikely to explain the lack of checkpoint signaling. However, it is possible that other proteins associated with the axis or post-translational modifications of these axial components are different on the single X compared to either asynapsed Xs or autosomes and may contribute to the ability of the single X to evade detection by the checkpoint machinery.
Analysis of chromatin marks and X-linked gene expression suggests that the chromatin/transcriptional status of X may distinguish the lone X from asynapsed chromosomes to prevent inappropriate checkpoint activation. We found that while the lone X in fem-3(lf) mutants, the asynapsed Xs in him-8, and the asynapsed Vs in zim-2 accumulate H3dimethylK9, a repressive mark associated with heterochromatin (Kelly et al. 2002), only the asynapsed chromosome pairs accumulate activating marks late in pachytene, induce gene expression, and activate checkpoints. Bean et al. (2004) suggested that the acquisition of activating marks in him-8 was a consequence of low levels of pairing observed in the him-8(e1489) mutant. However, both him-8(e1489) and him-8(me4) have as severe pairing defects as the deletion allele (Phillips et al. 2005). We suggest that late acquisition of activating marks and lack of gene transcription of the single X chromosome prevents detection of the unpaired X by the checkpoint machinery. Alternatively, upstream pathways that respond to X chromosome number, such as the dosage compensation machinery (Meyer 2005) or the mes (maternal-effect sterile) genes (Garvin et al. 1998), may function to block checkpoint signaling when the X is unpaired in addition to influencing the transcriptional status of the X.
In mammalian males, there is also accumulation of H3dimethylK9 and transcriptional silencing of the X and Y chromosomes (Namekawa et al. 2006). It was proposed that MSCI is related to general silencing of unpaired DNA or MSUC. Consistent with this, in both C. elegans and mammals unpaired autosomes as well as the sex chromosomes attract H3dimethylK9 (Bean et al. 2004; Schimenti 2005; Figure 6). However, MSCI of the single X in C. elegans males appears to be a more stable transcriptionally repressed state than unpaired Xs or autosomes in hermaphrodites. Transcriptional repression of the single X suggests the possibility that there is an X-linked gene required in trans for checkpoint signaling that is not expressed in males. However, we do not favor this hypothesis as checkpoint activation occurs in fem-3(lf) X0 worms in the presence of asynapsed autosomes even though there is transcriptional repression of the X. Our results instead suggest that transcriptional repression of the X functions in cis to prevent detection of the asynapsed X by the checkpoint machinery. Further analyses of chromatin modifications, transcriptional profiles and upstream pathways will need to be performed to determine the relationship between the transcriptional status of the single X chromosome and checkpoint signaling.
It was previously proposed that the single X chromosome of C. elegans males is analogous to the sex body in mammals (Kelly et al. 2002). In mammalian spermatogenesis the sex body is transcriptionally silenced and is separated from the autosomes in its own nuclear domain (Handel 2004; Turner et al. 2005). In addition to the accumulation of H3dimethylK9, the sex body chromatin domain also accumulates BRCA1, ATR, and the phosphorylated form of H2AX, γH2AX; proteins essential for MSCI but are also involved in checkpoints (Turner et al. 2004, 2005). BRCA1 is not required for X silencing in C. elegans males but its localization pattern has not been determined (Kelly and Aramayo 2007). On the other hand, while ATR is required for DNA damage checkpoint signaling in C. elegans (Garcia-Muse and Boulton 2005) it does not accumulate on the single X chromosome of males (A. Jaramillo-Lambert and J. Engebrecht, unpublished results) and H2AX is not found in C. elegans (Boulton 2006; Kelly and Aramayo 2007). It appears that in mammalian male germ cells, the sex body is recognized as unpaired; ATR and γH2AX are recruited to the sex body, but the checkpoint machinery has been assimilated to function in transcriptional silencing and does not promote checkpoint activation in this context. The single X chromosome of C. elegans males is transcriptionally silenced, but does not recruit checkpoint proteins and nonetheless evades checkpoint activation. While checkpoint proteins are not required for transcriptional silencing in C. elegans, the same transcriptional silencing and checkpoint evasion outcome is achieved similarly to what occurs in the male mammalian germ line.
Acknowledgments
We thank A. Dernburg, A. Villeneuve, and M. Zetka for generously providing antibodies. We also thank T. Schedl and the Caenorhabditis Genetic Center for strains and helpful discussion and S. Burgess, D. Starr, and J. Vitt for comments on the manuscript. This work was supported by National Institutes of Health (NIH) GM086505 and the Agricultural Experimental Station CA-D*MCB-7237-H (to J.E.). A.J.L. was supported by NIH T32GM070377 and a University of California, Davis Office of Graduate Studies dissertation-year fellowship.
References
- Alpi, A., P. Pasierbek, A. Gartner and J. Loidl, 2003. Genetic and cytological characterization of the recombination protein RAD-51 in Caenorhabditis elegans. Chromosoma 112 6–16. [DOI] [PubMed] [Google Scholar]
- Andux, S., and R. E. Ellis, 2008. Apoptosis maintains oocyte quality in aging Caenorhabditis elegans females. PLoS Genet. 4 e1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind, L., and E. V. Koonin, 1998. The HORMA domain: a common structural denominator in mitotic checkpoints, chromosome synapsis and DNA repair. Trends Biochem. Sci. 23 284–286. [DOI] [PubMed] [Google Scholar]
- Ashley, T., A. W. Plug, J. Xu, A. J. Solari, G. Reddy et al., 1995. Dynamic changes in Rad51 distribution on chromatin during meiosis in male and female vertebrates. Chromosoma 104 19–28. [DOI] [PubMed] [Google Scholar]
- Barton, M. K., and J. Kimble, 1990. fog-1, a regulatory gene required for specification of spermatogenesis in the germ line of Caenorhabditis elegans. Genetics 125 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton, M. K., T. B. Schedl and J. Kimble, 1987. Gain-of-function mutations of fem-3, a sex-determination gene in Caenorhabditis elegans. Genetics 115 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean, C. J., C. E. Schaner and W. G. Kelly, 2004. Meiotic pairing and imprinted X chromatin assembly in Caenorhabditis elegans. Nat. Genet. 36 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla, N., and A. F. Dernburg, 2005. A conserved checkpoint monitors meiotic chromosome synapsis in Caenorhabditis elegans. Science 310 1683–1686. [DOI] [PubMed] [Google Scholar]
- Blank, R. D., G. R. Campbell, A. Calabro and P. D'Eustachio, 1988. A linkage map of mouse chromosome 12: localization of Igh and effects of sex and interference on recombination. Genetics 120 1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton, S. J., 2006. BRCA1-mediated ubiquitylation. Cell Cycle 5 1481–1486. [DOI] [PubMed] [Google Scholar]
- Boulton, S. J., J. S. Martin, J. Polanowska, D. E. Hill, A. Gartner et al., 2004. BRCA1/BARD1 orthologs required for DNA repair in Caenorhabditis elegans. Curr. Biol. 14 33–39. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne, P. S., S. K. Mahadevaiah and J. M. Turner, 2009. The consequences of asynapsis for mammalian meiosis. Nat. Rev. Genet. 10 207–216. [DOI] [PubMed] [Google Scholar]
- Carlton, P. M., A. P. Farruggio and A. F. Dernburg, 2006. A link between meiotic prophase progression and crossover control. PLoS Genet. 2 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church, D. L., K. L. Guan and E. J. Lambie, 1995. Three genes of the MAP kinase cascade, mek-2, mpk-1/sur-1 and let-60 ras, are required for meiotic cell cycle progression in Caenorhabditis elegans. Development 121 2525–2535. [DOI] [PubMed] [Google Scholar]
- Colaiácovo, M. P., A. J. MacQueen, E. Martinez-Perez, K. McDonald, A. Adamo et al., 2003. Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev. Cell 5 463–474. [DOI] [PubMed] [Google Scholar]
- Couteau, F., K. Nabeshima, A. Villeneuve and M. Zetka, 2004. A component of C. elegans meiotic chromosome axes at the interface of homolog alignment, synapsis, nuclear reorganization, and recombination. Curr. Biol. 14 585–592. [DOI] [PubMed] [Google Scholar]
- Couteau, F., and M. Zetka, 2005. HTP-1 coordinates synaptonemal complex assembly with homolog alignment during meiosis in C. elegans. Genes Dev. 19 2744–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg, A. F., K. McDonald, G. Moulder, R. Barstead, M. Dresser et al., 1998. Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94 387–398. [DOI] [PubMed] [Google Scholar]
- Donis-Keller, H., P. Green, C. Helms, S. Cartinhour, B. Weiffenbach et al., 1987. A genetic linkage map of the human genome. Cell 51 319–337. [DOI] [PubMed] [Google Scholar]
- Eppig, J. J., R. M. Schultz and G. S. Kopf, 1996. Maturation of mouse oocytes in serum-free medium. Hum. Reprod. 11 1139–1140. [DOI] [PubMed] [Google Scholar]
- Garcia-Muse, T., and S. J. Boulton, 2005. Distinct modes of ATR activation after replication stress and DNA double-strand breaks in Caenorhabditis elegans. EMBO J. 24 4345–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner, A., A. J. MacQueen and A. M. Villeneuve, 2004. Methods for analyzing checkpoint responses in Caenorhabditis elegans. Methods Mol. Biol. 280 257–274. [DOI] [PubMed] [Google Scholar]
- Gartner, A., S. Milstein, S. Ahmed, J. Hodgkin and M. O. Hengartner, 2000. A conserved checkpoint pathway mediates DNA damage-induced apoptosis and cell cycle arrest in C. elegans. Mol. Cell 5 435–443. [DOI] [PubMed] [Google Scholar]
- Garvin, C., R. Holdeman and S. Strome, 1998. The phenotype of mes-2, mes-3, mes-4 and mes-6, maternal-effect genes required for survival of the germline in Caenorhabditis elegans, is sensitive to chromosome dosage. Genetics 148 167–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer, W., S. Kaitna, F. Couteau, J. D. Ward, S. J. Boulton et al., 2008. HTP-3 links DSB formation with homolog pairing and crossing over during C. elegans meiosis. Dev. Cell 14 263–274. [DOI] [PubMed] [Google Scholar]
- Greenstein, D., 2005. Control of oocyte meiotic maturation and fertilization. WormBook, 1–12. [DOI] [PMC free article] [PubMed]
- Gumienny, T. L., E. Lambie, E. Hartwieg, H. R. Horvitz and M. O. Hengartner, 1999. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development 126 1011–1022. [DOI] [PubMed] [Google Scholar]
- Hall, D. H., V. P. Winfrey, G. Blaeuer, L. H. Hoffman, T. Furuta et al., 1999. Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev. Biol. 212 101–123. [DOI] [PubMed] [Google Scholar]
- Handel, M. A., 2004. The XY body: a specialized meiotic chromatin domain. Exp. Cell Res. 296 57–63. [DOI] [PubMed] [Google Scholar]
- Handel, M. A., and J. J. Eppig, 1998. Sexual dimorphism in the regulation of mammalian meiosis. Curr. Top. Dev. Biol. 37 333–358. [DOI] [PubMed] [Google Scholar]
- Harrison, J. C., and J. E. Haber, 2006. Surviving the breakup: the DNA damage checkpoint. Annu. Rev. Genet. 40 209–235. [DOI] [PubMed] [Google Scholar]
- Hassold, T., and P. Hunt, 2001. To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2 280–291. [DOI] [PubMed] [Google Scholar]
- Hochwagen, A., and A. Amon, 2006. Checking your breaks: surveillance mechanisms of meiotic recombination. Curr. Biol. 16 R217–R228. [DOI] [PubMed] [Google Scholar]
- Hodgkin, J., 1986. Sex determination in the nematode C. elegans: analysis of tra-3 suppressors and characterization of fem genes. Genetics 114 15–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin, J. A., and S. Brenner, 1977. Mutations causing transformation of sexual phenotype in the nematode Caenorhabditis elegans. Genetics 86 275–287. [PMC free article] [PubMed] [Google Scholar]
- Hofmann, E. R., S. Milstein, S. J. Boulton, M. Ye, J. J. Hofmann et al., 2002. Caenorhabditis elegans HUS-1 is a DNA damage checkpoint protein required for genome stability and EGL-1-mediated apoptosis. Curr. Biol. 12 1908–1918. [DOI] [PubMed] [Google Scholar]
- Hollingsworth, N. M., L. Goetsch and B. Byers, 1990. The HOP1 gene encodes a meiosis-specific component of yeast chromosomes. Cell 61 73–84. [DOI] [PubMed] [Google Scholar]
- Hubbard, E. J., and D. Greenstein, 2005. Introduction to the germ line. WormBook, 1–4. [DOI] [PMC free article] [PubMed]
- Hunt, P. A., and T. J. Hassold, 2002. Sex matters in meiosis. Science 296 2181–2183. [DOI] [PubMed] [Google Scholar]
- Jaramillo-Lambert, A., M. Ellefson, A. M. Villeneuve and J. Engebrecht, 2007. Differential timing of S phases, X chromosome replication, and meiotic prophase in the C. elegans germ line. Dev. Biol. 308 206–221. [DOI] [PubMed] [Google Scholar]
- Kamath, R. S., A. G. Fraser, Y. Dong, G. Poulin, R. Durbin et al., 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421 231–237. [DOI] [PubMed] [Google Scholar]
- Kelly, W. G., and R. Aramayo, 2007. Meiotic silencing and the epigenetics of sex. Chromosome Res. 15 633–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, W. G., C. E. Schaner, A. F. Dernburg, M. H. Lee, S. K. Kim et al., 2002. X-chromosome silencing in the germline of C. elegans. Development 129 479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner, N., 1996. Meiosis: how could it work? Proc. Natl. Acad. Sci. USA 93 8167–8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler, K. E., C. L. Boulton, H. E. Collins, R. L. French, K. C. Herman et al., 1996. Spontaneous X chromosome MI and MII nondisjunction events in Drosophila melanogaster oocytes have different recombinational histories. Nat. Genet. 14 406–414. [DOI] [PubMed] [Google Scholar]
- L'Hernault, S. W., 2006. Spermatogenesis. WormBook, 1–14. [DOI] [PMC free article] [PubMed]
- Lamb, N. E., S. B. Freeman, A. Savage-Austin, D. Pettay, L. Taft et al., 1996. Susceptible chiasmate configurations of chromosome 21 predispose to non-disjunction in both maternal meiosis I and meiosis II. Nat. Genet. 14 400–405. [DOI] [PubMed] [Google Scholar]
- Lee, B., and A. Amon, 2001. Meiosis: how to create a specialized cell cycle. Curr. Opin. Cell Biol. 13 770–777. [DOI] [PubMed] [Google Scholar]
- Lee, M. H., and T. Schedl, 2006. RNA in situ hybridization of dissected gonads. WormBook, 1–7. [DOI] [PMC free article] [PubMed]
- Lee, M. H., M. Ohmachi, S. Arur, S. Nayak, R. Francis et al., 2007. Multiple functions and dynamic activation of MPK-1 extracellular signal-regulated kinase signaling in Caenorhabditis elegans germline development. Genetics 177 2039–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, A. J., 1997. p53, the cellular gatekeeper for growth and division. Cell 88 323–331. [DOI] [PubMed] [Google Scholar]
- Lim, J. G., R. R. Stine and J. L. Yanowitz, 2008. Domain-specific regulation of recombination in Caenorhabditis elegans in response to temperature, age and sex. Genetics 180 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen, A. J., M. P. Colaiacovo, K. McDonald and A. M. Villeneuve, 2002. Synapsis-dependent and -independent mechanisms stabilize homolog pairing during meiotic prophase in C. elegans. Genes Dev. 16 2428–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen, A. J., C. M. Phillips, N. Bhalla, P. Weiser, A. M. Villeneuve et al., 2005. Chromosome sites play dual roles to establish homologous synapsis during meiosis in C. elegans. Cell 123 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madl, J. E., and R. K. Herman, 1979. Polyploids and sex determination in Caenorhabditis elegans. Genetics 93 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevaiah, S. K., D. Bourc'his, D. G. de Rooij, T. H. Bestor, J. M. Turner et al., 2008. Extensive meiotic asynapsis in mice antagonises meiotic silencing of unsynapsed chromatin and consequently disrupts meiotic sex chromosome inactivation. J. Cell Biol. 182 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manandhar, G., H. Schatten and P. Sutovsky, 2005. Centrosome reduction during gametogenesis and its significance. Biol. Reprod. 72 2–13. [DOI] [PubMed] [Google Scholar]
- Martinez-Perez, E., M. Schvarzstein, C. Barroso, J. Lightfoot, A. F. Dernburg et al., 2008. Crossovers trigger a remodeling of meiotic chromosome axis composition that is linked to two-step loss of sister chromatid cohesion. Genes Dev. 22 2886–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Perez, E., and A. M. Villeneuve, 2005. HTP-1-dependent constraints coordinate homolog pairing and synapsis and promote chiasma formation during C. elegans meiosis. Genes Dev. 19 2727–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui, Y., and H. J. Clarke, 1979. Oocyte maturation. Int. Rev. Cytol. 57 185–282. [DOI] [PubMed] [Google Scholar]
- McCarter, J., B. Bartlett, T. Dang and T. Schedl, 1999. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev. Biol. 205 111–128. [DOI] [PubMed] [Google Scholar]
- Meneely, P. M., A. F. Farago and T. M. Kauffman, 2002. Crossover distribution and high interference for both the X chromosome and an autosome during oogenesis and spermatogenesis in Caenorhabditis elegans. Genetics 162 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, B. J., 2005. X–Chromosome dosage compensation. WormBook, 1–14. [DOI] [PMC free article] [PubMed]
- Moens, P. B., D. J. Chen, Z. Shen, N. Kolas, M. Tarsounas et al., 1997. Rad51 immunocytology in rat and mouse spermatocytes and oocytes. Chromosoma 106 207–215. [DOI] [PubMed] [Google Scholar]
- Morelli, M. A., and P. E. Cohen, 2005. Not all germ cells are created equal: aspects of sexual dimorphism in mammalian meiosis. Reproduction 130 761–781. [DOI] [PubMed] [Google Scholar]
- Morgan, T. H., 1912. Special Articles. Science 36 718–720. [DOI] [PubMed] [Google Scholar]
- Namekawa, S. H., P. J. Park, L. F. Zhang, J. E. Shima, J. R. McCarrey et al., 2006. Postmeiotic sex chromatin in the male germline of mice. Curr. Biol. 16 660–667. [DOI] [PubMed] [Google Scholar]
- Niu, H., L. Wan, B. Baumgartner, D. Schaefer, J. Loidl et al., 2005. Partner choice during meiosis is regulated by Hop1-promoted dimerization of Mek1. Mol. Biol. Cell. 16 5804–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, J., A. Viera, M. T. Parra, R. de la Fuente, J. A. Suja et al., 2006. Involvement of synaptonemal complex proteins in sex chromosome segregation during marsupial male meiosis. PLoS Genet. 2 e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasierbek, P., M. Jantsch, M. Melcher, A. Schleiffer, D. Schweizer et al., 2001. A Caenorhabditis elegans cohesion protein with functions in meiotic chromosome pairing and disjunction. Genes Dev. 15 1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, J., S. Palmer, A. Gabriel and A. Ashworth, 2001. A short pseudoautosomal region in laboratory mice. Genome Res. 11 1826–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, C. M., and A. F. Dernburg, 2006. A family of zinc-finger proteins is required for chromosome-specific pairing and synapsis during meiosis in C. elegans. Dev. Cell 11 817–829. [DOI] [PubMed] [Google Scholar]
- Phillips, C. M., C. Wong, N. Bhalla, P. M. Carlton, P. Weiser et al., 2005. HIM-8 binds to the X chromosome pairing center and mediates chromosome-specific meiotic synapsis. Cell 123 1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke, V., H. E. Smith, J. Nance, J. Wang, C. Van Doren et al., 2000. A global profile of germline gene expression in C. elegans. Mol. Cell 6 605–616. [DOI] [PubMed] [Google Scholar]
- Rhind, N., and P. Russell, 2000. Chk1 and Cds1: linchpins of the DNA damage and replication checkpoint pathways. J. Cell Sci. 113(Pt 22): 3889–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder, G. S., 1997. Meiotic chromosomes: it takes two to tango. Genes Dev. 11 2600–2621. [DOI] [PubMed] [Google Scholar]
- Ross, L. O., R. Maxfield and D. Dawson, 1996. Exchanges are not equally able to enhance meiotic chromosome segregation in yeast. Proc. Natl. Acad. Sci. USA 93 4979–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimenti, J., 2005. Synapsis or silence. Nat. Genet. 37 11–13. [DOI] [PubMed] [Google Scholar]
- Schoenmakers, S., E. Wassenaar, J. W. Hoogerbrugge, J. S. Laven, J. A. Grootegoed et al., 2009. Female meiotic sex chromosome inactivation in chicken. PLoS Genet. 5 e1000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson, A. F., L. Ling, V. van Zuylen and B. J. Meyer, 2009. The axial element protein HTP-3 promotes cohesin loading and meiotic axis assembly in C. elegans to implement the meiotic program of chromosome segregation. Genes Dev. 23 1763–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakes, D. C., J. C. Wu, P. L. Sadler, K. Laprade, L. L. Moore et al., 2009. Spermatogenesis-specific features of the meiotic program in Caenorhabditis elegans. PLoS Genet. 5 e1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, A., H. Perlman, Y. Yan, C. Walker, G. Corley-Smith et al., 2002. Sex-specific recombination rates in zebrafish (Danio rerio). Genetics 160 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolikov, S., A. Eizinger, A. Hurlburt, E. Rogers, A. M. Villeneuve et al., 2007. Synapsis-defective mutants reveal a correlation between chromosome conformation and mode of double-strand break repair during Caenorhabditis elegans meiosis. Genetics 176 2027–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolikov, S., K. Schild-Prufert and M.P. Colaiacovo, 2009. A yeast two-hybrid screen for SYP-3 interactors identifies SYP-4, a component required for synaptonemal complex assembly and chiasma formation in Caenorhabditis elegans meiosis. PLoS Genet. 5 e1000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed, R. M., 1986. Oocyte development in XO foetuses of man and mouse: the possible role of heterologous X-chromosome pairing in germ cell survival. Chromosoma 94 115–124. [DOI] [PubMed] [Google Scholar]
- Stergiou, L., K. Doukoumetzidis, A. Sendoel and M. O. Hengartner, 2007. The nucleotide excision repair pathway is required for UV-C-induced apoptosis in Caenorhabditis elegans. Cell Death Differ. 14 1129–1138. [DOI] [PubMed] [Google Scholar]
- Timmons, L., D. L. Court and A. Fire, 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263 103–112. [DOI] [PubMed] [Google Scholar]
- Turner, J. M., O. Aprelikova, X. Xu, R. Wang, S. Kim et al., 2004. BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr. Biol. 14 2135–2142. [DOI] [PubMed] [Google Scholar]
- Turner, J. M., S. K. Mahadevaiah, O. Fernandez-Capetillo, A. Nussenzweig, X. Xu et al., 2005. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat. Genet. 37 41–47. [DOI] [PubMed] [Google Scholar]
- Walworth, N. C., and R. Bernards, 1996. rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science 271 353–356. [DOI] [PubMed] [Google Scholar]
- Wignall, S. M., and A. M. Villeneuve, 2009. Lateral microtubule bundles promote chromosome alignment during acentrosomal oocyte meiosis. Nat. Cell Biol. 11 839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolke, U., E. A. Jezuit and J. R. Priess, 2007. Actin-dependent cytoplasmic streaming in C. elegans oogenesis. Development 134 2227–2236. [DOI] [PubMed] [Google Scholar]
- Wu, T. F., and D. S. Chu, 2008. Sperm chromatin: fertile grounds for proteomic discovery of clinical tools. Mol. Cell Proteomics 7 1876–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetka, M. C., and A. M. Rose, 1990. Sex-related differences in crossing over in Caenorhabditis elegans. Genetics 126 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetka, M. C., I. Kawasaki, S. Strome and F. Muller, 1999. Synapsis and chiasma formation in Caenorhabditis elegans require HIM-3, a meiotic chromosome core component that functions in chromosome segregation. Genes Dev. 13 2258–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Z., E. Hartwieg and H. R. Horvitz, 2001. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell 104 43–56. [DOI] [PubMed] [Google Scholar]
- Zickler, D., and N. Kleckner, 1999. Meiotic chromosomes: integrating structure and function. Annu. Rev. Genet. 33 603–754. [DOI] [PubMed] [Google Scholar]