Abstract
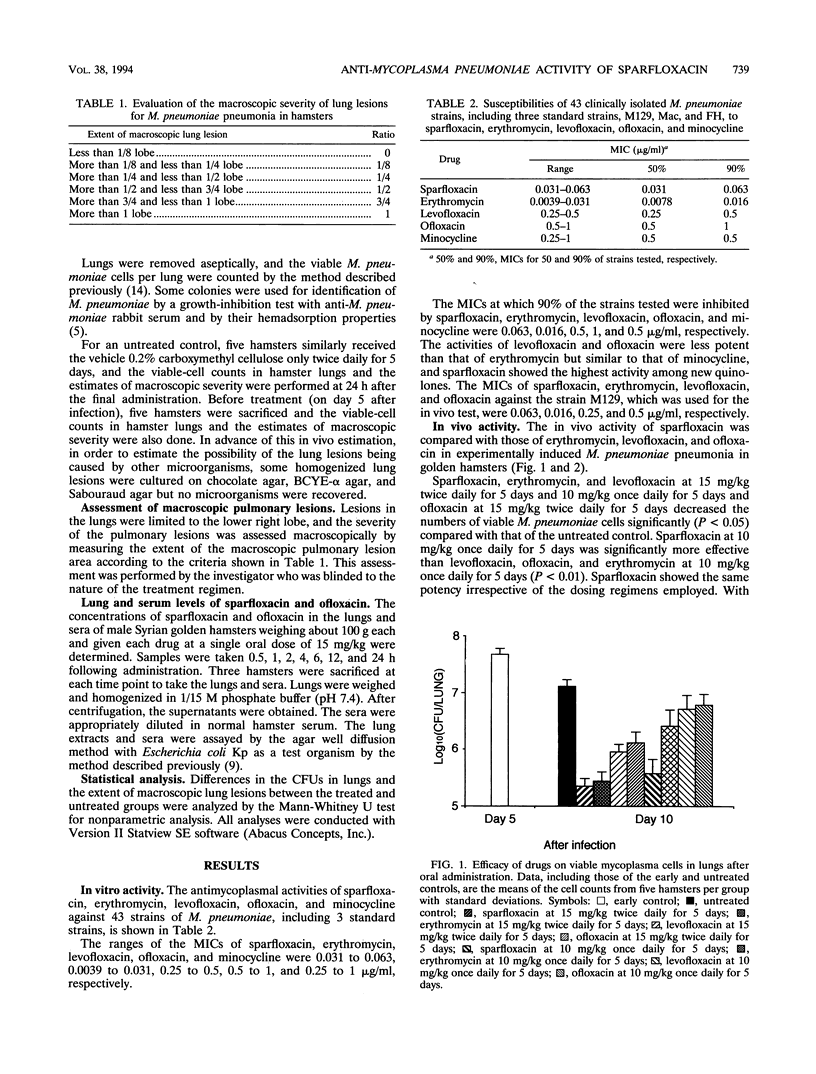
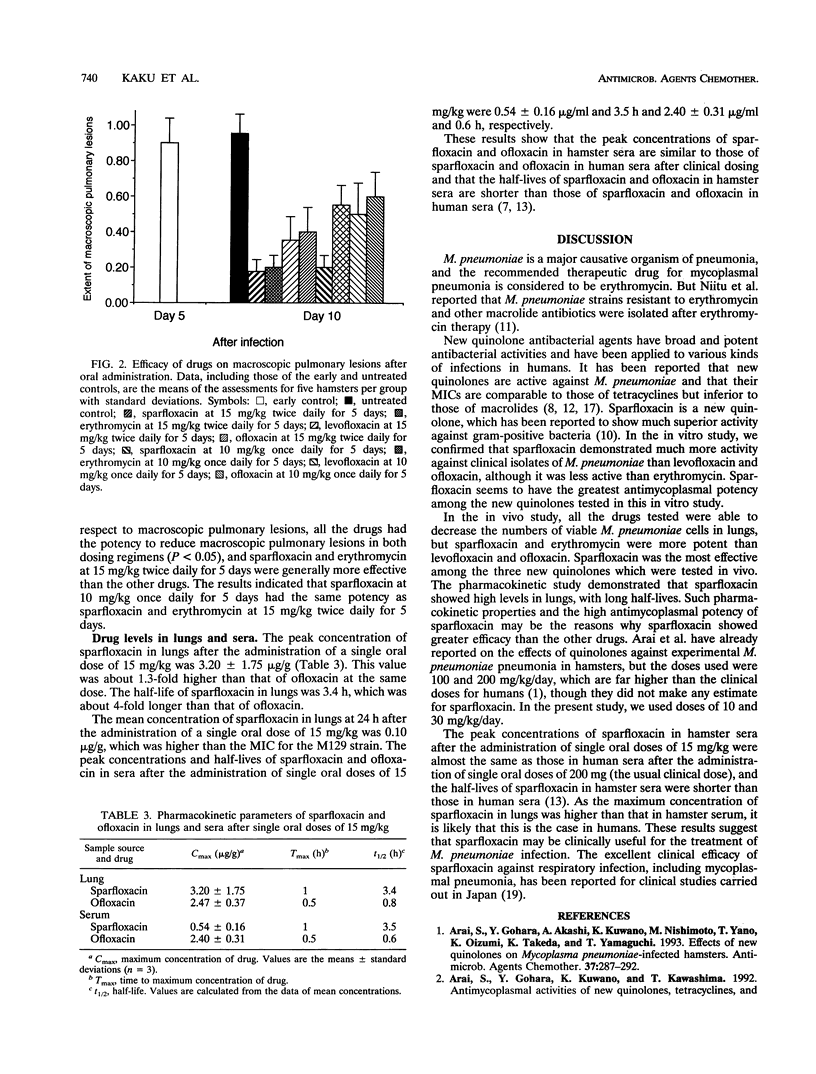
The in vitro and in vivo activities of sparfloxacin against Mycoplasma pneumoniae were compared with those of erythromycin, levofloxacin, ofloxacin, and minocycline. The MICs of sparfloxacin, erythromycin, levofloxacin, ofloxacin, and minocycline for 90% of the 43 M. pneumoniae strains tested were 0.063, 0.016, 0.5, 1, and 0.5 microgram/ml, respectively. In the experimental pulmonary M. pneumoniae infection model in Syrian golden hamsters, sparfloxacin was as effective as erythromycin when orally administered at 15 mg/kg twice daily for 5 days and more effective than erythromycin when orally administered at 10 mg/kg once daily for 5 days. Sparfloxacin was more effective than levofloxacin and ofloxacin in both dosing regimens. The peak concentrations of sparfloxacin in hamster sera after administration of single oral doses of 15 mg/kg were almost the same as those in human sera after administration of single oral doses of 200 mg (the usual clinical dose), and the half-life of sparfloxacin in hamster serum was shorter than that in human serum after administration of a single oral dose of 200 mg. These results suggest that sparfloxacin may be clinically useful for the treatment of M. pneumoniae infections.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai S., Gohara Y., Akashi A., Kuwano K., Nishimoto M., Yano T., Oizumi K., Takeda K., Yamaguchi T. Effects of new quinolones on Mycoplasma pneumoniae-infected hamsters. Antimicrob Agents Chemother. 1993 Feb;37(2):287–292. doi: 10.1128/aac.37.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S., Gohara Y., Kuwano K., Kawashima T. Antimycoplasmal activities of new quinolones, tetracyclines, and macrolides against Mycoplasma pneumoniae. Antimicrob Agents Chemother. 1992 Jun;36(6):1322–1324. doi: 10.1128/aac.36.6.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile M. F., Chandler D. K., Yoshida H., Grabowski M. W., Harasawa R., Razin S. Parameters of Mycoplasma pneumoniae infection in Syrian hamsters. Infect Immun. 1988 Sep;56(9):2443–2449. doi: 10.1128/iai.56.9.2443-2449.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLYDE W. A., Jr MYCOPLASMA SPECIES IDENTIFICATION BASED UPON GROWTH INHIBITION BY SPECIFIC ANTISERA. J Immunol. 1964 Jun;92:958–965. [PubMed] [Google Scholar]
- Foy H. M., Kenny G. E., Cooney M. K., Allan I. D. Long-term epidemiology of infections with Mycoplasma pneumoniae. J Infect Dis. 1979 Jun;139(6):681–687. doi: 10.1093/infdis/139.6.681. [DOI] [PubMed] [Google Scholar]
- Kenny G. E., Cartwright F. D. Susceptibility of Mycoplasma pneumoniae to several new quinolones, tetracycline, and erythromycin. Antimicrob Agents Chemother. 1991 Mar;35(3):587–589. doi: 10.1128/aac.35.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Kurobe N., Ohue T., Hashimoto M., Shimizu M. Pharmacokinetics of a novel quinolone, AT-4140, in animals. Antimicrob Agents Chemother. 1990 Jan;34(1):89–93. doi: 10.1128/aac.34.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Minami A., Nakata K., Kurobe N., Kouno K., Sakaguchi Y., Kashimoto S., Yoshida H., Kojima T., Ohue T. In vitro and in vivo antibacterial activities of AT-4140, a new broad-spectrum quinolone. Antimicrob Agents Chemother. 1989 Aug;33(8):1167–1173. doi: 10.1128/aac.33.8.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niitu Y., Hasegawa S., Suetake T., Kubota H., Komatsu S., Horikawa M. Resistance of Mycoplasma pneumoniae to erythromycin and other antibiotics. J Pediatr. 1970 Mar;76(3):438–443. doi: 10.1016/s0022-3476(70)80485-1. [DOI] [PubMed] [Google Scholar]
- Osada Y., Ogawa H. Antimycoplasmal activity of ofloxacin (DL-8280). Antimicrob Agents Chemother. 1983 Mar;23(3):509–511. doi: 10.1128/aac.23.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Ogura A., Nakayama K., Noguchi Y., Matsuno K., Saito M. Susceptibility of newly established mouse strain MPS to Mycoplasma pneumoniae infection. Microbiol Immunol. 1991;35(3):247–252. doi: 10.1111/j.1348-0421.1991.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Shames J. M., George R. B., Holliday W. B., Rasch J. R., Mogabgab W. J. Comparison of antibiotics in the treatment of mycoplasmal pneumonia. Arch Intern Med. 1970 Apr;125(4):680–684. [PubMed] [Google Scholar]
- Slotkin R. I., Clyde W. A., Jr, Denny F. W. The effect of antibiotics on Mycoplasma pneumoniae in vitro and in vivo. Am J Epidemiol. 1967 Jul;86(1):225–237. doi: 10.1093/oxfordjournals.aje.a120727. [DOI] [PubMed] [Google Scholar]
- Waites K. B., Duffy L. B., Schmid T., Crabb D., Pate M. S., Cassell G. H. In vitro susceptibilities of Mycoplasma pneumoniae, Mycoplasma hominis, and Ureaplasma urealyticum to sparfloxacin and PD 127391. Antimicrob Agents Chemother. 1991 Jun;35(6):1181–1185. doi: 10.1128/aac.35.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev. 1989 Oct;2(4):378–424. doi: 10.1128/cmr.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]


