Abstract
The opportunistic pathogen Candida albicans can grow over a wide pH range, which is associated with its ability to colonize and infect distinct host niches. C. albicans growth in neutral-alkaline environments requires proteolytic activation of the transcription factor Rim101. Rim101 activation requires Snf7, a member of the endosomal sorting complex required for transport (ESCRT) pathway. We hypothesized that Snf7 has distinct functions in the Rim101 and ESCRT pathways, which we tested by alanine-scanning mutagenesis. While some snf7 alleles conferred no defects, we identified alleles with solely ESCRT-dependent, solely Rim101-dependent, or both Rim101- and ESCRT-dependent defects. Thus, Snf7 function in these two pathways is at least partially separable. Both Rim101- and ESCRT-dependent functions require Snf7 recruitment to the endosomal membrane and alleles that disrupted both pathways were found to localize normally, suggesting a downstream defect. Most alleles that conferred solely Rim101-dependent defects were still able to process Rim101 normally under steady-state conditions. However, these same strains did display a kinetic defect in Rim101 processing. Several alleles with solely Rim101-dependent defects mapped to the C-terminal end of Snf7. Further analyses suggested that these mutations disrupted interactions with bro-domain proteins, Rim20 and Bro1, in overlapping but slightly divergent Snf7 domains.
Candida albicans is a common cause of nosocomial, hematogenously disseminated systemic infection, which has an attributable mortality of up to 50% even with antifungal therapy (Perlroth et al. 2007; Pfaller and Diekema 2007). The success of C. albicans as a pathogen is principally due to its success as a human commensal. As a commensal, C. albicans colonizes diverse surfaces, including the oral, intestinal, or vaginal mucosa in at least 80% of the adult human population (Pfaller and Diekema 2007; Southern et al. 2008). While C. albicans primarily causes non-life-threatening infections at these sites, life-threatening systemic infections can arise through escape of commensals from mucosal sites (Andrutis et al. 2000; Mavor et al. 2005). Thus, C. albicans must be able to thrive in diverse host environments to survive as a commensal and cause disease as a pathogen.
One environmental condition that varies markedly in sites colonized by C. albicans is pH. C. albicans can survive and thrive in the most acidic host sites, such as the stomach and vaginal cavity, and the most alkaline sites, such as the colon. C. albicans can grow over a wide pH range in vitro (pH 2–10), demonstrating the flexibility of C. albicans in the face of environmental pH. The ability to adapt to distinct environmental pH is critical for survival and pathogenesis for several reasons. First, environmental pH is a potent inducer of the C. albicans yeast-to-hyphae transition, which is crucial for pathogenesis (Davis et al. 2000a; Liu 2001, 2002; Gow et al. 2002; Davis 2003). Second, the expression profile of gene families relevant to pathogenesis, such as the secreted aspartyl protease family, is regulated by extracellular pH (Borg-von Zepelin et al. 1998; Bensen et al. 2004). Third, environmental pH affects the kinetics of extracellular enzymes, including virulence factors (Borg-von Zepelin et al. 1998). Fourth, environmental pH affects nutrient uptake, as many plasma membrane transporters use the proton gradient, which is not maintained at alkaline pH (King et al. 2004). Nutrient solubility is also affected in neutral-alkaline environments, making their uptake more difficult (Howard 1999; Bensen et al. 2004; Baek et al. 2008). Therefore, to survive and infect the host, C. albicans must respond appropriately to environmental pH.
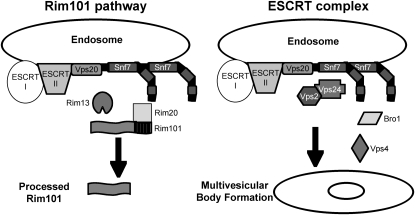
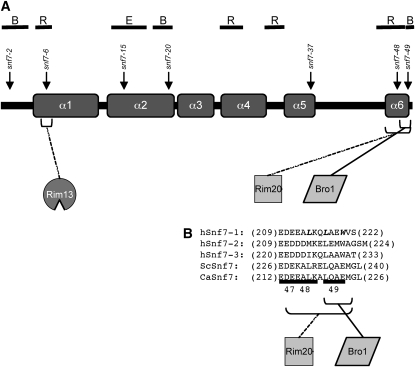
Several distinct pH-sensing systems that are required for adaptation of C. albicans to neutral-alkaline pH environments have been identified (Porta et al. 1999; Davis et al. 2000b, 2002; Davis 2003, 2009; Kullas et al. 2007; Sheth et al. 2008). One system, the Rim101 signal transduction pathway, regulates activity of the transcription factor Rim101. A similar pH-dependent Rim101/PacC pathway has been detected in a number of ascomycetes and basidiomycetes, including Saccharomyces cerevisiae, Aspergillus nidulans, and Ustillago maydis (Lambert et al. 1997; Penalva and Arst 2004; Arechiga-Carvajal and Ruiz-Herrera 2005). Rim101 is activated at neutral-alkaline pH by the proteolytic removal of an inhibitory C-terminal domain (Figure 1) (Davis 2003). Proteolytic activation requires upstream members, including Rim13, which acts as the putative protease (Li et al. 2004), and Rim20, which interacts with a PEST-like motif in the Rim101 C-terminal domain (Xu and Mitchell 2001; Vincent et al. 2003). Rim101 activation also requires Snf7, which interacts with Rim13 and Rim20 (Ito et al. 2001; Xu and Mitchell 2001; Bowers et al. 2004; Blanchin-Roland et al. 2008). Therefore, Snf7 is predicted to facilitate interaction between the protease Rim13 and its substrate Rim101 via Rim20. Rim101 activation is required for growth in neutral-alkaline environments and is required for C. albicans virulence in animal models of both systemic and mucosal disease (Porta et al. 1999; Ramon et al. 1999; Davis et al. 2000a,b; Mitchell et al. 2007; Villar et al. 2007). Thus, the sensing and adaptation to environmental pH through the Rim101 pathway is essential for C. albicans pathogenesis.
Figure 1.—
Model of Snf7 role in Rim101 processing and in ESCRT complex functions. On the left, ESCRT-I and -II recruitment of Vps20–Snf7 to the endosomal membrane leads to Snf7 interaction with the protease Rim13 and scaffold protein Rim20. Rim20 interacts with the C-terminal PEST-like domain of Rim101, and these interactions lead to Rim101 processing to its active form. On the right, ESCRT-I and -II recruitment of Vps20–Snf7 leads to downstream recruitment of Vps2/Vps24 and Bro1. Vps4 interacts with Snf7 to facilitate ESCRT-III dissociation from the membrane, and these interactions lead to multivesicular body formation.
Another response to alkaline pH in yeast is an increased reliance on endocytosis and vacuolar acidification for nutrient acquisition (Munn and Riezman 1994; Giaever et al. 2002). Because alkaline conditions do not generate a favorable proton gradient, plasma membrane transporters are shut down and cells rely on the internal vacuolar proton gradient. In fact, endocytosis and vacuolar acidification are essential processes for fungal growth in alkaline but not acidic environments (Munn and Riezman 1994). To deliver endosomes containing extracellular material to the vacuole, cells use the endocytic sorting complex required for transport (ESCRT) pathway. This pathway consists of the cytoplasmic protein complexes, ESCRT-0, -I, and -II, that are sequentially recruited to ubiquitylated cargo proteins at endocytic vesicle membranes (Figure 1) (Williams and Urbe 2007). This then recruits the ESCRT-III heterodimer Vps20–Snf7 (Katzmann et al. 2001; Babst et al. 2002a,b; Bilodeau et al. 2002; Katzmann et al. 2003), which initiates Snf7 oligomerization (Teis et al. 2008). Vps20–Snf7 then recruits the second half of ESCRT-III, the Vps2–Vps24 heterodimer (Babst et al. 2002a), which recruits downstream ESCRT members, including Bro1 and Vps4. Bro1 recruits a deubiquitinase that removes ubiquitin from the cargo protein, while Vps4 is an AAA-ATPase that dissociates ESCRT-III from the endosomal membrane, promoting multivesicular body (MVB) formation and fusion with the vacuole (Fujita et al. 2003; Yeo et al. 2003). Using the ESCRT pathway, cells are able to acquire and deliver nutrients to the vacuole, where the internal proton gradient facilitates delivery of cargo nutrients to the cytoplasm of the cell (Ohsumi and Anraku 1981; Ohsumi and Anraku 1983).
ESCRT-I and -II, as well as Vps20 and Snf7, are required for Rim101 processing (Xu et al. 2004). Although strains lacking ESCRT-I and -II do not recruit Snf7 to the endosomal membrane, Snf7 is expressed. This suggests that Snf7 must be localized to the endosomal membrane for its function in the Rim101 pathway, where it may serve as a scaffold for the Rim101 processing machinery at the membrane surface. This idea is supported by the colocalization of Rim101 pathway member Rim20 with Snf7 in a punctate pattern when cells are grown under alkaline conditions (Boysen and Mitchell 2006). Thus, Snf7 localization is important for function both in the ESCRT and in the Rim101 pathways.
In addition to colocalizing, Snf7 and Rim20 interact through the yeast two-hybrid and split-ubiquitin assays (Ito et al. 2001; Nikko and Andre 2007). Snf7–Rim20 interactions likely occur through the Rim20 bro1-domain. The bro1-domain was first identified in the ESCRT pathway member Bro1 as a Snf7-interaction domain. (Kim et al. 2005; McCullough et al. 2008). As both Bro1 and Rim20 act as scaffold proteins, Bro1 and Rim20 have been proposed to act as adaptors, promoting downstream Snf7 function toward the ESCRT pathway or the Rim101 pathway, respectively (Boysen and Mitchell 2006).
Because Snf7 is required for both ESCRT-mediated MVB formation and for Rim101 activation, we wanted to more precisely characterize the role of Snf7 in these two processes in C. albicans. We hypothesized that the function of Snf7 in the ESCRT and Rim101 pathways is distinct. To test this hypothesis, we generated a series of snf7 mutant alleles and identified specific alleles whose products disrupted the ESCRT pathway, the Rim101 pathway, or both pathways. Phenotypic analyses of our alleles have revealed that Snf7 function in the ESCRT pathway is separable from Snf7 function in the Rim101 pathway. Further analyses of these alleles have uncovered a slight variation in the bro1-domain interactions at the C-terminal end of Snf7.
MATERIALS AND METHODS
Strains and plasmids:
All C. albicans strains from this study are derived from BWP17 (Wilson et al. 1999) and are listed in Table 1. To generate the SNF7 complementation plasmid pDDB426, wild-type SNF7 sequence was amplified by the PCR from BWP17 genomic DNA using primers 5′ SNF7 comp and 3′ SNF7 comp (Table 2). The resulting PCR product and NotI/EcoRI-digested pDDB78 were transformed into S. cerevisiae to generate pDDB426 by in vivo recombination (Muhlrad et al. 1992). Plasmid pDDB426, and all additional plasmids generated by in vivo recombination in S. cerevisiae, were recovered from S. cerevisiae and transformed into DH5α Escherichia coli by electroporation for amplification.
TABLE 1.
Strains used in this study
| Name | Genotype | Reference |
|---|---|---|
| BWP17 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG his1∷hisG/his1∷hisG | Wilson et al. (1999) |
| DAY5 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG his1∷hisG/his1∷hisG rim101∷ARG4/rim101∷URA3-dpl200 | Wilson et al. (1999) |
| DAY185 | ura3∷λimm434/ura3∷λimm434 ARG4∷URA3∷arg4∷hisG/arg4∷hisG HIS1∷DDB78∷his1∷hisG/his1∷hisG | Davis et al. (2000a) |
| DAY534 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | Kullas et al. (2004) |
| DAY537 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG his1∷hisG/his1∷hisG vps4∷ARG4/vps4∷URA3-dpl200 | Kullas et al. (2004) |
| DAY763 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG HIS1∷DDB78∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | Kullas et al. (2004) |
| DAY980 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG SNF7-V5∷HIS1∷DDB427∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY981 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-1∷HIS1∷DDB428∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY982 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-2∷HIS1∷DDB429∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY983 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-3∷HIS1∷DDB430∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY984 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-4∷HIS1∷DDB431∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY985 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-5∷HIS1∷DDB432∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY986 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-6∷HIS1∷DDB433∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY987 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-8∷HIS1∷DDB434∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY988 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-9∷HIS1∷DDB435∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY989 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-10∷HIS1∷DDB436∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY990 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-11∷HIS1∷DDB437∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY991 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-12∷HIS1∷DDB438∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY992 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-13∷HIS1∷DDB439∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY993 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-14∷HIS1∷DDB440∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY994 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-15∷HIS1∷DDB441∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY995 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-16∷HIS1∷DDB442∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY996 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-17∷HIS1∷DDB443∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY997 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-18∷HIS1∷DDB444∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY998 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-19∷HIS1∷DDB445∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY999 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-20∷HIS1∷DDB446∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1000 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-21∷HIS1∷DDB447∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1001 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-22∷HIS1∷DDB448∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1002 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-23∷HIS1∷DDB449∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1003 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-24∷HIS1∷DDB450∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1004 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-25∷HIS1∷DDB451∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1005 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-26∷HIS1∷DDB452∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1006 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-27∷HIS1∷DDB453∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1007 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-28∷HIS1∷DDB454∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1008 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-29∷HIS1∷DDB455∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1009 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-30∷HIS1∷DDB456∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1010 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-31∷HIS1∷DDB457∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1011 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-32∷HIS1∷DDB458∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1012 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-33∷HIS1∷DDB459∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1013 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-34∷HIS1∷DDB460∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1014 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-35∷HIS1∷DDB461∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1015 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-36∷HIS1∷DDB462∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1016 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-37∷HIS1∷DDB463∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1017 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-38∷HIS1∷DDB464∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1018 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-39∷HIS1∷DDB465∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1019 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-40∷HIS1∷DDB466∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1020 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-41∷HIS1∷DDB467∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1021 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-42∷HIS1∷DDB468∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1022 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-43∷HIS1∷DDB469∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1023 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-44∷HIS1∷DDB470∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1024 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-45∷HIS1∷DDB471∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1025 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-46∷HIS1∷DDB472∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1026 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-47∷HIS1∷DDB473∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1027 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-48∷HIS1∷DDB474∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1028 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-49∷HIS1∷DDB475∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1029 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-50∷HIS1∷DDB476∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷URA3-dpl200 | This study |
| DAY1113 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG his1∷hisG/his1∷hisG vps4∷ARG4/vps4∷dpl200 | This study |
| DAY1114 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG SNF7-V5∷HIS1∷DDB427∷his1∷hisG/his1∷hisG vps4∷ARG4/vps4∷dpl200 | This study |
| DAY1115 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-6∷HIS1∷DDB433∷his1∷hisG/his1∷hisG vps4∷ARG4/vps4∷dpl200 | This study |
| DAY1116 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-15∷HIS1∷DDB441∷his1∷hisG/his1∷hisG vps4∷ARG4/vps4∷dpl200 | This study |
| DAY1117 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-20∷HIS1∷DDB446∷his1∷hisG/his1∷hisG vps4∷ARG4/vps4∷dpl200 | This study |
| DAY1126 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| DAY1127 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1128 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG SNF7∷HIS1∷DDB426∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1129 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-11∷HIS1∷DDB437∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1130 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-35∷HIS1∷DDB461∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1131 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-6∷HIS1∷DDB433∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1132 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-32∷HIS1∷DDB458∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1133 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-45∷HIS1∷DDB471∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1134 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-46∷HIS1∷DDB472∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1135 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-47∷HIS1∷DDB473∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1136 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-48∷HIS1∷DDB474∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1137 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-49∷HIS1∷DDB475∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1138 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-14∷HIS1∷DDB440∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1139 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-15∷HIS1∷DDB441∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1140 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-17∷HIS1∷DDB443∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1141 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-29∷HIS1∷DDB455∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1142 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-50∷HIS1∷DDB476∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1143 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-2∷HIS1∷DDB429∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1144 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-20∷HIS1∷DDB446∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1145 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG his1∷hisG/his1∷hisG rim101∷ARG4/rim101∷dpl200 rim101-281∷URA3∷pDDB479∷RIM101/RIM101 | This study |
| DAY1146 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 rim101-281∷URA3∷pDDB479∷RIM101/RIM101 | This study |
| DAY1147 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-48∷HIS1∷DDB474∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 rim101-281∷URA3∷pDDB479∷RIM101/RIM101 | This study |
| DAY1148 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-20.1∷HIS1∷DDB481∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1149 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-35.1∷HIS1∷DDB483∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1150 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-48.1∷HIS1∷DDB485∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1151 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG SNF7-V5∷HIS1∷DDB427∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1153 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG HIS1∷DDB78∷his1∷hisG/his1∷hisG rim20∷ARG4/rim20∷URA3 | This study |
| DAY1155 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG HIS1∷DDB78∷his1∷hisG/his1∷hisG vps4∷ARG4/vps4∷URA3-dpl200 | This study |
| DAY1156 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG HIS1∷DDB78∷his1∷hisG/his1∷hisG bro1∷ARG4/bro1∷URA3-dpl200 | This study |
| DAY1213 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-47.1∷HIS1∷DDB494∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 | ||
| DAY1214 | ura3∷λimm434/ura3∷λimm434 arg4∷hisG/arg4∷hisG snf7-49.1∷HIS1∷DDB495∷his1∷hisG/his1∷hisG snf7∷ARG4/snf7∷dpl200 | This study |
| RIM101-V5∷URA3∷DDB478∷RIM101/RIM101 |
TABLE 2.
Primers used in this study
| Primer name | Sequence 5′−3′ |
|---|---|
| 5′ Snf7-HpaI-V5 | TGTATCAAGAGAAGAAGAGTTACCACAATTCCCATCTGTTGGTAAGCCTATCCCTAACCC |
| 3′ Snf7-HpaI-V5 | CTTCATCTTCATCTTCTTCTACTACTGGAGCTTTCTTGTTATGGTGATGGTGATGATGAC |
| 5′ Snf7 comp | AAGCTCGGAATTAACCCTCACTAAAGGGAACAAAAGCTGGGCCTCATTGAGCAACTTGAG |
| 3′ Snf7 comp | ACGACGGCCAGTGAATTGTAATACGACTCACTATAGGGCGTAATCGACATTAAAGGACTC |
| 5′ Snf7.1 | TAGTAAACAGGCCTAGGATCGCGGGAGCTTTTTTTGGAGGAAATAGCCA |
| 3′ Snf7.1 | TGGCTATTTCCTCCAAAAAAAGCTCCCGCCATCCTAGGCCTGTTTACTA |
| 5′ Snf7.2 | GGCCTAGGATGTGGGGATATGCTGCTGGAGGAAATAGCCAACAAAA |
| 3′ Snf7.2 | TTTTGTTGGCTATTTCCTCCAGCAGCATATCCCCACATCCTAGGCC |
| 5′ Snf7.3 | ATTTTTTTGGAGGAAATAGCGCAGCAAAGAAAGATTTACCAAAGAA |
| 3′ Snf7.3 | TTCTTTGGTAAATCTTTCTTTGCTGCGCTATTTCCTCCAAAAAAAT |
| 5′ Snf7.4 | TTGGAGGAAATAGCCAACAAGCGCGGCAGCTTTACCAAAGAAGGCAATAGT |
| 3′ Snf7.4 | ACTATTGCCTTCTTTGGTAAAGCTGCCGCTTGTTGGCTATTTCCTCCAA |
| 5′ Snf7.5 | AACAAAAGAAAGATTTACCAGCGGCGGCAATAGTGGAATTGCGAGA |
| 3′ Snf7.5 | TCTCGCAATTCCACTATTGCCGCCGCTGGTAAATCTTTCTTTTGTT |
| 5′ Snf7.6 | TACCAAAGAAGGCAATAGTGGCATTGGCAGCACACATACAAACACTAAACAA |
| 3′ Snf7.6 | TTGTTTAGTGTTTGTATGTGTGCTGCCAATGCCACTATTGCCTTCTTTGGTA |
| 5′ Snf7.7 | CAATAGTGGAATTGCGAGAAGCCATAGCAACACTAAACAAGAAGAAGAA |
| 3′ Snf7.7 | TTCTTCTTCTTGTTTAGTGTTGCTATGGCTTCTCGCAATTCCACTATTG |
| 5′ Snf7.8 | AACACATACAAACACTAAACGCGGCGGCGAACCATTTGCAACAGCAAAT |
| 3′ Snf7.8 | ATTTGCTGTTGCAAATGGTTCGCCGCCGCGTTTAGTGTTTGTATGTGTT |
| 5′ Snf7.9 | ACAAGAAGAAGAACCATTTGGCAGCGGCAATGGATGACCAAGAT |
| 3′ Snf7.9 | AACTGATCTTGGTCATCCATTGCCGCTGCCAAATGGTTCTTCTTCTTGT |
| 5′ Snf7.10 | AGAACCATTTGCAACAGCAAGCGGCTGCCCAAGATCAGTTGGCCAGAAA |
| 3′ Snf7.10 | TTTCTGGCCAACTGATCTTGGGCAGCCGCTTGCTGTTGCAAATGGTTCT |
| 5′ Snf7.11 | TGCAACAGCAAATGGATGACGCAGCTGCGTTGGCCAGAAAATATGTTAG |
| 3′ Snf7.11 | CTAACATATTTTCTGGCCAACGCAGCTGCGTCATCCATTTGCTGTTGCA |
| 5′ Snf7.12 | ATGACCAAGATCAGTTGGCCGCAGCAGCTGTTAGTTCAAAACAAACAAC |
| 3′ Snf7.12 | GTTGTTTGTTTTGAACTAACAGCTGCTGCGGCCAACTGATCTTGGTCAT |
| 5′ Snf7.13 | CCAGAAAATATGTTAGTTCAGCAGCAACAACTTTAGCTAAAAGTGC |
| 3′ Snf7.13 | GCACTTTTAGCTAAAGTTGTTGCTGCTGAACTAACATATTTTCTGG |
| 5′ Snf7.14 | CAAAACAAACAACTTTAGCTGCAGCTGCTTTAAAAAGAAAAAAGGG |
| 3′ Snf7.14 | CCCTTTTTTCTTTTTAAAGCAGCTGCAGCTAAAGTTGTTTGTTTTG |
| 5′ Snf7.15 | CAACTTTAGCTAAAAGTGCTGCAGCAGCAAAAAAGGGGTATGAATCTAA |
| 3′ Snf7.15 | TTAGATTCATACCCCTTTTTTGCTGCTGCAGCACTTTTAGCTAAAGTTG |
| 5′ Snf7.16 | AGTGCTTTAAAAAGAGCAGCGGGGTATGCATCTAATCTATTAAAA |
| 3′ Snf7.16 | TTTTAATAGATTAGATGCATACCCCGCTGCTCTTTTTAAAGCACT |
| 5′ Snf7.17 | GAATCTAATCTATTAGCAGTGGCAAACGCGATTGAAACTTTGGAA |
| 3′ Snf7.17 | TTCCAAAGTTTCAATCGCGTTTGCCACTGCTAATAGATTAGATTC |
| 5′ Snf7.18 | GTGGAAAACCAGATTGCAACTGCGGCAACACAATTAATT |
| 3′ Snf7.18 | ACTAATTAATTGTGTTGCCGCAGTTGCAATCTGGTTTTCCAC |
| 5′ Snf7.19 | ACCAGATTGAAACTTTGGAAGCAGCAGCAATTAGTATCGAAGGAGCAAA |
| 3′ Snf7.19 | TTTGCTCCTTCGATACTAATTGCTGCTGCTTCCAAAGTTTCAATCTGGT |
| 5′ Snf7.20 | CTTTGGAAACACAATTAATTGCTGCCGCAGGAGCAAACTTGAACTTGGA |
| 3′ Snf7.20 | TCCAAGTTCAAGTTTGCTCCTGCGGCAGCAATTAATTGTGTTTCCAAAG |
| 5′ Snf7.21 | AAGGAGCAAACTTGAACTTGGCAACTGCGGCAGCTATGAAACAAGGAG |
| 3′ Snf7.21 | TTTGCTCCTTGTTTCATAGCTGCCGCAGTTGCCAAGTTCAAGTTTGCTCCTT |
| 5′ Snf7.22 | ACTTGGAAACTATGAAAGCTGCGGCAGCAGGAGCAAAGGCCATGAAACA |
| 3′ Snf7.22 | TGTTTCATGGCCTTTGCTCCTGCTGCCGCAGCTTTCATAGTTTCCAAGT |
| 5′ Snf7.23 | ATGAAACAAGGAGCAAAGGCCATGAAACAAATACATGGGGAATAC |
| 3′ Snf7.23 | GTATTCCCCATGTATTGCTGCCATGGCCGCTGCTCCTTGTTTCAT |
| 5′ Snf7.24 | CAAAGGCCATGAAACAAATAGCTGCGGCATACGATGTAGACAAAGTTGA |
| 3′ Snf7.24 | TCAACTTTGTCTACATCGTATGCCGCAGCTATTTGTTTCATGGCCTTTG |
| 5′ Snf7.25 | CAAATACATGGGGAATACGCTGTAGCCGCAGTTGAAGATACTATGGATG |
| 3′ Snf7.25 | CATCCATAGTATCTTCAACTGCGGCTACAGCGTATTCCCCATGTATTTG |
| 5′ Snf7.26 | AATACGATGTAGACAAAGTTGCAGCTACTGCGGATGAAATAAGAGAACAA |
| 3′ Snf7.26 | TTGTTCTCTTATTTCATCCGCAGTAGCTGCAACTTTGTCTACATCGTATT |
| 5′ Snf7.27 | CAAAGTTGAAGATACTATGGCTGCAATAGCAGAACAAGTAGAGTTAGCC |
| 3′ Snf7.27 | GGCTAACTCTACTTGTTCTGCTATTGCAGCCATAGTATCTTCAACTTTG |
| 5′ Snf7.28 | TACTATGGATGAAATAAGAGCAGCAGTAGCGTTAGCCGATGAAATCAGT |
| 3′ Snf7.28 | ACTGATTTCATCGGCTAACGCTACTGCTGCTCTTATTTCATCCATAGTA |
| 5′ Snf7.29 | CAAGTAGAGTTAGCCGCTGCAATCAGTGCAGCTATATCGAGGCCC |
| 3′ Snf7.29 | GGGCCTCGATATAGCTGCACTGATTGCAGCGGCTAACTCTACTTG |
| 5′ Snf7.30 | ATGAAATCAGTGAAGCTATAGCGGCGCCCGTTGGTAATGAATTTGT |
| 3′ Snf7.30 | ACAAATTCATTACCAACGGGCGCCGCTATAGCTTCACTGATTTCAT |
| 5′ Snf7.31 | CTATATCGAGGCCCGTTGGTAATGAATTTGTTGATGAAGATGAATT |
| 3′ Snf7.31 | AATTCATCTTCATCAACAAATGCAGCACCAACGGGCCTCGATATAG |
| 5′ Snf7.32 | CCGTTGGTAATGAATTTGTTGCTGCAGATGAATTGGACGAAGAATT |
| 3′ Snf7.32 | AATTCTTCGTCCAATTCATCTGCAGCAACAAATTCATTACCAACGG |
| 5′ Snf7.33 | GTAATGAATTTGTTGATGAAGCTGCATTGGACGAAGAATTGAAAGA |
| 3′ Snf7.33 | TCTTTCAATTCTTCGTCCAATGCAGCTTCATCAACAAATTCATTAC |
| 5′ Snf7.34 | TTGTTGATGAAGATGAATTGGCCGCAGCATTGAAAGAGTTGGAGGCAGA |
| 3′ Snf7.34 | TCTGCCTCCAACTCTTTCAATGCTGCGGCCAATTCATCTTCATCAACAA |
| 5′ Snf7.35 | AAGATGAATTGGACGAAGAAGCGGCAGCGTTGGAGGCAGAAGCTAAAGA |
| 3′ Snf7.35 | TCTTTAGCTTCTGCCTCCAACGCTGCCGCTTCTTCGTCCAATTCATCTT |
| 5′ Snf7.36 | TGGACGAAGAATTGAAAGAGGCGGCGGCAGAAGCTAAAGAACA |
| 3′ Snf7.36 | TCTTGTTCTTTAGCTTCTGCCGCCGCCTCTTTCAATTCTTCGTCCA |
| 5′ Snf7.37 | TTGAAAGAGTTGGAGGCAGCAGCTGCAGCACAAGAACAAGAACATAGA |
| 3′ Snf7.37 | TCTATGTTCTTGTTCTTGTGCTGCAGCTGCTGCCTCCAACTCTTTCAA |
| 5′ Snf7.38 | TGGAGGCAGAAGCTAAAGAAGCAGCAGCAGAACATAGAGTGCCAGCTCA |
| 3′ Snf7.38 | TGAGCTGGCACTCTATGTTCTGCTGCTGCTTCTTTAGCTTCTGCCTCCA |
| 5′ Snf7.39 | AAGCTAAAGAACAAGAACAAGCAGCTGCAGTGCCAGCTCAAAAGGCAAA |
| 3′ Snf7.39 | TTTGCCTTTTGAGCTGGCACTGCAGCTGCTTGTTCTTGTTCTTTAGCTT |
| 5′ Snf7.40 | GAACATAGAGTGCCAGCTGCAGCGGCAGCACCACAACCTGTATCAAGA |
| 3′ Snf7.40 | TCTTGATACAGGTTGTGGTGCTGCCGCTGCAGCTGGCACTCTATGTTC |
| 5′ Snf7.41 | TGCCAGCTCAAAAGGCAAAAGCAGCACCTGTATCAAGAGAAGAAGA |
| 3′ Snf7.41 | TCTTCTTCTCTTGATACAGGTGCTGCTTTTGCCTTTTGAGCTGGCA |
| 5′ Snf7.42 | AGGCAAAACCACAACCTGTAGCAGCAGAAGAAGAGTTACCACAATT |
| 3′ Snf7.42 | AATTGTGGTAACTCTTCTTCTGCTGCTACAGGTTGTGGTTTTGCCT |
| 5′ Snf7.43 | AACCACAACCTGTATCAAGAGCAGCAGCGTTACCACAATTCCCATCTGT |
| 3′ Snf7.43 | ACAGATGGGAATTGTGGTAACGCTGCTGCTCTTGATACAGGTTGTGGTT |
| 5′ Snf7.44 | CAAGAGAAGAAGAGTTACCAGCATTCCCATCTGTTGGTAAGCC |
| 3′ Snf7.44 | GGCTTACCAACAGATGGGAATGCTGGTAACTCTTCTTCTCTTG |
| 5′ Snf7.45 | ATCATCACCATCACCATAACGCGGCAGCTCCAGTAGTAGAAGAAGA |
| 3′ Snf7.45 | TCTTCTTCTACTACTGGAGCTGCCGCGTTATGGTGATGGTGATGAT |
| 5′ Snf7.46 | AAGAAAGCTCCAGTAGTAGCAGCAGCTGAAGATGAAGAAGCATTG |
| 3′ Snf7.46 | TTTCAATGCTTCTTCATCTTCAGCTGCTGCTACTACTGGAGCTTTCTT |
| 5′ Snf7.47 | CCAGTAGTAGAAGAAGATGCAGCTGCAGAAGCATTGAAAGCATTGCAAG |
| 3′ Snf7.47 | CTTGCAATGCTTTCAATGCTTCTGCAGCTGCATCTTCTTCTACTACTGG |
| 5′ Snf7.48 | GAAGAAGATGAAGATGAAGCAGCAGCGGCAGCATTGCAAGCTGAAATG |
| 3′ Snf7.48 | CATTTCAGCTTGCAATGCTGCCGCTGCTGCTTCATCTTCATCTTCTTC |
| 5′ Snf7.49 | GAAGAAGCATTGAAAGCAGCGGCAGCTGCAATGGGATTATGATGTGTT |
| 3′ Snf7.49 | AACACATCATAATCCCATTGCAGCTGCCGCTGCTTTCAATGCTTCTTC |
| 5′ Snf7.50 | TAATTAGTATCGAAGGAGCAAACTTGAACTTGGAAACTATGAAAGCTAT |
| 3′ Snf7.50 | ATAGCTTTCATAGTTTCCAAGGCCGCGGCTGCTCCTTCGATACTAATTA |
To generate an epitope-tagged SNF7 allele, the V5-His6 tag was amplified in the PCR from the pTRACER-EF plasmid (Invitrogen) using primers 5′ Snf7-V5 and 3′ Snf7-V5. The resulting PCR product and HpaI-digested pDDB426 were transformed into S. cerevisiae to generate pDDB427. Purified pDDB427 was digested with NruI and transformed into DAY534 to generate DAY980.
To generate the snf7 alanine-scanning alleles, two overlapping PCR products were generated using plasmid pDDB427 as template. For example, snf7-1 was amplified from pDDB427 in two PCR reactions, the first using primers 5′ SNF7 comp and 3′ snf7-1, and the second using primers 5′ snf7-1 and 3′ SNF7 comp (Table 2). The two PCR products and NotI-/EcoRI-digested pDDB78 were transformed into S. cerevisiae to generate plasmid pDDB428 by in vivo recombination. This approach was used to generate plasmids pDDB428–pDDB476. Purified plasmids were digested with NruI and transformed into C. albicans strain DAY534 to generate DAY981–DAY1029. The snf7 alleles lacking the V5 epitope (snf7-20.1, snf7-35.1, snf7-48.1, snf7-47.1, and snf7-49.1) were generated using the same approach except pDDB426 as the template sequence to produce pDDB481, 483, 485, 494, and 495, respectively. All mutant snf7 alleles were sequenced to ensure that only specifically engineered mutations were present.
Specific snf7 alleles were transformed into the vps4Δ/Δ background as follows. First, the vps4Δ/Δ strain DAY537 (Kullas et al. 2004) was plated on 5-FOA-containing YPD plates to select for loss of the URA3 marker through homologous recombination to generate DAY1113. DAY1113 was transformed with pDDB427, 433, 441, and 446 to generate DAY1114–DAY1117.
To generate a Ura marker plasmid, first C. albicans URA3 was amplified from pGEM–URA3 using primers pRS/pGEMT-5 and pRS/pGEMT-3 (Spreghini et al. 2003). The resulting PCR product and NgoMI-linearized pRS314 (Sikorski and Hieter 1989) were transformed into S. cerevisiae to generate pDDB76 by in vivo recombination. Next, pDDB200 (Li et al. 2004) was digested with PvuII and the RIM101-containing fragment was purified. The purified product and NotI-digested pDDB76 were transformed into S. cerevisiae to generate pDDB477 by in vivo recombination (Muhlrad et al. 1992). Finally, the V5 epitope sequence was amplified from pTRACER-EF in a PCR using primers AgeI 5′ V5 and AGEI 3′ V5 (Li et al. 2004) and the resulting PCR product was transformed with AgeI-digested pDDB477 into S. cerevisiae to generate pDDB478.
Specific snf7 mutant alleles were expressed with RIM101-V5 as follows. First, the snf7Δ/Δ strain DAY534 (Kullas et al. 2004) was plated on 5-FOA-containing YPD plates to select for loss of the URA3 marker through homologous recombination to generate DAY1126. DAY1126 was then transformed with BstEII-digested pDDB478 to generate DAY1127. DAY1127 was then transformed with pDDB427, 426, 437, 461, 433, 458, 471, 472, 473, 474, 475, 440, 441, 443, 455, 476, 429, 446, 481, 483, 485, 494, and 495 to generate DAY1151, DAY1128–DAY1144, DAY1148–DAY1150, DAY1213, and DAY1214, respectively.
To generate prototrophic vps4Δ/Δ, bro1Δ/Δ, and rim20Δ/Δ mutant strains, DAY23 (Davis et al. 2000b), DAY537, and DAY653 (Kullas et al. 2004) were transformed with NruI-cut DDB78 and selected on SC-his medium to generate DAY1153, DAY1155, and DAY1156.
Growth and filamentation assays:
Strains were regularly propagated in YPD medium (2% Bacto Peptone, 1% yeast extract, 2% dextrose). To test for growth phenotypes, YPD was buffered with 150 mm HEPES to pH 9 with NaOH, or contained 150 mm lithium chloride. M199 medium (Gibco BRL) was buffered with 150 mm HEPES and pH adjusted as described in the text. Transformants were selected on synthetic medium (SC, 0.67% yeast nitrogen base with ammonium sulfate and without amino acids, 2% dextrose) supplemented as required for the auxotrophic requirements for the cells (Adams et al. 1997). To select for Ura- transformants, strains were streaked on synthetic complete medium containing 0.1% 5-FOA (MP Biomedicals). All media, except that selecting for Ura+ transformants, was supplemented with 80 μg of uridine/ml. Solid medium contained 2% Bacto-agar.
FM 4-64 staining:
Twenty-five microliters of a YPD overnight culture was inoculated into 1 ml M199 pH 8 medium and incubated at 30° for 4.5 hr. Two microliters of 16 mm FM 4-64 (Invitrogen) in DMSO was added to each tube and the cells were incubated on ice for 15 min. Cells were then washed and resuspended in 1 ml fresh M199 pH 8 medium and incubated at 30° for 90 min. Eighty microliters of cells was transferred to a tube containing 10 μl each of 100 mm NaN3 and 100 mm NaF. Cells were stored on ice and examined by fluorescent microscopy.
Protein preparation:
Fifty microliters of YPD medium was inoculated from an overnight culture to an OD600 0.05–0.07 and grown to an OD600 0.5–0.7. Cells were washed with 1 mm phenylmethylsulphonyl fluoride (PMSF) and then resuspended in radioimmunoprecipitation assay buffer (50 mm Tris pH 8, 150 mm NaCl, 1% NP-40, 3 mm EDTA, 0.5% deoxycholate, 0.1% SDS) with protease inhibitors (1 mm PMSF, 10 mm dithiothreitol [DTT], 1 μg/ml each of leupeptin, aprotinin, and pepstatin). Cells were lysed by glass bead disruption by vortexing four times for 2 min each. Cell debris was removed by 15 min centrifugation at 13000 × g. Supernatants were collected and protein concentration was determined by Bradford assay (Bio-Rad).
Cell fractionation:
Overnight cultures (0.6 ml) were diluted into 100 ml M199 medium at pH 4 or pH 8 and grown roughly 6 hr at 30°. Five OD600 cells were washed in cold 10 mm NaF/10 mm NaN3 and pelleted. Cells were resuspended in 10 mm Tris pH 7.5/100 mm EDTA/0.5% β-mercaptoethanol/10 mm NaN3/10 mm NaF and shaken 20 min at 37°. Cells were collected and resuspended in isotonic S buffer (40 mm Tris pH 7.5/1.2 m sorbitol/0.5 mm MgCl2/10 mm NaN3/10 mm NaF/800 μg/ml yeast lytic enzyme [MPBio]). Spheroplasts were gently pelleted and resuspended in Lysis Buffer (50 mm Tris pH 7.5/0.2 m sorbitol/2 mm EDTA) containing 10 mm DTT and protease inhibitors (1 mm PMSF, 1 μg/ml each of leupeptin, aprotinin, and pepstatin), and homogenized with 30 dounces in a glass homogenizer. Cell debris was removed by centrifugation for 10 min at 500 × g to generate the cleared homogenate. One hundred microliters of the supernatant was removed and saved at −80° as total sample. The remainder of the supernatant was centrifuged 15 min at 13,000 × g. The supernatant was removed and stored at −80° and the pellets were washed twice with 1 ml lysis buffer, centrifuged 15 min at 13,000 × g, and resuspended in 0.5 ml lysis buffer. A 50-μl sample from each fraction (supernatant and pellet, as well as cleared homogenate) was used for Western blot analysis.
Western blot analysis:
Fifty microliters of crude protein or cell fractionation sample was separated by SDS–polyacrylamide gel electrophoresis (SDS–PAGE). Resolving gels were made to 12% or 6% polyacrylamide for Rim101 or Snf7 visualization, respectively. Gels were transferred to a nitrocellulose membrane and blots were blocked 1 hr at room temperature with 5% milk in Tris-buffered saline containing 0.05% Tween-20 (TBS-T). Blots were probed in blocking solution containing a 1:5000 monoclonal anti-V5-HRP (Invitrogen) antibody. Blots were washed with TBS-T, incubated with ECL reagent (Amersham), and exposed to film.
Immunofluorescence:
Cells were grown to mid-log phase and fixed with 4% formaldehyde for 15 min. Spheroplasts were generated using 5 μg/ml YLE (MPBio) in Solution B (100 mm potassium phoshate pH 7.5, 1.2 m sorbitol) for 15 min. Cells were pelleted at 0.5 × g for 5 min, washed twice in 1 ml Solution B, and spotted onto polylysine-coated slides. Samples were blocked 10 min with 5% BSA and incubated 1 hr with 1:100 anti-V5 antibody. This was followed by a 1-hr incubation with 1:200 anti-mouse IgG conjugated to alexafluor 488 (Invitrogen). Cells were visualized on a Zeiss Imager.M1 microscope and images were captured using Axiovision Release 4.6.3 software.
Filamentation assays:
For filamentation assays on solid medium, 3 μl of overnight YPD cultures was spotted onto M199 pH 8 agar plates. Plates were incubated 5–7 days at 37° and photographed. For liquid filamentation assays, overnight YPD cultures were inoculated 1:100 into M199 pH 8 medium and grown for 4 hr at 37°. A total of 500 μl of cells were fixed with 1 ml ethanol for 30 min at 23°. Cell morphology was analyzed microscopically and at least 300 cells per sample were counted.
FaDu cell damage assay:
FaDu cells (ATCC) were grown in 24-well tissue culture dishes and incubated at 37° 5% CO2 in modified eagle medium (MEM) with 10% final concentration FBS and 5 ml antibiotic/antimycotic cocktail (Invitrogen). At 90% monolayer confluence, FaDu cells were incubated in 0.5 ml medium containing 0.5 μCi 51Cr for 16 hr. After washing FaDu with PBS, 1 × 105 C. albicans cells were added in MEM with 10% FBS and 5 ml antibiotic cocktail and incubated 10 hr. Some FaDu were untreated to measure spontaneous 51Cr release. After incubation, 0.5 ml supernatant was moved to a 13-ml glass (supernatant) tube. One-half milliliter of 6 m NaOH was added to FaDu and moved to a separate (debris) tube. Final monolayer was removed to the debris tube in 0.5 ml Liftaway (RPI Corp). Specific release was calculated as [(2 × supernatant) − (2 × spontaneous release)]/[(2 × total)−(2 × spontaneous release)].
RESULTS
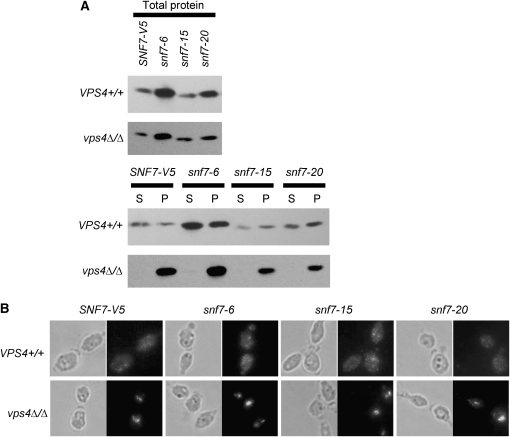
To determine how Snf7 functions in the Rim101 and vacuolar transport pathways, we first generated a V5 epitope-tagged SNF7 allele. Since C-terminal Snf7-V5 fusions were not functional (data not shown), we used the internal HpaI site to generate an in-frame fusion. This SNF7-V5 allele fully complemented the endocytosis and growth defects observed in the snf7Δ/Δ strain (Figures 2 and 3, Table 4), demonstrating that Snf7-V5 is functional.
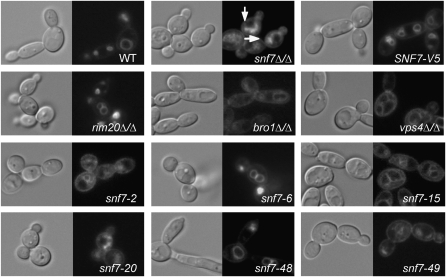
Figure 2.—
FM 4-64 localization in snf7 mutants, including WT (DAY185), snf7Δ/Δ (DAY763), snf7Δ/Δ + SNF7-V5 (DAY980), rim20Δ/Δ (DAY1153), bro1Δ/Δ (DAY1156), vps4Δ/Δ (DAY1155), snf7Δ/Δ + snf7-2 (DAY982), snf7Δ/Δ + snf7-6 (DAY986), snf7Δ/Δ + snf7-15 (DAY994), snf7Δ/Δ + snf7-20 (DAY 999), snf7Δ/Δ + snf7-48 (DAY1027), and snf7Δ/Δ + snf7-49 (DAY1028). Strains were grown at 30° to mid-log phase in M199 (pH 8) medium and exposed to FM 4-64 for 15 min. Cells were washed and incubated in fresh M199 pH 8 medium for 90 min at 30°. Cells were placed on ice and NaF and NaN3 were added prior to photographing. Arrows indicate Class E-like accumulations in snf7Δ/Δ strain.
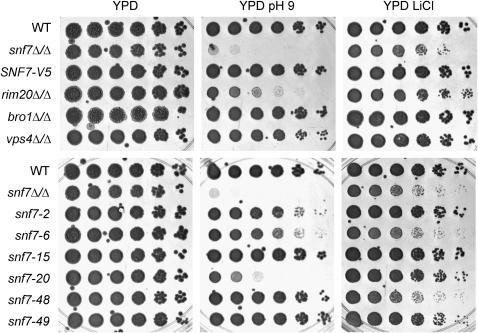
Figure 3.—
Growth phenotypes of snf7 mutants. Strains pictured include the wild type (WT) (DAY185), snf7Δ/Δ (DAY763), snf7Δ/Δ + SNF7-V5 (DAY980), rim20Δ/Δ (DAY1153), bro1Δ/Δ (DAY1156), vps4Δ/Δ (DAY1155), snf7Δ/Δ + snf7-2 (DAY982), snf7Δ/Δ + snf7-6 (DAY986), snf7Δ/Δ + snf7-15 (DAY994), snf7Δ/Δ + snf7-20 (DAY999), snf7Δ/Δ + snf7-48 (DAY1027), and snf7Δ/Δ + snf7-49 (DAY1028). Strains were grown on YPD, YPD pH 9, and YPD + LiCl for 2 days at 37° prior to photographing.
TABLE 4.
Filamentation Assay
| Strain | Genotype | Alkaline agar filamentation | Acidic agar filamentation | % germ tubesa |
|---|---|---|---|---|
| DAY185 | WT | + | − | 96 ± 3 |
| DAY763 | snf7Δ/Δ | − | + | 0 ± 0 |
| DAY980 | SNF7-V5 | + | − | 96 ± 1 |
| DAY25 | rim101Δ/Δ | − | − | 0 ± 0 |
| DAY537 | vps4Δ/Δ | ± | + | 82 ± 2 |
| DAY982 | snf7-2 | + | − | 2 ± 2 |
| DAY986 | snf7-6 | − | − | 1 ± 1 |
| DAY994 | snf7-15 | + | − | 78 ± 4 |
| DAY999 | snf7-20 | − | − | 13 ± 7 |
| DAY1028 | snf7-48 | + | − | 86 ± 6 |
Percentage germ tube formation ± standard deviation from two independent experiments.
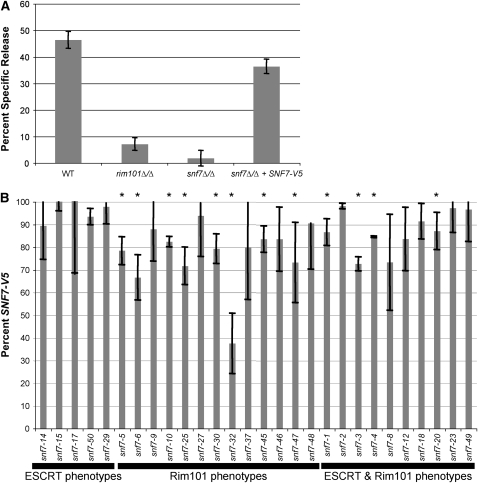
We predicted that distinct domains of Snf7 contribute to vacuolar transport and Rim101 activation. To address this hypothesis, we used alanine-scanning mutagenesis to generate a series of mutant snf7-V5 alleles. Alanine residues were substituted at up to three charged residues within a span of five amino acids. Using this approach, 49 alleles were generated in the SNF7-V5 background that span the length of the 226 residues of Snf7 protein (Table 3). To determine if these alleles affect Snf7 function in the vacuolar transport pathway, Rim101 pathway, or both pathways, the alleles were transformed into a snf7Δ/Δ mutant and tested for complementation of vacuolar transport and Rim101 processing defects (referred to as ESCRT-dependent and Rim101-dependent phenotypes below, although we recognize that Rim101-dependent phenotypes also depend on upstream ESCRT function).
TABLE 3.
Snf7 mutant sequences
| Allele | WT sequencea | Allele | WT sequence |
|---|---|---|---|
| snf7-1 | 1-MWGYF | snf7-26 | 125-VEDTM |
| snf7-2 | 3-GYFFG | snf7-27 | 129-MDEIR |
| snf7-3 | 10-SQQKK | snf7-28 | 133-REQVE |
| snf7-4 | 12-QKKDL | snf7-29 | 140-DEISE |
| snf7-5 | 17-PKKAI | snf7-30 | 146-ISRPV |
| snf7-6 | 22-VELRE | snf7-31 | 151-GNEFV |
| snf7-8 | 32-NKKKN | snf7-32 | 155-VDEDE |
| snf7-9 | 38-LQQQM | snf7-33 | 157-EDELD |
| snf7-10 | 41-QMDDQ | snf7-34 | 160-LDEEL |
| snf7-11 | 44-DQDQL | snf7-35 | 163-ELKEL |
| snf7-12 | 49-ARKYV | snf7-36 | 166-ELEAE |
| snf7-13 | 55-SKQTT | snf7-37 | 169-AEAKE |
| snf7-14 | 61-AKSAL | snf7-38 | 173-EQEQE |
| snf7-15 | 64-ALKRK | snf7-39 | 176-QEHRV |
| snf7-16 | 68-KKGYE | snf7-40 | 182-AQKAK |
| snf7-17 | 77-KVENQ | snf7-41 | 186-KPQPV |
| snf7-18 | 82-IETLE | snf7-42 | 190-VSREE |
| snf7-19 | 86-ETQLI | snf7-43 | 192-REEEL |
| snf7-20 | 90-ISIEG | snf7-44 | 196-LPQFP |
| snf7-50 | 95-ANLNL | snf7-45 | 202-VNKKA |
| snf7-21 | 100-ETMKA | snf7-46 | 209-VEEDE |
| snf7-22 | 105-AMKQG | snf7-47 | 212-DEDEE |
| snf7-23 | 111-KAMKQ | snf7-48 | 215-EEALK |
| snf7-24 | 116-IHGEY | snf7-49 | 220-ALQAE |
| snf7-25 | 120-YDVDK |
The number represents the N-terminal Snf7 residue. Underlined residues are changes to alanine in the mutant.
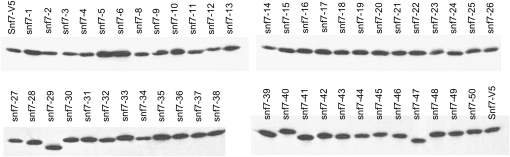
To determine if a given mutation affected Snf7 protein stability, we performed Western blot analysis on crude protein extracts from our mutant strains. All strains containing an alanine-scanning allele produced abundant Snf7 protein (Figure 4). However, we noted that specific alleles revealed differences in mobility, such as snf7-29, snf7-40, and snf7-47. These differences are likely due to alterations of SDS binding; however, we cannot rule out the possibility that the altered mobilities are due to disruption of an as-yet-unknown Snf7 post-translational modification. Regardless, these results indicate that the alanine-scanning alleles are expressed and that phenotypes observed in the mutant alleles are not due to the instability of the Snf7 protein.
Figure 4.—
Mutant snf7 alleles produce detectable protein. A total of 250 μg protein collected from mid-log cultures was loaded for each sample and run on 10% SDS–PAGE. Blots were probed with anti-V5-HRP antibody. Protein loading was normalized to anti-tubulin signal (data not shown).
When grown on rich YPD medium, the wild-type strain and the snf7Δ/Δ + SNF7-V5 strain grew comparably (Figure 3). However, the snf7Δ/Δ mutant grew more slowly, as reported previously (Kullas et al. 2004). All of the snf7Δ/Δ + snf7 alanine-scanning allele strains rescued this growth defect on rich medium. This result, and the fact that Snf7 protein is expressed at similar levels in all strains, suggests that none of the snf7 alleles is a true null.
snf7 alleles affecting vacuolar transport:
We first analyzed the snf7 alleles for ESCRT-dependent phenotypes. Endocytosis is required to move plasma membrane components to the vacuole and can be monitored using the fluorescent lipophilic dye, FM 4-64 (Vida and Emr 1995). In wild-type and snf7Δ/Δ +SNF7-V5 cells, FM 4-64 associated with the plasma membrane was taken up by endocytosis and was ultimately localized to the vacuolar membrane, resulting in the ring-like pattern around the vacuole (Figure 2). In snf7Δ/Δ cells, FM 4-64 associated with the plasma membrane and was taken up by endocytosis, but was unable to localize to the vacuole, resulting in a more diffuse staining pattern and the apparent formation of class E-like exclusion bodies around the perimeter of the vacuole (Figure 2). These exclusion bodies likely consist of endosomes unable to fully mature to MVBs and thus unable to efficiently fuse with the vacuole (Kranz et al. 2001). We next tested a rim20Δ/Δ mutant, which does not process Rim101 but has no detected role in MVB formation (Davis et al. 2000a; Kullas et al. 2004). This strain localized FM 4-64 to the vacuole like wild-type, as previously reported. We also tested a bro1Δ/Δ strain, which affects MVB formation but has no detected role in Rim101 processing (Kullas et al. 2004 and data not shown). The bro1Δ/Δ mutant showed stronger vacuolar staining than the snf7Δ/Δ mutant but similarly displayed class E-like bodies around the periphery of the vacuole and cytoplasmic punctate spots not observed in wild-type cells (Figure 2). This suggests that bro1Δ/Δ mutants have a defect in vacuolar trafficking, but that this defect is not as severe as the snf7Δ/Δ mutant, as previously described in C. albicans and homologous S. cerevisiae mutants (Odorizzi et al. 2003; Kullas et al. 2004). Finally, we tested a vps4Δ/Δ strain, which also had a defect in vacuolar trafficking that was less pronounced than the snf7Δ/Δ mutant defect, as reported previously (Kullas et al. 2004). Thus Snf7 and downstream ESCRT proteins, but not Rim101 pathway members, are required for FM 4-64 localization to the vacuole.
The snf7Δ/Δ + snf7 alanine-scanning alleles were assayed for FM 4-64 localization and characterized as nonfunctional, partially functional, or fully functional. Nonfunctional alleles were defined as those that have a staining pattern that mimics the snf7Δ/Δ strain, such as snf7-2 and snf7-20 (Figure 2). Partially functional alleles were those that showed some vacuolar staining but variously retained FM 4-64 in the cytoplasm or had slightly aberrant vacuolar staining patterns, such as snf7-15 and snf7-49. We noted similar patterns between some partially functional alleles, such as snf7-15, and the vps4Δ/Δ strain, which had a less severe FM 4-64 trafficking defect than the snf7Δ/Δ mutant (Figure 2). Fully functional alleles were defined as those that localized FM 4-64 to the vacuole similar to the snf7Δ/Δ +SNF7-V5 strain, such as snf7-6 and snf7-48. Thus, we were able to test the ESCRT-dependent function of our snf7 alleles and identified several alleles from each of three categories among our snf7 alleles (Figure 5).
Figure 5.—
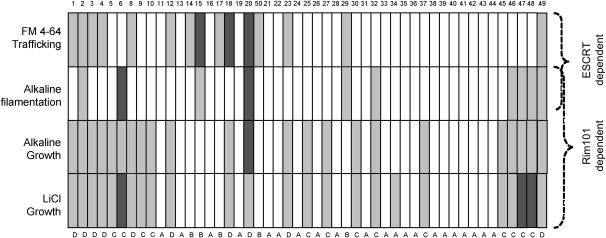
Comparison of mutant snf7 alleles facilitates categorization into functional groups. The cartoon represents the N- to C-terminal sequence of Snf7, with the alleles placed in order along the protein sequence. Assays are listed to the left of each row while snf7 mutant allele number is listed above each column. Rim101- and ESCRT-dependent assays are labeled to the right side. Open blocks indicate that the mutant snf7 allele behaves like wild-type SNF7, dark shaded blocks indicate that the mutant allele behaves like snf7Δ/Δ, and light shaded blocks indicate an intermediate phenotype. For FM 4-64 trafficking, the output is specific vacuolar staining. For alkaline filamentation, the output is percentage germ tube formation. For alkaline and LiCl growth assays, the output is colony size.
snf7 alleles affecting the Rim101 pathway:
We next analyzed the alanine-scanning snf7 alleles for Rim101-dependent phenotypes with fivefold serial dilution growth assays. Rim101 activation is required for growth on YPD buffered to pH 9 and on YPD containing lithium chloride (LiCl) (Figure 3). These growth defects are also observed on streaked agar plates (supporting information, Figure S1). On YPD pH 9 medium, the wild-type and snf7Δ/Δ + SNF7-V5 strains grew similarly, but the snf7Δ/Δ strain did not produce isolated colonies (Figure 3). The rim20Δ/Δ mutant also displayed a growth defect, although not as severe as the snf7Δ/Δ strain. Both the bro1Δ/Δ and vps4Δ/Δ mutant strains were able to form wild-type-sized colonies on this alkaline medium (Figure 3). Thus, Snf7 and downstream Rim101 pathway members are required for alkaline growth, and downstream ESCRT pathway members are not.
The snf7Δ/Δ + snf7 alanine-scanning alleles showed a range of growth phenotypes on alkaline medium that were categorized as nonfunctional, partially functional, or fully functional. Nonfunctional alleles were defined as those that, like the snf7Δ/Δ mutant, were unable to form individual colonies after 2 days growth, such as snf7-20 (Figure 3). Partially functional alleles were defined as those that could form isolated colonies smaller than the colonies produced by the snf7Δ/Δ + SNF7-V5 strain, such as snf7-2 and snf7-6, and, to a lesser degree, snf7-48 and snf7-49 (Figure 3 and Supplemental Figure 1). Fully functional alleles were defined as those that could form isolated colonies similar in size to those produced by the snf7Δ/Δ + SNF7-V5 strain, such as snf7-15. We identified several alleles from all three categories among our snf7 alleles using this Rim101-dependent growth assay (Figure 5).
We next assayed growth on LiCl medium. On LiCl medium, the wild-type and snf7Δ/Δ + SNF7-V5 strains grew similarly and the snf7Δ/Δ mutant had a severe defect, forming only pinprick-sized colonies (Figure 3). The rim20Δ/Δ mutant strain showed a slightly less severe growth defect, forming only very small colonies, while the bro1Δ/Δ and vps4Δ/Δ mutant strains showed no growth defects on LiCl medium (Figure 3). Thus, Snf7 and downstream Rim101 pathway members, but not downstream ESCRT members, are required for LiCl growth.
The snf7Δ/Δ + snf7 alanine-scanning alleles again showed a range of growth phenotypes on LiCl medium that were categorized as nonfunctional, partially functional, or fully functional. Nonfunctional alleles were defined as those whose colonies grew similarly to the snf7Δ/Δ mutant, such as snf7-6 and snf7-48. Partially functional alleles were defined as those that promoted colony growth of a size intermediate between the snf7Δ/Δ +SNF7-V5 and the snf7Δ/Δ strains, such as snf7-2, snf7-20 and snf7-49 (Figure 3). Fully functional alleles were defined as those that grew similarly to the snf7Δ/Δ +SNF7-V5 strain, such as snf7-15. Again, we identified several alleles from all three categories among our snf7 alleles using this assay (Figure 5).
While most snf7 alanine-scanning alleles conferred similar growth defects on both pH 9 medium and LiCl medium (Figure 5), we did note that certain alleles showed variation between these two assays. For example, snf7-48 was scored as partially functional on pH 9 medium but as nonfunctional on LiCl medium (Figures 3 and 5). However, no allele was fully functional in one growth assay and nonfunctional in the other. Thus, we infer that the few observed differences between these two growth assays reflect sensitivities between the growth assays. Alleles that conferred clear growth defects on both pH 9 and LiCl media were candidates for Rim101 pathway disruption.
We noted that some alleles displayed both ESCRT-dependent defects and Rim101-dependent defects, such as snf7-20, while some alleles displayed ESCRT-dependent defects yet showed no detectable Rim101-dependent defects, such as snf7-15. Conversely, some alleles showing no ESCRT-dependent defects conferred Rim101-dependent defects, such as snf7-6 and snf7-48 (Figure 5). Thus, among these snf7 alleles, we have identified candidate alleles with differential ESCRT- and Rim101-dependent phenotypes that are strong candidates for separation of function.
Filamentation-related snf7 phenotypes:
Vesicle trafficking and Rim101 activation are both required for filamentation, a critical virulence trait of C. albicans (Bruckmann et al. 2000; Kullas et al. 2004; Palmer et al. 2005; Bernardo et al. 2008). We wished to further investigate the role of these processes and their relative contributions to filamentation. Since Snf7 is required for both ESCRT trafficking and Rim101 processing, we predicted that snf7 alanine-scanning alleles defective in either ESCRT function or Rim101 activation would show defects in filamentation. To test this possibility, we assayed the snf7 alanine-scanning alleles for filamentation in liquid and solid M199 pH 8 medium.
We first assessed filamentation using a quantitative liquid assay. Strains were incubated 4 hr in M199 pH 8 medium and germ tube formation was assessed for each strain. The wild-type strain produced ∼95% germ tubes (Table 4). The snf7Δ/Δ mutant produced <0.01% germ tubes under these conditions, while the complemented snf7Δ/Δ + SNF7-V5 strain rescued germ tube production. The rim101Δ/Δ and rim20Δ/Δ mutant strains were both severely deficient in germ tube production, with no detectable germ tubes after a 4-hr incubation (Table 4). The bro1Δ/Δ strain produced wild-type levels of germ tubes, while the vps4Δ/Δ strain produced ∼80% germ tubes. Nonfunctional alleles were those that produced <5% germ tubes, such as snf7-6. Partially functional alleles were those that produced an intermediate number, 5–90%, of germ tubes, such as snf7-15 and snf7-48 with 78 and 88% germ tubes, respectively. Fully functional alleles were defined as those that formed >90% germ tubes like the snf7Δ/Δ +SNF7-V5. We noted that some alleles with filamentation defects also conferred ESCRT-dependent defects, such as snf7-15; that some also conferred Rim101-dependent defects, such as snf7-48; and that some also conferred defects in both, such as snf7-20. We did not find any alleles conferring solely filamentation defects. Although both Rim101- and ESCRT-related defects are reported to affect filamentation, we noted fewer pheonotypic defects in this assay than in the FM 4-64 assay or growth assays (Figure 5).
We also assessed colony filamentation by spotting strains on solid alkaline or acidic medium and measuring the ability of the strains to form peripheral filamentation. On alkaline medium, the wild-type and snf7Δ/Δ + SNF7-V5 strains formed peripheral filamentous rings (Table 4). The snf7Δ/Δ, rim20Δ/Δ, and rim101Δ/Δ mutants did not produce a ring of peripheral filaments, as expected due to the inability of these strains to process Rim101. The bro1Δ/Δ strain produced a wild-type filamentation pattern, and the vps4Δ/Δ mutant produced an erratic ring of peripheral filaments, as previously reported (Kullas et al. 2004). The snf7Δ/Δ + snf7 alanine-scanning alleles were assayed and categorized as functional or nonfunctional, as no intermediate phenotypes were observed. Nonfunctional alleles were defined as alleles that did not produce peripheral filaments, such as snf7-6 and snf7-20. Fully functional alleles produced filamentous rings similar to those observed in the snf7Δ/Δ +SNF7-V5 strain, such as snf7-2, snf7-15, and snf7-48.
On acidic solid medium, neither the wild-type, snf7Δ/Δ + SNF7-V5, rim101Δ/Δ, rim20Δ/Δ, nor the bro1Δ/Δ strains formed peripheral filaments (Table 4). However, we noted that both the snf7Δ/Δ and vps4Δ/Δ strains formed robust filaments. None of the mutant snf7 alanine alleles promoted acidic filamentation, supporting our previous premise that no snf7 mutation results in completely abolished Snf7 function. Because both Snf7 and Vps4 are required for ESCRT function and Rim101 plays no role in ESCRT function (Kullas et al. 2004), our data suggest that vacuolar transport influences filamentation at acidic pH.
To define regions that may function specifically in either the ESCRT or Rim101 pathways, we mapped the results of the phenotypic assays in relation to the position of snf7 alanine-scanning alleles (Figure 5). Alleles were classified into categories on the basis of their phenotypic profile. These included alleles that showed either no phenotypic defects or a mild defect in a single Rim101-dependent assay (group A), alleles that showed only ESCRT-dependent defects (group B), alleles that showed only Rim101-dependent defects (group C), and alleles that showed both ESCRT- and Rim101-dependent defects (group D). We then used these groups as a guide for investigating the mechanisms behind our alanine-scanning snf7 allele phenotypes.
Snf7 protein localization:
In wild-type cells, Snf7 cycles between the cytoplasm and endosomal membrane, and its release requires Vps4. We tested Snf7 localization using cell fractionations to separate the membrane-bound organelles, including endosomes, from the cytoplasm. We first used plasma membrane protein Pma1 and cytoplasmic protein Pgk1, which localized to the pellet or supernatant fraction, respectively (data not shown), as control proteins for our fractionation protocol. Snf7-V5 was observed in both pellet and supernatant (Figure 6A), indicating that Snf7-V5 is able to cycle on and off endosomal membranes, as reported in S. cerevisiae (Babst et al. 1998). Snf7-V5 localization in a vps4Δ/Δ background resulted in a strong pellet signal but no supernatant signal, indicating that Snf7-V5 is unable to dissociate from endosomal membranes in the absence of Vps4, also as reported in S. cerevisiae (Babst et al. 1998). This inability to dissociate is independent of extracellular pH (data not shown). We further confirmed a Vps4-dependent Snf7 release from endosomes using immunofluorescence. Snf7-V5 fluorescence in a wild-type background showed staining throughout the cytoplasm as well as concentrated spots, which likely represent foci of Snf7 endosomal recruitment (Figure 6B). Snf7-V5 fluorescence in a vps4Δ/Δ background showed little cytoplasmic staining and 1 or 2 very highly concentrated spots, which likely are class E-like compartments. These two methods confirm that Snf7 localization is regulated similarly to previously described systems.
Figure 6.—
(A) Snf7 localization remains normal in alanine-scanning snf7 mutants during cell fractionation. Mid-log cultures were gently lysed and separated by centrifugation to generate a cytoplasm-containing supernatant (S) and an organelle-containing pellet (P). Fifty microliters of each fraction was run on 10% SDS–PAGES. Blots were probed with anti-V5-HRP antibody. (B) Snf7 localization remains normal in alanine-scanning snf7 mutants during immunofluorescence. Strains were grown to mid-log phase in M199 (pH 8) medium, fixed with 4% formaldehyde, spheroplasted, and attached to polylysine-treated wells for immunofluorescence. Samples were treated with anti-V5 antibody, followed by anti-mouse-IgG-alexafluor 488 (green).
We identified 17 group D snf7 alleles that affected Snf7 function in both the Rim101 and ESCRT pathways. Two simple models can explain the group D phenotypes. In the first model, group D alleles are defective in overall Snf7 function due to a failure of Snf7 protein to interact with the upstream ESCRT pathway member Vps20 or with Snf7 itself. Vps20 has been shown to facilitate initial Snf7 endosomal association, followed by the formation of a Snf7 lattice through Snf7-Snf7 interactions (Babst et al. 2002a; Teis et al. 2008). Thus, snf7 alleles unable to interact with either Vps20 or itself would not be able to properly localize, and would be expected to have a defective phenotype in any assay for Snf7 function. In the second model, group D alleles are defective in overall Snf7 function due to disruption of a Snf7 region required for interaction with downstream components of both pathways. For example, Snf7 interacts with Bro1 and Rim20 via a bro1-domain found in both proteins (Vincent et al. 2003; Kim et al. 2005). To distinguish between these two possible explanations, Snf7 protein from the group D alleles was localized through cell fractionations.
We predicted that if group D alleles affected upstream Snf7 interactions, Snf7 protein would not be properly recruited to the endosomal membrane. To test this, we introduced the group D alleles into a vps4Δ/Δ strain, in which Snf7 protein is unable to dissociate from the endosomal membrane. If the group D allele blocked recruitment to the endosome, Snf7 should be detected only in the supernatant fraction. If the group D allele blocked downstream interactions with members of both pathways, Snf7 should be enriched in the pellet fraction. Strains containing snf7-20 showed primarily pellet-associated Snf7 (Figure 6A), indicating that normal recruitment and retention of Snf7 in a vps4Δ/Δ background was retained. Results for the other group D strains were similar to snf7-20 (data not shown). We used immunofluorescence to confirm that snf7-20 expression leads to normal Snf7 protein localization in both a VPS4+/+ and vps4Δ/Δ background (Figure 6B). This suggests the mutations in these alleles affect an epitope necessary for downstream Snf7 function in both the Rim101 and ESCRT pathway.
We identified five group B alleles that affected only ESCRT-dependent Snf7 function. Because Rim101 function requires endosomal Snf7 localization, and because these alleles did not affect Rim101 function, we predicted that alleles in this category affected interactions with downstream ESCRT pathway members, such as Vps2, Vps24, or Vps4. Because we had noted a similar FM 4-64 staining pattern between the snf7-15 and vps4Δ/Δ strains (Figure 2), we considered snf7-15 to be a strong candidate for interrupted Snf7-Vps4 interactions. To test this possibility, we investigated Snf7-15 localization by cell fractionation. Alanine scanning alleles affecting Snf7-Vps4 interactions should have enriched Snf7 protein in the pellet fraction, as the vps4Δ/Δ strain does, while mutant alleles not affecting Snf7-Vps4 interactions should have Snf7 in both the pellet and supernatant fractions, as the wild-type strain (Figure 6A). Snf7-15 protein was found in both pellet and cytoplasmic fractions, suggesting that Snf7-Vps4 interactions are not inhibited in this strain. We expected group C alleles, which conferred only Rim101-dependent defect, would promote Snf7 protein localization similar to wild-type and we detected both pellet- and supernatant-associated Snf7 protein in all group C alleles, such as snf7-6 (Figure 6A). Normally regulated Snf7 protein localization from both the snf7-6 and snf7-15 alleles was confirmed with immunofluorescence (Figure 6B). Thus, we did not find any snf7 alleles that significantly affected protein localization patterns.
Rim101 processing and localization:
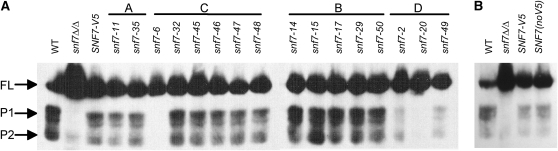
We identified thirteen group C alleles that disrupted only Rim101-dependent Snf7 function, including snf7-6 and snf7-48. Because Snf7 interacts with Rim101 processing machinery, we predicted that alleles in this category would produce less active, processed Rim101, and that this decrease would explain the phenotypic defects observed in our screens. To test this prediction, we used Western blot analysis to investigate Rim101 processing in snf7Δ/Δ RIM101-V5 strains containing mutant snf7 alleles (Figure 7A). We noted that the SNF7-V5 strain contained less processed Rim101 (both 74 and 65 kD forms) and more full-length Rim101 compared to wild-type cells. However, similar results were observed using an untagged SNF7 complementation strain (Figure 7B), suggesting that decreased Rim101 processing in the SNF7-V5 strain is not due to the V5 epitope.
Figure 7.—
(A) Rim101 processing is affected by some snf7 mutants. Strains were grown to mid-log phase in M199 pH 8 medium before protein preparation. Equivalent protein amounts were analyzed by Western blotting analysis. FL, full length Rim101 (85 kDa); P1, processed form 1 of Rim101, the active form (74 kDa); P2, processed form 2 of Rim101, with unknown function (65 kDa). (B) Rim101 processing is decreased in the presence of only one SNF7 allele regardless of V5 epitope.
We tested all snf7 mutant alleles for their ability to promote Rim101 processing. As expected, all group A and group B alleles, such as snf7-35 and snf7-15 respectively, processed Rim101 similarly to the SNF7-V5 strain. (Figure 7A). We expected group C alleles, which conferred only Rim101-dependent defects, to show decreased levels of Rim101 processing compared to the SNF7-V5 strain. However, we found only one allele, snf7-6, that abolished Rim101 processing, similar to the snf7Δ/Δ strain (Figure 7A). Other group C alleles, such as snf7-48, processed levels of Rim101 similar to the SNF7-V5 strain. We expected group D alleles, which conferred both ESCRT- and Rim101-dependent defects, to also have decreased levels of Rim101 processing compared to the SNF7-V5 strain. This was observed for all group D alleles, including snf7-20 (Figure 7A). Thus, while alleles in group B and group D behaved as expected, a decrease in processed Rim101 does not explain the growth defects seen in all group C alleles.
We considered two models to explain the processing seen in group C alleles. First, group C snf7 alleles that process Rim101 do not promote translocation to the nucleus. Second, group C snf7 alleles have Rim101 processing defects that were missed at steady-state growth. To test these models, we generated alanine-scanning snf7 alleles without the V5 epitope and expressed them into a snf7Δ/Δ RIM101-V5 strain. We grew our strains in acidic medium to mid-log phase and shifted them to alkaline medium for 30 min to investigate the initial stages of alkaline adaptation. We then tested Rim101 localization through immunofluorescence and Rim101 processing through Western blot analysis.
In a strain with wild-type SNF7 grown at pH 4, Rim101-V5 showed cytoplasmic staining (data not shown). This same strain shifted to pH 8 showed V5 staining that colocalized with the DNA stain DAPI (Figure 8A), indicating nuclear localization of Rim101-V5 in these cells. The snf7Δ/Δ strain, which does not process Rim101, showed cytoplasmic V5 staining with no specific DAPI colocalization, indicating cytoplasmic retention of Rim101-V5 in this strain. We next analyzed the snf7Δ/Δ mutant complemented with wild-type SNF7 or the group A allele snf7-35.1. As expected, strains containing either the wild-type SNF7 allele or the snf7-35.1 allele displayed wild-type Rim101-V5 staining patterns (Figure 8A), indicating nuclear localization of Rim101 in these strains. This correlates well with the robust processing promoted by these alleles (Figure 7A).
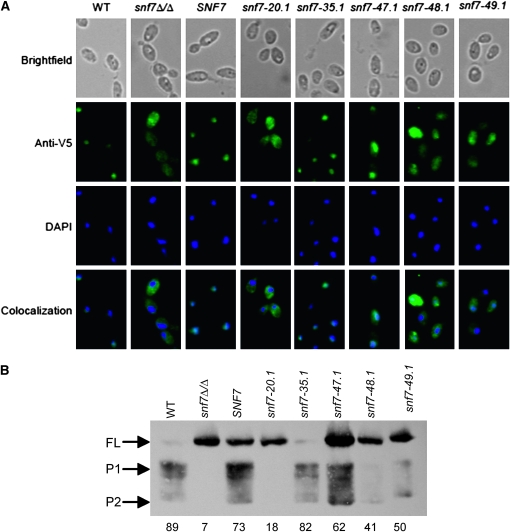
Figure 8.—
(A) Rim101 localization in group C alleles may be impaired after a shift to alkaline pH. Strains were grown to mid-log phase in M199 (pH 4) medium and shifted to pH 8 for 30 min. Strains were fixed with 4% formaldehyde, spheroplasted, and attached to polylysine-treated wells for immunofluorescence. Samples were treated with anti-V5 antibody, followed by anti-mouse-IgG-alexafluor 488 (green). Nuclei were visualized by DAPI staining (blue). Strains investigated include SNF7+/+ RIM101-V5 (WT) (DAY1212), snf7Δ/Δ RIM101-V5 (DAY1127), snf7Δ/Δ SNF7 RIM101-V5 (DAY1128), snf7Δ/Δ snf7-20.1 RIM101-V5 (DAY1148), snf7Δ/Δ snf7-35.1 RIM101-V5 (DAY1149), snf7Δ/Δ snf7-47.1 RIM101-V5 (DAY1213), snf7Δ/Δ snf7-48.1 RIM101-V5 (DAY1150), and snf7Δ/Δ snf7-49.1 RIM101-V5 (DAY1214). (B) Rim101 processing in group C alleles may be impaired after a shift to alkaline pH. Strains were grown as in A before protein preparation. Equivalent protein amounts were analyzed by Western blotting analysis. Numbers under each column represent percentage P1 signal over total Rim101 (FL + P1 + P2) signal.
Next, we tested the group D alleles snf7-20.1 and snf7-49.1. The snf7-20.1 allele conferred severe growth defects (Figure 3) and contained no processed, active Rim101 (Figure 7). Cells expressing snf7-20 displayed a V5 staining pattern similar to the snf7Δ/Δ strain, with no DAPI colocalization, indicating non-nuclear localization in these cells. The snf7-49 allele conferred intermediate growth defects (Figure 3) and decreased active Rim101 (Figure 7). Cells expressing snf7-49.1 displayed an intermediate V5 staining pattern with both DAPI-overlapping and non-DAPI overlapping staining (Figure 8A). This indicated partial nuclear localization and partial cytoplasmic retention of Rim101 in this strain. Thus, the Rim101-V5 localization in these group D strains correlated with the growth phenotype and Rim101 processing observed.
We then tested the group C alleles snf7-47.1 and snf7-48.1 that conferred Rim101-dependent defects yet had normal Rim101 processing (Figures 3 and 7 and data not shown). Strains expressing either of these alleles displayed strong cytoplasmic V5 compared with snf7-35.1 and wild-type SNF7 strains. While some cells showed slight V5 colocalization with DAPI, all cells maintained cytoplasmic staining (Figure 8A). We noted a stronger cytoplasmic staining pattern in the snf7-48.1 strain compared to the snf7-47.1 strain, which correlated with the slight difference in Rim101-dependent phenotypic defects observed between these strains (data not shown). This indicates partial nuclear localization and partial cytoplasmic retention of Rim101-V5 in the snf7-47.1 and snf7-48.1 strains.
To determine how Rim101 processing is affected during adaptation to alkaline pH by these snf7 alleles, we also examined Rim101 processing following a 30 min shift to alkaline pH. As previously observed (Figure 7), the wild-type strain processed Rim101-V5 and the snf7Δ/Δ strain did not (Figure 8B). Also, addition of SNF7 or the group A snf7-35.1 allele restored processing. Both group D alleles, snf7-20.1 and snf7-49.1, conferred processing defects. Thus, these alleles promoted similar Rim101 processing under steady state and adaptive growth conditions.
However, we found that the group C allele snf7-48.1 had considerably less Rim101-V5 processing than wild-type after a 30-minute shift to pH 8 compared to wild-type cells or compared to steady state growth at pH 8 (Figure 8B). This suggests that processing occurs more slowly in a snf7-48 strain, but active Rim101 levels eventually reach wild-type levels. We observed more processed Rim101 in the snf7-47.1 strain, but noted that this strain still had less processed Rim101 (P1 + P2) relative to the amount of total Rim101 (FL + P1 + P2) than the contorl (Figure 8B). When we tested our 13 group C alleles, we observed a decrease in processed Rim101 relative to total Rim101 in 7 of 13 group C alleles (unpublished data). Taken together, these data suggest that many group C alleles, including snf7-48, fail to process Rim101 at the same rate as wild-type, but that Rim101-dependent phenotypic defects become less pronounced as processed Rim101 accumulates in the cell during steady-state growth.
Epithelial cell damage:
C. albicans causes epithelial cell damage, and this damage requires active Rim101 (Villar et al. 2007; Nobile et al. 2008). We wished to test the ability of our mutant snf7 alleles to mediate epithelial cell damage in a Rim101-dependent and Rim101-independent manner. To do this, we utilized a radiolabeled FaDu oropharyngeal epithelial cell line. When incubated with the wild-type C. albicans strain, we observed ∼45% specific release (Figure 9A). We observed significantly less damage when FaDu were incubated with the rim101Δ/Δ or snf7Δ/Δ strains. The addition of the SNF7-V5 allele rescued most, but not all, of the damage defect in the snf7Δ/Δ strain (Figure 9A). Because the mutant snf7 alleles were generated in the SNF7-V5 background, we measured epithelial damage mediated by these mutant alleles and compared it to damage mediated by SNF7-V5.
Figure 9.—
C. albicans-mediated FaDu cell damage is affected by Rim101-dependent defects. (A) Both the rim101Δ/Δ mutant and snf7Δ/Δ mutant have severe FaDu cell damage defects, and the SNF7-V5 allele rescues most of the snf7Δ/Δ defect. FaDu monolayers were Cr51 labeled overnight, then washed and incubated with 1 × 105 cells/ml C. albicans for 10 hr. Strains were tested in triplicate during each assay and compared with media-alone wells to measure specific Cr51 release. The assay was repeated at least three times; the figure denotes one representative assay. (B) Only mutant snf7 alleles with Rim101-dependent defects have damage defects. Assays were run as described in A, and mutant snf7 allele damage was compared to SNF7-V5 damage. Each strain was tested in triplicate during each assay, and assays were repeated twice for each mutant snf7 allele.
We predicted that the group C alleles, which conferred only Rim101-dependent defects, would cause decreased epithelial cell damage due to their Rim101-related phenotypes. We tested thirteen group C alleles, of which eight caused less damage than the SNF7-V5 allele (p < 0.05) (Figure 9B). We noted that snf7-6, the only group C allele to completely abolish Rim101 processing, did not have the strongest damage defect. In fact, none of the group C alleles were as diminished in damage as the rim101Δ/Δ, suggesting that all alleles maintained at least low levels of Rim101 activity. Overall, 62% of group C alleles caused less FaDu cell damage than the SNF7-V5 allele.
Endocytosis plays an important role in receptor down-regulation and nutrient acquisition. We predicted that full ESCRT function would be required for wild-type epithelial cell damage. However, because the group B allele growth and filamentation defects were less severe than the group C defects, we predicted the group B alleles would play a lesser role in epithelial cell damage than the group C alleles. In fact, we did not see a significant decrease in damage in any of the five alleles tested (Figure 9B). This suggests either that the ESCRT pathway does not contribute to FaDu cell damage or that the snf7 alanine mutations do not abolish ESCRT function to levels that affect C. albicans-epithelial cell interactions.
Finally, we tested the group D alleles, which conferred both ESCRT- and Rim101-dependent defects. Four of ten group D alleles tested caused decreased FaDu damage (p < 0.05), and none of the defects were more severe than those conferred by the group C alleles (Figure 9B). Thus 40% of the group D alleles caused less damage than the SNF7-V5 allele, likely due to Rim101-dependent defects.
DISCUSSION
Adaptation of C. albicans to a neutral-alkaline environment requires several cellular responses. One critical response is the activation of the transcription factor Rim101, which regulates gene expression in an extracellular pH-dependent manner. Another cellular change is the dependence on endocytosis and endosomal trafficking for uptake of otherwise inaccessible nutrients. Snf7 protein plays a pivotal role in both of these processes, and is therefore essential for the cellular adaptation to a neutral-alkaline environment. We investigated the role of Snf7 in these two distinct processes using alanine-scanning mutagenesis, which allowed us to examine the function of discrete regions of Snf7. Our analyses demonstrate that Snf7 function in the Rim101 pathway is separable from Snf7 function in the ESCRT pathway.
Separation of function and identification of functional domains:
Our phenotypic results allowed us to characterize the mutant snf7 alleles as nonfunctional, partially functional, or fully functional, with respect to the different phenotypic assays. To disrupt discrete domains, each mutation changed up to three amino acids. While we did not test Snf7 interactions directly due to the failure to generate functional tagged constructs, disruption of a interaction domain remains the simplest explanation for many of our partially complementary alleles.
The largest number of alleles (21) was classified into group A, the group of alleles conferring no defects or only one Rim101-dependent phenotypic defect. Of the 21 alleles included in this group, 19 have no apparent defect in any phenotypic assay tested. The largest cluster of group A alleles was a span of 25 amino acids in the C-terminal half of the protein. The J-Pred software program (Cuff and Barton 2000) predicted six alpha-helices spanning the coding sequence (Figure 10), which is similar to the predicted human Snf7 structures (Shim et al. 2007). We aligned our phenotypic data with the predicted helical structures to correlate Snf7 structures and function. Interestingly, the cluster of group A alleles coincided with a region containing no predicted helices, suggesting that this lack of defined structure may increase tolerance for mutation in this region. The functional importance of the identified helices may explain their conservation of through evolutionary time, while the less conserved unstructured regions suggest a linker sequence function.
Figure 10.—
Model of Snf7 interactions. (A) Snf7 interacts with ESCRT member Bro1, or Rim101 members Rim13 and Rim20, through distinct interaction domains. Putative Snf7 helical structure is shown with several snf7 alleles marked for reference. Also noted are the domains predicted from our studies to be involved in ESCRT-specific (E), Rim101-specific (R), or both (B) processes. Solid lines represent interactions conserved in other species. Dashed lines represent interactions predicted from our data. (B) Alignment of Snf7 C-terminal sequence with snf7 allele numbers noted below. Mutation of red residues abolished hSnf7-1–Bro1 domain interactions (McCullough et al. 2008). C. albicans snf7 mutation of these residues has differential effect on bro1-domain protein interaction.
We noted regions where adjacent alleles displayed similar phenotypic effects. There was one cluster of group D alleles at the N-terminus, indicating that this region of Snf7 is important for Rim101 and ESCRT functions. This region may function in Snf7 recruitment to the endosomal membrane through upstream interactions or it could be involved with a common downstream interaction. Because Snf7-bro1-domain interactions are the only common interactions yet identified between the C. albicans Rim101 and ESCRT pathways, and because Snf7-bro1-domain interactions have been mapped to the C-terminal end of Snf7 (see below), this region is more likely to be involved in upstream Snf7 function, such as recruitment by Vps20 or self-oligomerization. These alleles caused intermediate phenotypes, suggesting partial Snf7 activity, which may explain why we didn't observe differences in Snf7 localization (Figure 6 and data not shown).
The majority of group B alleles fell within the second predicted alpha helix, which spans residues 58-94 and encompasses snf7-14 through snf7-20. CHMP3, the human Vps24 homolog, has a helical structure related to CHMP4a, one of three human Snf7 homologs, and the second alpha helix of CHMP3 is important for self-interactions (Muziol et al. 2006; Shim et al. 2007). We predict this region of Snf7 is important for an ESCRT-specific interaction, such as with the Vps2/Vps24 heterodimer or Vps4, which are necessary for endosomal trafficking but do not prevent Rim101 processing (Xu et al. 2004). We demonstrated that Snf7-15 is able to cycle between endosome and cytoplasm by cell fractionation and immunofluorescence. These data indicate that Snf7-15 does not abolish Vps4 interactions. Failure of snf7-14, snf7-15, or snf7-17 to efficiently self-oligomerize or to interact with downstream ESCRT-III members could account for the aberrant FM 4-64 trafficking seen in these strains (Figure 2), although the normal Snf7 protein localization pattern observed (Figure 6) indicates that minimally some Snf7 recycling occurs. Rim101-dependent growth and Rim101 processing appear normal in these strains, indicating that Rim101 activation is supported by the endosome-associated Snf7 present in these strains.
Strikingly, many group C alleles, which affect only Rim101-dependent phenotypes, cluster at the C-terminal alpha helix of Snf7. The clustering of group C alleles over this helix suggests that this structure may be specific for Snf7 interaction with Rim101 pathway proteins. Thus, this region of Snf7 is also a good candidate for interaction with a Rim101 pathway member, such as Rim13 or Rim20. The bro1-domain of the human Bro1 homolog (ALIX) was shown to interact with the C-terminal end of all three human Snf7 homologs, CHMP4a, 4b, and 4c (McCullough et al. 2008). The CHMP4 region important for ALIX interactions corresponds to the C-terminal group C cluster of C. albicans Snf7. This suggests the C-terminal region of Snf7 may be important for interactions with bro1-domain-containing proteins. However, we predicted that disrupting Snf7-bro1-domain interactions would affect both Rim101- and ESCRT-dependent phenotypes, which was only observed at the very C-terminal mutation, snf7-49. Why would mutations affecting interactions with one bro1-containing protein, Rim20, not affect interactions with another bro1-containing protein, Bro1? One possibility is that different bro1-containing proteins interact at this site using slightly different residues; residues changed in snf7-48 may interrupt only Rim20 interactions, while those changed in snf7-49 may interrupt both Rim20 and Bro1 interactions. This is supported by the fact that while all ALIX-CHMP4 interactions occurred at the C-terminal end of CHMP4, the critical residues for interaction varied between the three CHMP4 homologs (McCullough et al. 2008). Alternatively, the residues involved may be important for both Rim20 and Bro1 interactions, but the minor phenotypic defects of a failed Snf7-Bro1 interaction were not detected by our system for alleles other than snf7-49. The importance of this region for overall protein function is emphasized by the failure of our C-terminally tagged Snf7 to fully complement a snf7Δ/Δ strain (data not shown). A third possibility is that the C-terminal group C cluster interacts with an unidentified Rim101 pathway member in C. albicans. In A. nidulans, Yarrowia lipolytica, and S. cerevisiae, an additional Rim101 pathway member, PalC/Rim23, has been identified which contains a bro1-domain and is able to interact with Snf7 (Tilburn et al. 2005; Galindo et al. 2007; Blanchin-Roland et al. 2008). We identified C. albicans orf19.2914 through a blast search with ScRim23 sequence. If Orf19.2914 interacts with Snf7 through its bro1-domain, this interaction should occur at the Snf7 C-terminus, either in conjunction with Rim20 or on separate molecules of Snf7, which oligomerizes on the endosomal membrane.
Abnormal Snf7-Rim20 or Snf7-Rim13 interactions are possible explanations for group C phenotypes. However, it is possible that Rim13 interacts with a domain of Snf7 that also interacts with an ESCRT pathway member. A human homolog of Rim13, Calpain 7, was shown to interact with CHMP4b and CHMP4c through a microtubule interacting and trafficking (MIT) domain (Shim et al. 2008). Vps4-ESCRT III member interactions also occur through a MIT domain at the N-terminus of Vps4 (Scott et al. 2005; Tsang et al. 2006). Calpain 7 contains two MIT domains, while Vps4 contains one. However, while the A. nidulans Rim13 homolog, PalB, has recently been shown to interact with an ESCRT-III member through an MIT domain, no MIT domains were identified in S. cerevisiae or C. albicans Rim13 (Rodriguez-Galan et al. 2008). While this does not rule out a shared Snf7-interaction domain between Rim13 and an ESCRT pathway member, it suggests that C. albicans Rim13 may interact with Snf7 in a manner not conserved among ESCRT pathway member interactions. Group C alleles, such as snf7-6, may affect these Rim13-Snf7 interactions.
Effects on Rim101 processing and regulation:
Several mutant snf7 alleles caused Rim101-dependent phenotypes without causing overt ESCRT-specific phenotypes (group C alleles). As described above, snf7-6 may interrupt a domain necessary for Snf7 interactions with Rim101 processing machinery. We predict that the C terminus of Snf7 interacts with the bro1-domain of Rim20. Thus, we predict that snf7-6 interrupts interaction with putative protease Rim13. Surprisingly, several C-terminal mutant alleles (snf7-45, -46, -47, and -48) showed Rim101-dependent defects but had Rim101 processing at levels comparable to the complemented strain during steady-state growth. We found that these strains have a Rim101 processing defect when shifted briefly to alkaline pH, indicating that these strains have a kinetic defect not observed during steady-state growth. Therefore, defective Rim101 processing accounts for all group C allele phenotypes.
Our phenotypic analyses, combined with structural and biochemical analyses, have allowed us to generate a model of Snf7 interactions (Figure 10). Our model posits that after Snf7 has been recruited to the endosomal membrane, Snf7–Rim13 interactions occur at the region containing snf7-6, and that disruption of this region results in abrogation of Rim101 processing. Further, Snf7–Rim20 interactions occur at the C-terminal end, in a region spanning the area required for Snf7–Bro1 interactions. We can predict essential residues for Snf7–Bro1 domain interaction by aligning C. albicans Snf7 with Homo sapiens Snf7 proteins. McCullough et al. (2008) found three critical residues required for bro1-domain interaction with hSnf7-1 (Figure 10B). Our work suggests that these residues remain critical, but that Rim20–Snf7 interaction requires the entire sequence while Bro1–Snf7 interaction requires only the very C-terminal sequence. Rim20 and Bro1 colocalization has been observed under alkaline conditions (Boysen and Mitchell 2006), suggesting that Rim101 processing and ESCRT function can occur simultaneously at the same Snf7 oligomerization site. Thus, under alkaline conditions, an undetermined factor, such as modified Rim8/PalF (Herranz et al. 2005), may promote Rim20–Snf7 interactions over Bro1–Snf7 interactions.
Effects on epithelial cell damage:
Our data support the idea that Rim101 is necessary for wild-type levels of epithelial cell damage (Figure 9A). As we predicted, the majority of group C snf7 alleles conferred reduced damage. We noted that the allele snf7-6, which abolished all Rim101 processing, caused more damage than the rim101Δ/Δ mutant. One explanation for this result is that the snf7-6 allele processes very low levels of Rim101 undetectable by Western blot analysis. However, we favor the model that full-length Rim101 can mediate partial damage. This idea is supported in an endothelial cell damage model where a rim8Δ/Δ mutant caused less host cell damage than the rim101Δ/Δ mutant (Davis et al. 2000a). Further, the fact that the group C snf7 alleles are not as deficient as the snf7Δ/Δ strain supports the idea that these alleles are at least partially functional.
We were surprised to find that group B alleles did not affect host cell damage in this model. MVB formation and vesicle trafficking are important for cell physiology. Several explanations may account for this observation. First, ESCRT function and MVB formation may not play an important role during epithelial cell damage. Epithelial cell damage depends on the ability of C. albicans to filament, and no group B allele affected filamentation as severely as the group C alleles. Second, ESCRT function may be important for acquiring nutrients, such as iron, for which the cells are not starved during the course of these assays. Third, the alleles may not fully knock down ESCRT function, as most snf7 alleles affecting solely ESCRT function conferred intermediate FM 4-64 trafficking defects (Figure 2). Regardless, our data suggest that ESCRT function does not play a role during epithelial cell damage.
Effects on filamentation:
Numerous redundant signals play a role in the regulation of filamentation, including temperature, nutrient availability, and cell density. Defects in both vesicle trafficking and pH sensing have been shown to affect hyphal formation (Davis et al. 2000b; Gunther et al. 2005; Palmer et al. 2005; Bernardo et al. 2008). Few of our alanine-scanning snf7 alleles conferred strong defects in filamentation, which reinforces that few snf7 alleles completely abolished Snf7 function. We were surprised to observe acidic filamentation of the snf7Δ/Δ and vps4Δ/Δ strains, suggesting that MVB trafficking may play an inhibitory function under acidic conditions. This was particularly remarkable as snf7Δ/Δ and vps4Δ/Δ strains have opposite effects on Rim101 processing: a snf7Δ/Δ strain is unable to process Rim101 and a vps4Δ/Δ strain constitutively processes Rim101 (Kullas et al. 2004; Hayashi et al. 2005). Although these null mutations have opposite effects on Rim101 processing, they have similar effects on ESCRT trafficking: both null mutations result in accumulation of endosomes unable to fuse with the vacuole. This suggests that aberrant ESCRT function results in acidic filamentation independent of Rim101 processing.
The intimate relationship between endocytosis and signal transduction has been established in various signaling pathways in many different organisms (Polo and Di Fiore 2006; Kirkin and Dikic 2007; von Zastrow and Sorkin 2007); however, much remains unknown regarding the exact nature of these relationships. Endocytosis can serve to downregulate receptors, to promote extended downstream signaling through “signaling endosomes,” or to generate physical proximity between members of a signaling pathway. The Rim101 pathway is likely a case of the latter model, with the signaling complex brought proximal to the processing complex to promote Rim101 processing. When Rim101 and ESCRT pathway members intersect remains unclear. The work done here demonstrates several functional regions of Snf7 that are specific for the Rim101 pathway, and suggests that bro1-domain containing proteins may interact at slightly divergent, though overlapping, Snf7 sites, leaving open the possibility that Rim13 and Rim20 interact with a single Snf7 monomer to facilitate Rim101 processing early in MVB formation.
Authors' note: While this article was under review, a similar study was accepted investigating S. cerevisiae SNF7 (Weiss et al. 2009). Weiss et al. identified snf7 alleles dysfunctional for MVB formation but functional for Rim101-dependent phenotypes. In agreement with our findings here, this study showed that S. cerevisiae SNF7 is also genetically separable, notably at the very N- and C-terminal ends, and that the phenotypes may be due to faulty Snf7–Vps4 interactions.
Acknowledgments
We thank members of the Davis lab for helpful discussion on this work and Lucia Zacchi and Dr. Kirsten Nielsen for critical review of the manuscript. We are grateful to Dr. Nielsen and Laura Okagaki for technical assistance with the microscopy. We are grateful to members of the numerous groups in the Candida community involved in establishing an accessible genome database. This research of Dana A. Davis was supported in part by the Investigators in Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund and by the National Institutes of Health (NIH) National Institute of Allergy and Infectious Disease award 1R01-AI064054-01 as well as T32DE007288 from the National Institute of Dental and Craniofacial Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental and Craniofacial Research or the National Institutes of Health.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.112029/DC1.
References
- Adams, A., D. E. Gottschling, C. A. Kaiser and T. Stearns (Editors), 1997. Methods in Yeast Genetics, 1997: A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Andrutis, K. A., P. J. Riggle, C. A. Kumamoto and S. Tzipori, 2000. Intestinal lesions associated with disseminated candidiasis in an experimental animal model. J. Clin. Microbiol. 38 2317–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arechiga-Carvajal, E. T., and J. Ruiz-Herrera, 2005. The RIM101/pacC homologue from the basidiomycete Ustilago maydis is functional in multiple pH-sensitive phenomena. Eukaryot. Cell 4 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst, M., D. J. Katzmann, E. J. Estepa-Sabal, T. Meerloo and S. D. Emr, 2002. a Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 3 271–282. [DOI] [PubMed] [Google Scholar]
- Babst, M., D. J. Katzmann, W. B. Snyder, B. Wendland and S. D. Emr, 2002. b Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell 3 283–289. [DOI] [PubMed] [Google Scholar]
- Babst, M., B. Wendland, E. J. Estepa and S. D. Emr, 1998. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 17 2982–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek, Y. U., M. Li and D. A. Davis, 2008. Candida albicans ferric reductases are differentially regulated in response to distinct forms of iron limitation by the Rim101 and CBF transcription factors. Eukaryot. Cell 7 1168–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensen, E. S., S. J. Martin, M. Li, J. Berman and D. A. Davis, 2004. Transcriptional profiling in Candida albicans reveals new adaptive responses to extracellular pH and functions for Rim101p. Mol. Microbiol. 54 1335–1351. [DOI] [PubMed] [Google Scholar]
- Bernardo, S. M., Z. Khalique, J. Kot, J. K. Jones and S. A. Lee, 2008. Candida albicans VPS1 contributes to protease secretion, filamentation, and biofilm formation. Fungal Genet. Biol. 45 861–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilodeau, P. S., J. L. Urbanowski, S. C. Winistorfer and R. C. Piper, 2002. The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat. Cell. Biol. 4 534–539. [DOI] [PubMed] [Google Scholar]
- Blanchin-Roland, S., G. Da Costa and C. Gaillardin, 2008. Ambient pH signalling in the yeast Yarrowia lipolytica involves YlRim23p/PalC, which interacts with Snf7p/Vps32p, but does not require the long C terminus of YlRim9p/PalI. Microbiology 154 1668–1676. [DOI] [PubMed] [Google Scholar]
- Borg-von Zepelin, M., S. Beggah, K. Boggian, D. Sanglard and M. Monod, 1998. The expression of the secreted aspartyl proteinases Sap4 to Sap6 from Candida albicans in murine macrophages. Mol. Microbiol. 28 543–554. [DOI] [PubMed] [Google Scholar]
- Bowers, K., J. Lottridge, S. B. Helliwell, L. M. Goldthwaite, J. P. Luzio et al., 2004. Protein–protein interactions of ESCRT complexes in the yeast Saccharomyces cerevisiae. Traffic 5 194–210. [DOI] [PubMed] [Google Scholar]
- Boysen, J. H., and A. P. Mitchell, 2006. Control of Bro1-domain protein Rim20 localization by external pH, ESCRT machinery, and the Saccharomyces cerevisiae Rim101 pathway. Mol. Biol. Cell 17 1344–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckmann, A., W. Kunkel, A. Hartl, R. Wetzker and R. Eck, 2000. A phosphatidylinositol 3-kinase of Candida albicans influences adhesion, filamentous growth and virulence. Microbiology 146(11): 2755–2764. [DOI] [PubMed] [Google Scholar]
- Cuff, J. A., and G. J. Barton, 2000. Application of multiple sequence alignment profiles to improve protein secondary structure prediction. Proteins 40 502–511. [DOI] [PubMed] [Google Scholar]
- Davis, D., 2003. Adaptation to environmental pH in Candida albicans and its relation to pathogenesis. Curr. Genet. 44 1–7. [DOI] [PubMed] [Google Scholar]
- Davis, D., J. E. Edwards, Jr., A. P. Mitchell and A. S. Ibrahim, 2000. a Candida albicans RIM101 pH response pathway is required for host–pathogen interactions. Infect. Immun. 68 5953–5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, D., R. B. Wilson and A. P. Mitchell, 2000. b RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol. Cell. Biol. 20 971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, D. A., 2009. How human pathogenic fungi sense and adapt to pH: the link to virulence. Curr. Opin. Microbiol. 12 365–370. [DOI] [PubMed] [Google Scholar]
- Davis, D. A., V. M. Bruno, L. Loza, S. G. Filler and A. P. Mitchell, 2002. Candida albicans Mds3p, a conserved regulator of pH responses and virulence identified through insertional mutagenesis. Genetics 162 1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, H., M. Yamanaka, K. Imamura, Y. Tanaka, A. Nara et al., 2003. A dominant negative form of the AAA ATPase SKD1/VPS4 impairs membrane trafficking out of endosomal/lysosomal compartments: class E vps phenotype in mammalian cells. J. Cell Sci. 116 401–414. [DOI] [PubMed] [Google Scholar]
- Galindo, A., A. Hervas-Aguilar, O. Rodriguez-Galan, O. Vincent, H. N. Arst, Jr. et al., 2007. PalC, one of two Bro1 domain proteins in the fungal pH signalling pathway, localizes to cortical structures and binds Vps32. Traffic 8 1346–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever, G., A. M. Chu, L. Ni, C. Connelly, L. Riles et al., 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418 387–391. [DOI] [PubMed] [Google Scholar]
- Gow, N. A., A. J. Brown and F. C. Odds, 2002. Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 5 366–371. [DOI] [PubMed] [Google Scholar]
- Gunther, J., M. Nguyen, A. Hartl, W. Kunkel, P. F. Zipfel et al., 2005. Generation and functional in vivo characterization of a lipid kinase defective phosphatidylinositol 3-kinase Vps34p of Candida albicans. Microbiology 151 81–89. [DOI] [PubMed] [Google Scholar]
- Hayashi, M., T. Fukuzawa, H. Sorimachi and T. Maeda, 2005. Constitutive activation of the pH-responsive Rim101 pathway in yeast mutants defective in late steps of the MVB/ESCRT pathway. Mol. Cell Biol. 25 9478–9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz, S., J. M. Rodriguez, H. J. Bussink, J. C. Sanchez-Ferrero, H. N. Arst, Jr. et al., 2005. Arrestin-related proteins mediate pH signaling in fungi. Proc. Natl. Acad. Sci. USA 102 12141–12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, D. H., 1999. Acquisition, transport, and storage of iron by pathogenic fungi. Clin. Microbiol. Rev. 12 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, T., T. Chiba, R. Ozawa, M. Yoshida, M. Hattori et al., 2001. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA 98 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann, D. J., M. Babst and S. D. Emr, 2001. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106 145–155. [DOI] [PubMed] [Google Scholar]
- Katzmann, D. J., C. J. Stefan, M. Babst and S. D. Emr, 2003. Vps27 recruits ESCRT machinery to endosomes during MVB sorting. J. Cell Biol. 162 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J., S. Sitaraman, A. Hierro, B. M. Beach, G. Odorizzi et al., 2005. Structural basis for endosomal targeting by the Bro1 domain. Dev. Cell 8 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, D. A., D. M. Hannum, J. S. Qi and J. K. Hurst, 2004. HOCl-mediated cell death and metabolic dysfunction in the yeast Saccharomyces cerevisiae. Arch. Biochem. Biophys. 423 170–181. [DOI] [PubMed] [Google Scholar]
- Kirkin, V., and I. Dikic, 2007. Role of ubiquitin- and Ubl-binding proteins in cell signaling. Curr. Opin. Cell Biol. 19 199–205. [DOI] [PubMed] [Google Scholar]
- Kranz, A., A. Kinner and R. Kolling, 2001. A family of small coiled-coil-forming proteins functioning at the late endosome in yeast. Mol. Biol. Cell 12 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullas, A. L., M. Li and D. A. Davis, 2004. Snf7p, a component of the ESCRT-III protein complex, is an upstream member of the RIM101 pathway in Candida albicans. Eukaryot. Cell 3 1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullas, A. L., S. J. Martin and D. Davis, 2007. Adaptation to environmental pH: integrating the Rim101 and calcineurin signal transduction pathways. Mol. Microbiol. 66 858–871. [DOI] [PubMed] [Google Scholar]
- Lambert, M., S. Blanchin-Roland, F. Le Louedec, A. Lepingle and C. Gaillardin, 1997. Genetic analysis of regulatory mutants affecting synthesis of extracellular proteinases in the yeast Yarrowia lipolytica: identification of a RIM101/pacC homolog. Mol. Cell. Biol. 17 3966–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M., S. J. Martin, V. M. Bruno, A. P. Mitchell and D. A. Davis, 2004. Candida albicans Rim13p, a protease required for Rim101p processing at acidic and alkaline pHs. Eukaryot. Cell 3 741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H., 2001. Transcriptional control of dimorphism in Candida albicans. Curr. Opin. Microbiol. 4 728–735. [DOI] [PubMed] [Google Scholar]
- Liu, H., 2002. Co-regulation of pathogenesis with dimorphism and phenotypic switching in Candida albicans, a commensal and a pathogen. Int. J. Med. Microbiol. 292 299–311. [DOI] [PubMed] [Google Scholar]
- Mavor, A. L., S. Thewes and B. Hube, 2005. Systemic fungal infections caused by Candida species: epidemiology, infection process and virulence attributes. Curr. Drug Targets 6 863–874. [DOI] [PubMed] [Google Scholar]
- McCullough, J., R. D. Fisher, F. G. Whitby, W. I. Sundquist and C. P. Hill, 2008. ALIX-CHMP4 interactions in the human ESCRT pathway. Proc. Natl. Acad. Sci. USA 105 7687–7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, B. M., T. G. Wu, B. E. Jackson and K. R. Wilhelmus, 2007. Candida albicans strain-dependent virulence and Rim13p-mediated filamentation in experimental keratomycosis. Invest. Ophthalmol. Vis. Sci. 48 774–780. [DOI] [PubMed] [Google Scholar]
- Muhlrad, D., R. Hunter and R. Parker, 1992. A rapid method for localized mutagenesis of yeast genes. Yeast 8 79–82. [DOI] [PubMed] [Google Scholar]
- Munn, A. L., and H. Riezman, 1994. Endocytosis is required for the growth of vacuolar H(+)-ATPase-defective yeast: identification of six new END genes. J. Cell Biol. 127 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muziol, T., E. Pineda-Molina, R. B. Ravelli, A. Zamborlini, Y. Usami et al., 2006. Structural basis for budding by the ESCRT-III factor CHMP3. Dev. Cell 10 821–830. [DOI] [PubMed] [Google Scholar]
- Nikko, E., and B. Andre, 2007. Split-ubiquitin two-hybrid assay to analyze protein–protein interactions at the endosome: application to Saccharomyces cerevisiae Bro1 interacting with ESCRT complexes, the Doa4 ubiquitin hydrolase, and the Rsp5 ubiquitin ligase. Eukaryot. Cell 6 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile, C. J., N. Solis, C. L. Myers, A. J. Fay, J. S. Deneault et al., 2008. Candida albicans transcription factor Rim101 mediates pathogenic interactions through cell wall functions. Cell. Microbiol. 10 2180–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi, G., D. J. Katzmann, M. Babst, A. Audhya and S. D. Emr, 2003. Bro1 is an endosome-associated protein that functions in the MVB pathway in Saccharomyces cerevisiae. J. Cell. Sci. 116 1893–1903. [DOI] [PubMed] [Google Scholar]
- Ohsumi, Y., and Y. Anraku, 1981. Active transport of basic amino acids driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J. Biol. Chem. 256 2079–2082. [PubMed] [Google Scholar]
- Ohsumi, Y., and Y. Anraku, 1983. Calcium transport driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J. Biol. Chem. 258 5614–5617. [PubMed] [Google Scholar]
- Palmer, G. E., M. N. Kelly and J. E. Sturtevant, 2005. The Candida albicans vacuole is required for differentiation and efficient macrophage killing. Eukaryot. Cell 4 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penalva, M. A., and H. N. Arst, Jr., 2004. Recent advances in the characterization of ambient pH regulation of gene expression in filamentous fungi and yeasts. Annu. Rev. Microbiol. 58 425–451. [DOI] [PubMed] [Google Scholar]
- Perlroth, J., B. Choi and B. Spellberg, 2007. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med. Mycol. 45 321–346. [DOI] [PubMed] [Google Scholar]
- Pfaller, M. A., and D. J. Diekema, 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20 133–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo, S., and P. P. Di Fiore, 2006. Endocytosis conducts the cell signaling orchestra. Cell 124 897–900. [DOI] [PubMed] [Google Scholar]
- Porta, A., A. M. Ramon and W. A. Fonzi, 1999. PRR1, a homolog of Aspergillus nidulans palF, controls pH-dependent gene expression and filamentation in Candida albicans. J. Bacteriol. 181 7516–7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon, A. M., A. Porta and W. A. Fonzi, 1999. Effect of environmental pH on morphological development of Candida albicans is mediated via the PacC-related transcription factor encoded by PRR2. J. Bacteriol. 181 7524–7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Galan, O., A. Galindo, A. Hervas-Aguilar, H. N. Arst and M. A. Penalva, 2008. Physiological involvement in pH signalling of Vps24-mediated recruitment of Aspergillus PalB cysteine protease to ESCRT-III. J. Biol. Chem. 284 4404–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, A., J. Gaspar, M. D. Stuchell-Brereton, S. L. Alam, J. J. Skalicky et al., 2005. Structure and ESCRT-III protein interactions of the MIT domain of human VPS4A. Proc. Natl. Acad. Sci. USA 102 13813–13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth, C. C., E. G. Mogensen, M. S. Fu, I. C. Blomfield and F. A. Muhlschlegel, 2008. Candida albicans HSP12 is co-regulated by physiological CO2 and pH. Fungal. Genet. Biol. 45 1075–1080. [DOI] [PubMed] [Google Scholar]
- Shim, S., L. A. Kimpler and P. I. Hanson, 2007. Structure/function analysis of four core ESCRT-III proteins reveals common regulatory role for extreme C-terminal domain. Traffic 8 1068–1079. [DOI] [PubMed] [Google Scholar]
- Shim, S., S. A. Merrill and P. I. Hanson, 2008. Novel Interactions of ESCRT-III with LIP5 and VPS4 and their implications for ESCRT-III disassembly. Mol. Biol. Cell 19 2661–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R. S., and P. Hieter, 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern, P., J. Horbul, D. Maher and D. A. Davis, 2008. C. albicans colonization of human mucosal surfaces. PLoS ONE 3 e2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreghini, E., D. A. Davis, R. Subaran, M. Kim and A. P. Mitchell, 2003. Roles of Candida albicans Dfg5p and Dcw1p cell surface proteins in growth and hypha formation. Eukaryot. Cell 2 746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teis, D., S. Saksena and S. D. Emr, 2008. Ordered assembly of the ESCRT-III complex on endosomes is required to sequester cargo during MVB formation. Dev. Cell 15 578–589. [DOI] [PubMed] [Google Scholar]
- Tilburn, J., J. C. Sanchez-Ferrero, E. Reoyo, H. N. Arst, Jr. and M. A. Penalva, 2005. Mutational analysis of the pH signal transduction component PalC of Aspergillus nidulans supports distant similarity to BRO1 domain family members. Genetics 171 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang, H. T., J. W. Connell, S. E. Brown, A. Thompson, E. Reid et al., 2006. A systematic analysis of human CHMP protein interactions: additional MIT domain-containing proteins bind to multiple components of the human ESCRT III complex. Genomics 88 333–346. [DOI] [PubMed] [Google Scholar]
- Vida, T. A., and S. D. Emr, 1995. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar, C. C., H. Kashleva, C. J. Nobile, A. P. Mitchell and A. Dongari-Bagtzoglou, 2007. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect. Immun. 75 2126–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent, O., L. Rainbow, J. Tilburn, H. N. Arst, Jr. and M. A. Penalva, 2003. YPXL/I is a protein interaction motif recognized by aspergillus PalA and its human homologue, AIP1/Alix. Mol. Cell Biol. 23 1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zastrow, M., and A. Sorkin, 2007. Signaling on the endocytic pathway. Curr. Opin. Cell. Biol. 19 436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P., S. Huppert and R. Kölling, 2009. Analysis of the dual function of the ESCRT-III protein Snf7 in endocytic trafficking and in gene expression. Biochem. J. 424 89–97. [DOI] [PubMed] [Google Scholar]
- Williams, R. L., and S. Urbe, 2007. The emerging shape of the ESCRT machinery. Nat. Rev. Mol. Cell Biol. 8 355–368. [DOI] [PubMed] [Google Scholar]
- Wilson, R. B., D. Davis and A. P. Mitchell, 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 181 1868–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, W., and A. P. Mitchell, 2001. Yeast PalA/AIP1/Alix homolog Rim20p associates with a PEST-like region and is required for its proteolytic cleavage. J. Bacteriol. 183 6917–6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, W., F. J. Smith, Jr., R. Subaran and A. P. Mitchell, 2004. Multivesicular body-ESCRT components function in pH response regulation in Saccharomyces cerevisiae and Candida albicans. Mol. Biol. Cell 15 5528–5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo, S. C., L. Xu, J. Ren, V. J. Boulton, M. D. Wagle et al., 2003. Vps20p and Vta1p interact with Vps4p and function in multivesicular body sorting and endosomal transport in Saccharomyces cerevisiae. J. Cell Sci. 116 3957–3970. [DOI] [PubMed] [Google Scholar]