Summary
5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICA riboside) has been extensively used in vitro and in vivo to activate the AMP-activated protein kinase (AMPK), a metabolic sensor involved in both cellular and whole body energy homeostasis. However, it has been recently highlighted that AICA riboside also exerts AMPK-independent effects, mainly on AMP-regulated enzymes and mitochondrial oxidative phosphorylation (OXPHOS), leading to the conclusion that new compounds with reduced off target effects are needed to specifically activate AMPK. Here, we review recent findings on newly discovered AMPK activators, notably on A-769662, a nonnucleoside compound from the thienopyridone family. We also report that A-769662 is able to activate AMPK and stimulate glucose uptake in both L6 cells and primary myotubes derived from human satellite cells. In addition, A-769662 increases AMPK activity and phosphorylation of its main downstream targets in primary cultured rat hepatocytes but, by contrast with AICA riboside, does neither affect mitochondrial OXPHOS nor change cellular AMP:ATP ratio. We conclude that A-769662 could be one of the new promising chemical agents to activate AMPK with limited AMPK-independent side effects.
Keywords: AMPK, AICA riboside, 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside, ZMP, A-769662, glucose uptake, hepatocytes, mitochondria
INTRODUCTION
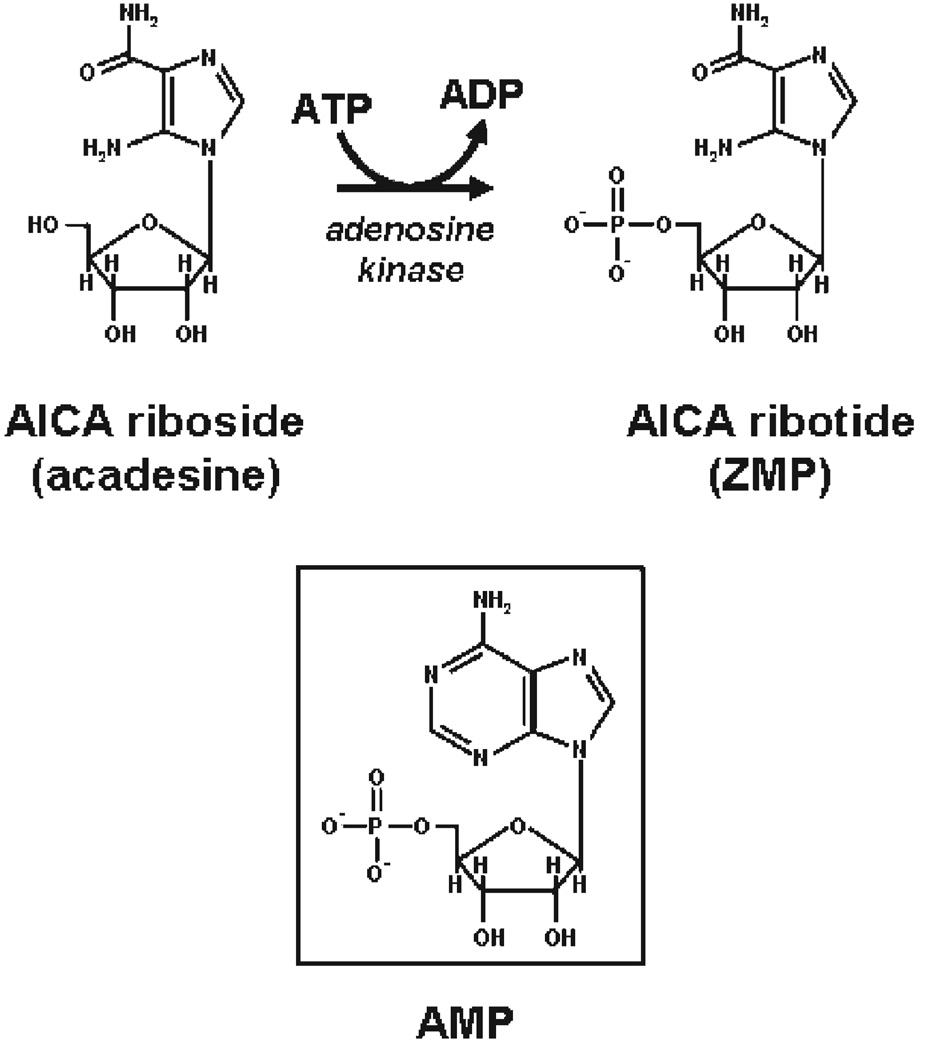
5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICA riboside) is a cell-permeable nucleoside which could be metabolically converted to 5-aminoimidazole-4-carboxamide ribotide (AICA ribotide or ZMP), the antepenultimate metabolic intermediate of the de novo purine synthesis pathway (Fig. 1). Indeed, because AICA riboside shares some structural similarities with adenosine it can enter most cells to be phosphorylated by adenosine kinase into ZMP, although a small amount being also further converted to ZTP (1). ZMP is an analogue of 5′-AMP and thus mimics several of its cellular effects. During the last decade, AICA riboside has been extensively used both in vitro (intact cells or tissues) and in vivo (whole animals) to activate the AMP-activated protein kinase (AMPK) and assess its function in a large number of pathways.
Figure 1.
Chemical structures of AICA riboside, ZMP, and AMP.
THE AMP-ACTIVATED PROTEIN KINASE, A KEY PLAYER REGULATING CELLULAR AND WHOLE BODY ENERGY HOMEOSTASIS
The AMPK is a well-conserved eukaryotic serine/threonine protein kinase which plays a central role in the regulation of cellular energy homeostasis (reviewed in (2–4)). AMPK is a heterotrimeric complex consisting of a catalytic (α) and two regulatory (β and γ) subunits. Each subunit has multiple isoforms encoded by several genes (α1, α2, β1, β2, γ1, γ2, γ3) giving 12 possible heterotrimeric combinations with different tissue distribution and cellular localization. The N-terminus of the α-subunit contains a Thr-172 residue in the activation loop, whose phosphorylation by upstream kinases is both sufficient and necessary for AMPK activation. The β-subunit acts as a scaffold for the two other subunits and contains a glycogen-binding domain that has been proposed to play a role in fuel sensing but whose exact function still remains to be clarified. The γ-subunit contains four CBS motifs able to bind adenine nucleotides, with a higher affinity for AMP than for ATP.
During metabolic stresses (nutrient or oxygen deprivation) or intense energetic demand (muscle contraction), AMPK is activated following rise in intracellular AMP concentration or increase in AMP:ATP ratio. As its name indicated, AMPK is allosterically stimulated by AMP but needs first to be phosphorylated on Thr-172 by upstream kinases. The first AMPK kinase (AMPKK) identified was LKB1, a tumor suppressor mutated in Peutz-Jeghers cancer syndrome, which seems to be constitutively active and mainly involved in Thr-172 phosphorylation following change in AMP:ATP ratio. A second AMPKK, the calcium/calmodulin-dependent protein kinase kinase β (CaMKKβ), was next found to phosphorylate Thr-172 and activate AMPK by an AMP-independent manner in response to increased intracellular calcium concentrations. Finally, a third putative AMPKK, the transforming growth factor-β-activated kinase (TAK1), has been recently reported but its exact regulation and physiological relevance remains unclear at present. On top of the regulation by AMPKK, it has been recently demonstrated that the rise in AMP also protects Thr-172 dephosphorylation by protein phosphatase 2C (PP2C). All these three effects, i.e. allosteric stimulation by AMP, phosphorylation of Thr-172 by AMPKKs and AMP-mediated inhibition of Thr-172 dephosphorylation by PP2C, contribute to regulate cellular AMPK activity (2–5).
In addition to metabolic stresses, AMPK activity is also modulated in a tissue-specific manner by either various hormones/cytokines, such as insulin, leptin, ghrelin, adiponectin, and interleukin-6, or signaling through α and β adrenergic receptors (2). Furthermore, AMPK activation was also reported after treatment with the antidiabetic drugs metformin and thiazolidinediones, constituting part of the rationale for using AMPK activators in the management of metabolic disorders and type 2 diabetes (2, 5). Whether all these agents control AMPK activity through changes in AMP:ATP ratio is however still a matter of debate.
Once activated, AMPK decreases ATP-consuming pathways and stimulates ATP-generating processes to restore energy balance, in line with the concept that it acts as a metabolic master switch promoting ATP conservation. The regulation involves phosphorylation by AMPK of both key enzymes controlling metabolic pathways (reviewed in (2, 4, 5)) and transcription factors modulating gene expression (reviewed in (6)). Because of space limitation, an exhaustive overview of the main cellular processes regulated by AMPK is not possible. However, in general, AMPK activation results in inhibition of lipid, glycogen, and protein synthesis as well as cell growth and proliferation, whereas fatty acid oxidation and glucose uptake are concomitantly stimulated. On top of regulating cellular metabolism, a broader role for AMPK in the control of feeding behavior and whole body energy expenditure was recently highlighted at the brain level (reviewed in (7)).
AICA RIBOSIDE, THE FIRST AND WIDELY USED CHEMICAL AMPK ACTIVATOR
The first report of AICA riboside as an AMPK activator was published in an international patent of 1994 where this cell-permeable compound was described as a ZMP-generating agent mimicking all of the allosteric effects of 5′-AMP on the AMPK system owing to its structural analogy with the adenine nucleotide (Fig. 1). Afterward, in vitro stimulation of AMPK by ZMP has been confirmed by several groups using purified AMPK from rat liver (8–10). In addition, administration of AICA riboside was shown to cause massive intracellular accumulation of ZMP in intact cells leading to activation of native AMPK and subsequent phosphorylation and inactivation of both acetyl-CoA carboxylase (ACC) and 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoA reductase) in hepatocytes and of hormone-sensitive lipase in adipocytes (8–10). These results clearly indicated that pharmacological stimulation of AMPK was possible by agents that, like AICA riboside, give rise to AMP or AMP mimetics (e.g., ZMP).
In Vitro
Most of cellular functions of AMPK have been discovered using AMPK-activating drugs in vitro and a large part of what has been learned about its downstream targets came from the extensive use of AICA riboside in intact cells. For example, as previously mentioned, AICA riboside provided direct evidence that AMPK plays a central role in the regulation of hepatic lipid metabolism by inhibiting fatty acid and cholesterol biosynthesis through phosphorylation and inactivation of ACC and HMG-CoA reductase, respectively (9, 10). The effect of AICA riboside on ACC phosphorylation is abolished in hepatocytes deleted of both AMPK catalytic subunits (11, 12), confirming that it is completely dependent on AMPK activation. On the other hand, AICA riboside stimulates long-chain fatty acid oxidation through its AMPK-mediated effect on ACC, relieving malonyl-CoA-dependent inhibition of carnitine palmitoyl-transferase-1 (CPT-1) (13). Protein synthesis is another biosynthetic pathway repressed by AICA riboside-induced AMPK activation through inactivation of both elongation via eukaryotic elongation factor 2 kinase (eEF2K) phosphorylation (14) and translation via inhibition of the mammalian target of rapamycin (mTOR) pathway (15). Although part of the hepatic action of AICA riboside is achieved by rapid and direct AMPK-mediated phosphorylation of metabolic enzymes, long-term effects of this compound have also been clearly demonstrated on the expression of many genes. Indeed, it induces modulation of transcriptional expression and/or activity of transcription factors and co-activators, such as HNF4, ChREBP, SREBP1c, SHP, or FOXO1a, leading to repression of glycolytic, lipogenic, and gluconeogenic genes (reviewed in (6)). In addition to its beneficial effect on hepatic glucose and lipid metabolism, recent report showed that AICA riboside inhibits several profibrogenic actions in human hepatic stellate cells, including cell proliferation and migration, chemokine secretion, and collagen production (16).
In both skeletal muscle and heart, AICA riboside increases glucose uptake, fatty acid uptake, and oxidation as well as suppression of protein synthesis, most of these effects being abolished in various AMPK knock-out mouse models (reviewed in (4)). On the other hand, AICA riboside caused phosphorylation and inactivation of glycogen synthase in perfused skeletal muscles, an effect that appears to be dependent on the AMPKα2 isoform (17). The stimulation of glucose transport by AICA riboside could be due to AMPK-mediated phosphorylation and inhibition of the Rab GTPase-activating proteins that are proposed to be involved in GLUT4 trafficking (18), Akt substrate of 160 kDa (AS160) and TBC1D1, because its effect was fully inhibited in transgenic mice expressing of dominant negative AMPKα2 mutant in muscle (19).
The role of AMPK in the regulation of adipocyte metabolism has been also initially studied using AICA riboside as an AMPK activator. It was reported to strongly inhibit β-adrenergic and isoproterenol-stimulated lipolysis in adipocytes (9, 20, 21), this being confirmed to be AMPK-mediated by the use of adenovirus-mediated expression of dominant positive and negative forms of AMPK (20). Furthermore, by contrast with liver, fatty acid oxidation was inhibited by AICA riboside in adipocytes (22), an effect completely prevented by compound C which is however not a highly selective AMPK inhibitor. Similarly, although AICA riboside stimulates glucose transport in the skeletal muscle through increased GLUT4 translocation, it inhibits both basal and insulin-stimulated glucose uptake in adipocytes (22). Finally, adipose tissue is also an active endocrine organ producing a variety of cytokines and chemokines. In cultured adipocytes, AICA riboside has been shown to increase the expression of the insulin-sensitizing hormone adiponectin and downregulate the secretion of pro-inflammatory cytokines, such as IL-6, IL-8, TNFα, and MCP-1 (23, 24).
In Vivo
Before the discovery of its effect on AMPK, many studies have shown that treatment with AICA riboside (also named acadesine) was an effective therapy for reducing myocardial ischemic injury in humans, also indicating that the compound is well tolerated and associated with minimal side effects (reviewed in (25)). Interest for AICA riboside rose again when pharmacological studies reported that its administration could also induce hypoglycemia in mice (26), presumably through AMPK activation in various tissues. It was later shown that AICA riboside treatment leads to concomitant increase in muscle glucose uptake and suppression of endogenous glucose production in lean and obese rats (27). Not long afterward, the inactivation of AMPK by a dominant inhibitory mutant in mouse skeletal muscle highlighted the role of AMPK-mediated stimulation of glucose uptake in part of the hypoglycemic effect of AICA riboside (28). To evaluate the respective contribution of the liver, we recently subjected mice lacking both α1 and α2 catalytic subunits in the liver (AMPKα1α2LS−/−) to acute AICA riboside injection. Interestingly, AMPKα1α2LS−/−, but not wild-type mice, showed resistance to AICA riboside-induced hypoglycemia, also indicating a significant contribution of hepatic AMPK in this effect (29).
Chronic treatment with AICA riboside was also shown to reduce intra-abdominal adiposity in obese rats (30) or to induce changes in skeletal muscle histological and metabolic characteristics, leading to a switch from type IIB to type IIX fibers and a significant increase in both glycolytic and oxidative enzyme activities (31). Interestingly, it also improved metabolic disturbances in animal models of type 2 diabetes, at least partly by improving whole body glucose tolerance and insulin sensitivity (30, 32–35). However, a recent study challenged the AMPK-mediated specific effect of AICA riboside on glucose uptake, reporting that its acute infusion in healthy humans is associated with increased skeletal muscle glucose uptake independently of any change in AMPK activity (36). The exact mechanism by which AICA riboside stimulates glucose uptake in human skeletal muscle is still unknown.
Thus, like all pharmacological approaches, it is obvious that results obtained with experiments using AICA riboside must be interpreted with caution and the question remains whether the pleiotropic effects of AICA riboside evidenced in more than 350 publications to date are mediated by AMPK.
AMPK-INDEPENDENT EFFECTS OF AICA RIBOSIDE
It was generally assumed that ZMP stimulates AMPK without affecting cellular levels of adenine nucleotides, including ATP, making AICA riboside a more specific method for activating AMPK than the use of agents inducing ATP depletion. However, we recently challenged this point by showing that AICA riboside, at least in hepatocytes, also decreased intracellular ATP levels at concentrations higher than 100 µM through an AMPK-independent mechanism (37). Indeed, in an attempt to investigate the role of AMPK in the control of hepatic glucose uptake, we elucidated the molecular mechanism involved in the AICA riboside-induced inhibition of glucose phosphorylation previously evidenced in hepatocytes (38). Using isolated hepatocytes from AMPKα1α2LS−/− mice, we demonstrated that AICA riboside inhibited glucose-induced translocation of glucokinase (GK) from the nucleus by an AMPK-independent mechanism linked to its effect on ATP depletion (11). It should be noted that two other recent papers also reported clear AMPK-independent effects of AICA riboside on both hepatic phosphatedylcholine synthesis (39) and autophagic proteolysis (40) that could be related to the nucleoside-induced ATP drop. Importantly, we next reported that AICA riboside induced a time and dose-dependent inhibition of cellular respiration associated with a decrease in cellular ATP concentrations in hepatocytes, both effects being clearly not mediated by AMPK because it persisted in hepatocytes from AMPKα1α2LS−/− mice (37). This inhibition resulted in part from a decrease in intracellular phosphate (Pi), one of the major mitochondrial oxidative phosphorylation (OXPHOS) cofactors, following the ATP-mediated phosphorylation of AICA riboside. On the other hand, accumulation of Z nucleotides may also play a role because we found that concentrations of ZMP in the range of those detected after incubation with AICA riboside exerted a direct inhibitory effect on the mitochondria respiratory chain complex 1 (37). In addition, ZTP also induced uncoupling of mitochondrial OXPHOS, an effect that could also worsen the change in cellular energetic by decreasing the yield of ATP synthesis (37). Altogether, these data demonstrated clearly for the first time that some of the cellular effects of AICA riboside are not necessarily caused by AMPK activation. It is important to note that there are other important AMPK-independent effects often overlooked in the interpretation of the results obtained with AICA riboside. Indeed, ZMP accumulation does also affect many enzymes with AMP-binding sites, such as GK (38), glycogen phosphorylase (41, 42), glycogen synthase (41), or fructose 1,6-bisphosphatase (43), their respective Km for ZMP being generally in the range of the cellular nucleotide concentration. In addition, it has been demonstrated that AICA riboside uptake and phosphorylation into cells could also be blocked by a number of protein kinase inhibitors, thus preventing ZMP accumulation and subsequent AMPK activation ((44) and B. Guigas, unpublished results).
Taken together, it is now clear that AICA riboside is not a specific AMPK activator and that some of the previously proposed functions of AMPK, established through experiments using AICA riboside, are actually mediated by other pathways. Thus, on top of classical genetic approaches (adenoviruses overexpressing negative or constitutively active forms of AMPK, small interfering RNA or knock-out or knock-in mice), it is obvious that more specific and cell-permeable pharmacological AMPK activators are required to investigate cellular functions of AMPK.
IDENTIFICATION AND CHARACTERIZATION OF NOVEL AMPK ACTIVATORS
A-769662
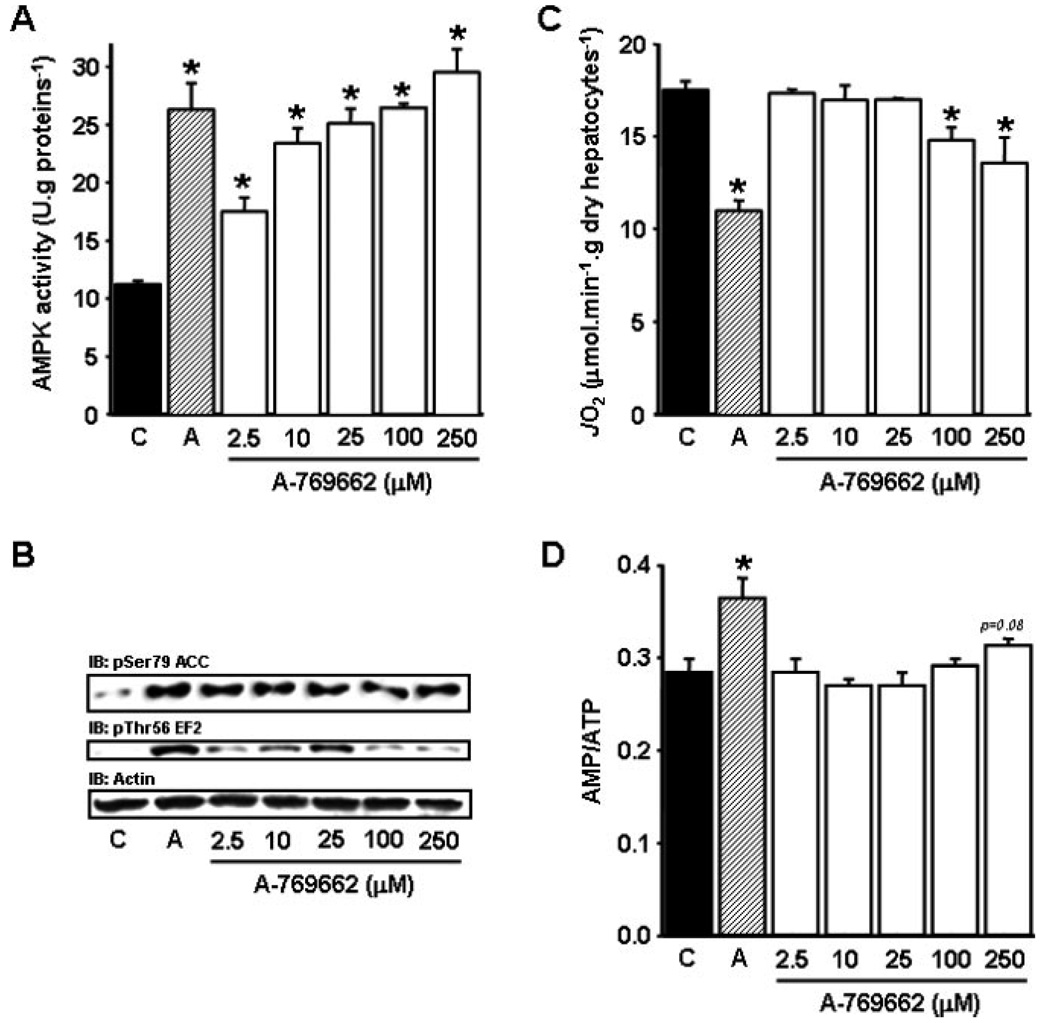
Recently, Cool et al. screened a chemical library of over 700,000 compounds using partially purified AMPKαβγ complex from rat liver and identified a nonnucleoside thienopyridone, A-769662, as a novel AMPK activator (45). Unlike other pharmacological AMPK activators, A-769662 directly activates native rat AMPK in cell-free assays, indicating that it is an allosteric activator. When AICA riboside or A-769662 was added to primary rat hepatocytes, both compounds stimulated ACC phosphorylation and decreased fatty acid synthesis, although A-769662 was ~20-fold more potent than AICA riboside (45). We recently tested A-769662 in primary rat hepatocytes and observed that it induced dose-dependent increase in AMPK activity and phosphorylation of its known downstream targets ACC and EF2 (Figs. 2A and 2B) and the optimal effect was obtained at concentration ~25 µM. More importantly, we found that A-769662 at concentrations below 100 µM, in contrast with AICA riboside (37), did not induce significant inhibition of mitochondrial oxygen consumption rate and increase in AMP:ATP ratio (Figs. 2C and 2D).
Figure 2.
Effects of AICA riboside and A-769662 on AMPK activity, phosphorylation of AMPK downstream targets, cellular oxygen consumption rate, and AMP-on-ATP ratio in isolated rat hepatocytes. Hepatocytes isolated from 24 h-starved rats were incubated at 37 °C in a Krebs/bicarbonate medium supplemented with 20/2 mM lactate/pyruvate and 4 mM octanoate and treated with 1 mM AICA riboside (hatched bars), the indicated concentrations of A-769662 (open bars) or its vehicle (black bars). After 30 min, cell samples were removed for determination of AMPK activity (A), Ser79-ACC and Thr56-EF2 phosphorylation (B) and intracellular adenine nucleotide concentrations (D). The oligomycin-sensitive oxygen consumption rate (JO2, C) was measured in separate experiments before and after the addition of 6 µg/mL oligomycin, as previously described (37). The results are expressed as means ± S.E.M. (n = 3). *P < 0.05 compared with vehicle.
Consistent with its inhibitory effect on ACC in cultured hepatocytes, Cool et al. also demonstrated that acute intraperitoneal injection of A-769662 in Sprague Dawley rats rapidly decreased hepatic malonyl CoA levels. Furthermore, administration of A-769662 for 5 days in diabetic ob/ob mice had several beneficial effects that would be expected from an AMPK activator, including decreased plasma glucose and triglyceride concentrations, lower hepatic triglycerides content, and slightly reduced weight gain (45). This treatment also resulted in decreased hepatic expression of the lipogenic enzyme fatty acid synthase, and of the gluconeogenic enzymes phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (45). However, it can be noted that in this study there was no data demonstrating that AMPK activity and phosphorylation of its downstream targets were stimulated in liver and skeletal muscle in response to acute or chronic treatment with A-769662. Whether major antidiabetic effects of A-769662 are mediated through the activation of AMPK in liver or other tissues are currently unknown and further studies are required.
Recently, Sanders et al. and Goransson et al. have further characterized A-769662 (12, 46). These studies revealed that similar to AMP, A-769662 activates AMPK both allosterically, and by inhibiting Thr-172 dephosphorylation of AMPK by protein phosphatase. Nonetheless, unlike AMP, A-769662 does not seem to bind to the Bateman domains of the γ-subunit (12). Furthermore, A-769662 activates AMPK harboring a mutation in the γ-subunit that abolishes activation by AMP, indicating that AMP and A-769662 stimulate AMPK through distinct mechanism (46). Cell-free studies have illustrated that A-769662 has no effect on an isolated α-subunit kinase domain (12, 46); however, an AMPK complex lacking the glycogen binding domain (GBD) of the β-subunit largely reduces the allosteric effect of A-769662, but not the allosteric activation by AMP (46). Interestingly, a point mutation of Ser-108 to alanine, an autophosphorylation site within the GBD of the β1-subunit, almost completely abolishes activation of AMPK by A-769662 in both cell-free assays and intact cells overexpressing the mutant. Moreover, mutation of Ser-108 caused a partial reduction of AMP-induced dephosphorylation of Thr-172 in cell-free assay and of AMPK activity and Thr-172 phosphorylation in both basal and activated states in cells (46). This indicates that intact β-subunit is required for the full action of A-769662. However, it does not prove that the compound is actually binding to the β-subunit. To more precisely understand the mechanism of action of A-769662, it would be necessary to crystallize the AMPK heterotrimer, complexed to this compound.
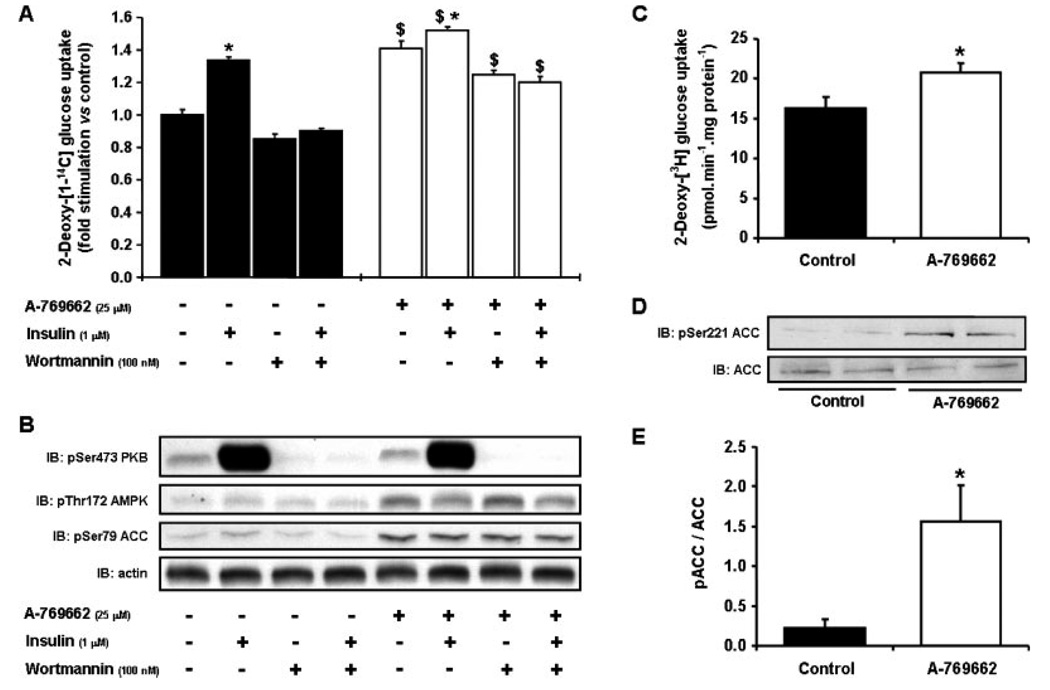
To determine the specificity of A-769662, we screened the compound in cell-free assays against a panel of 76 protein kinases and found that the majority of them are not significantly affected (12). We also found that the addition of A-769662 to mouse embryonic fibroblasts or primary mouse hepatocytes stimulate phosphorylation of ACC, effects that are completely abolished in AMPKα1α2−/− cells. Phosphorylation of AMPK and ACC in response to A-769662 is also abolished in isolated mouse skeletal muscle lacking LKB1, a major upstream kinase for AMPK in this tissue (47). However, in Hela cells, which lack LKB1 but express the alternative upstream kinase CaMKK, phosphorylation of AMPK and ACC in response to A-769662 still occurs. Taken together, these results indicate that in intact cells, the activation of AMPK by A-769662 is independent of upstream kinases utilized. To determine if the activation of AMPK in response to A-769662 results in enhanced glucose uptake in skeletal muscle, the compound was added to both cultured rat L6 myocytes and primary myotubes derived from satellite cells obtained through muscle biopsies of nondiabetic human subjects. We demonstrate here that A-769662 increases both AMPK/ACC phosphorylation and glucose uptake (Figs. 3A and 3B). This effect is additive with insulin and is clearly not mediated by changes in insulin signaling pathways because it was not abolished in the presence of the PI3K inhibitor wortmannin (Figs. 3A and 3B). We also found that A-769662 significantly stimulates glucose uptake (~30%, P < 0.05, Fig. 3C) and ACC phosphorylation (Figs. 3D and 3E) in human muscle cells. It would however be important to determine if A-769662 stimulates muscle glucose uptake in vivo.
Figure 3.
Effects of A-769662 on glucose uptake in L6 cells and human myotubes. Rat L6 myoblasts were differentiated into myotubes and treated with 25 µM A-769662 (black bars) or vehicle (0.1% DMSO, open bars) for 30 min. 2-deoxy-[1-14C]-glucose uptake (A) and Ser473-PKB, Thr172-AMPK, and Ser79-ACC phosphorylation (B) were then measured after preincubation with or without 100 nM wortmanin for 15 min and subsequent addition or not of 1 µM insulin for 20 min, as previously described (48). The results are expressed as means ± S.E.M. (n = 3). *P < 0.05 compared with no insulin; $P < 0.05 compared with vehicle. Primary human myotubes derived from normal glucose-tolerant subjects were treated with 100 µM A-769662 or vehicle (0.1% DMSO) for 1 h, and 2-deoxy-[1-3H]-glucose uptake (C) and Ser221-ACC phosphorylation (D,E) were measured as previously described (49). The results are expressed as means ± S.E.M. (n = 4). *P < 0.05 compared with vehicle.
In addition to the established roles in regulating key metabolic processes, AMPK has been also implicated in the control of cellular growth through the regulation of mTOR complex-1 (mTORC1) signaling pathway in response to energy-depleting stresses (reviewed in (50)). Recently, Huang et al. reported that inhibition of AMPK, resulting from a hypomorphic mutation that decreases LKB1 expression, significantly accelerated tumor development in PTEN-deficient mice in which mTORC1 activity is abnormally upregulated (51). In contrast, activating the AMPK pathway by treating PTEN-deficient mice with A-769662 or metformin/phenformin significantly delayed tumor onset. This study provides genetic evidence that AMPK activators can be effective drugs in suppressing abnormal cell growth/tumors caused by deregulation of mTORC1 pathway.
Taken together, A-769662 is a direct, specific, and potent AMPK activator. Results obtained from the study by Cool et al. (45) and Huang et al. (51) validate the hypothesis that activation of AMPK pathway in vivo is a viable approach for treatment of type 2 diabetes, metabolic diseases, and possibly cancers.
PT1
Very recently, Pang et al. reported a new AMPK activator with a different mechanism of action than A-769662 (52). Indeed, after random screening of a chemical library of 3,600 organic compounds using a truncated (residues 394 to 548) and inactive form of human AMPKα (α1394), they identified a small molecule, called PT1, able to directly activate recombinant human AMPKα1β1γ1 but not other AMPK-related kinases. Interestingly, PT1 also stimulates native AMPK and increases phosphorylation of its downstream target ACC in a dose-dependent manner in either L6 myotubes or HepG2 cells without significant modification of AMP:ATP ratio (52). Moreover, in Hela cells lacking LKB1, phosphorylation of AMPK and ACC in response to PT1 was significantly reduced by addition of STO-609, a CaMKK inhibitor, indicating that in intact cells AMPK activation by PT1 requires upstream kinase(s). As suggested by the authors, and by contrast with A-769662, it seems that PT1 may interact with some residues located near the autoinhibitory domain on the α-subunit and thus directly activates AMPK by relieving the autoinhibition through conformational change of the catalytic subunit of the kinase (52).
Whether all the effects of PT1 are mediated through AMPK is still unknown and it would be crucial to check that the cellular effects observed with this compound are completely abolished in AMPK-deficient systems.
CONCLUDING REMARKS
Although AICA riboside was undoubtly useful to advance our knowledge on AMPK effects during the last decade, we believe that it should be used with considerable cautions because of many AMPK-independent side-effects. New AMPK activators were recently discovered but further studies are still required to better understand their specific effects, notably using AMPK-deficient cell lines and animal models. Elucidation of more complete mechanism of action of these new drugs could also give insight into developing even more potent specific AMPK activators in the future. Nevertheless, it can be noted that these compounds, especially A-769662, hold considerable promise as an improved experimental tool for the study of the role of AMPK on multiple signaling pathways and cellular processes owing to their apparent reduced off targets effects.
ACKNOWLEDGEMENTS
This work was supported by FNRS, the Inter University pole of attraction, the European Union FP6 program (EXGENESIS Integrated Project LSHM-CT-2004-005272), and ALFEDIAM (Association de Langue Française pour l’Etude du Diabète et des Maladies Métaboliques). KS was supported by Diabetes UK (07/0003529) and the UK Medical Research Council and by the companies (AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck & Co. Inc, Merck KGaA and Pfizer). N.M. was supported by NIH grants AG030979 and DK080157, and S.M.R. was supported by NIH grant HL086089. BG was recipient of the ICP “Michel de Visscher” Fellowship. We apologize to all the authors whose primary researches were unable to be cited here and also thank Liliane Maisin, Martine de Cloedt, and Jan Kriek for technical assistance and Natalia Shpiro for the synthesis of A-769662.
Abbreviations
- ACC
acetyl-CoA carboxylase
- AICA riboside
5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside
- AS160
Akt substrate of 160 kDa
- AMPK
AMP-activated protein kinase
- AMPKK
AMPK kinase
- CaMKKβ
calcium/calmodulin-dependent protein kinase kinaseβ
- CBS
cystathionine-β-synthase
- CPT-1
carnitine palmitoyl-transferase-1
- eEF2K
eukaryotic elongation factor 2 kinase
- EF2
eukaryotic elongation factor 2
- GBD
glycogen binding domain
- GK
glucokinase
- HMG-CoA reductase
3-hydroxy-3-methylglutaryl-CoA reductase
- mTOR
mammalian target of rapamycin
- mTORC1
mTOR complex-1
- OXPHOS
oxidative phosphorylation
- PEPCK
phosphoenolpyruvate carboxykinase
- PP2C
protein phosphatase 2C
- SREBP-1c
sterol regulatory element binding protein-1c
- TAK1
transforming growth factor-β-activated kinase
- ZMP
5-aminoimidazole-4-carboxamide ribotide
REFERENCES
- 1.Vincent MF, Bontemps F, Van den Berghe G. Substrate cycling between 5-amino-4-imidazolecarboxamide riboside and its monophosphate in isolated rat hepatocytes. Biochem. Pharmacol. 1996;52:999–1006. doi: 10.1016/0006-2952(96)00413-3. [DOI] [PubMed] [Google Scholar]
- 2.Hardie DG. AMP-activated protein kinase as a drug target. Annu. Rev. Pharmacol. Toxicol. 2007;47:185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- 3.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 4.Winder WW, Thomson DM. Cellular energy sensing and signaling by AMP-activated protein kinase. Cell Biochem. Biophys. 2007;47:332–347. doi: 10.1007/s12013-007-0008-7. [DOI] [PubMed] [Google Scholar]
- 5.Viollet B, Foretz M, Guigas B, Horman S, Dentin R, Bertrand L, Hue L, Andreelli F. Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J. Physiol. 2006;574:41–53. doi: 10.1113/jphysiol.2006.108506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGee SL, Hargreaves M. AMPK and transcriptional regulation. Front. Biosci. 2008;13:3022–3033. doi: 10.2741/2907. [DOI] [PubMed] [Google Scholar]
- 7.Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J. Physiol. 2006;574:73–83. doi: 10.1113/jphysiol.2006.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan JE, Brocklehurst KJ, Marley AE, Carey F, Carling D, Beri RK. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994;353:33–36. doi: 10.1016/0014-5793(94)01006-4. [DOI] [PubMed] [Google Scholar]
- 9.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside—a specific method for activating AMP-activated protein-kinase in intact-cells. Eur. J. Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 10.Henin N, Vincent MF, Gruber HE, Van den Berghe G. Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. Faseb J. 1995;9:541–546. doi: 10.1096/fasebj.9.7.7737463. [DOI] [PubMed] [Google Scholar]
- 11.Guigas B, Bertrand L, Taleux N, Foretz M, Wiernsperger N, Vertommen D, Andreelli F, Viollet B, Hue L. 5-Aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside and metformin inhibit hepatic glucose phosphorylation by an AMP-activated protein kinase-independent effect on glucokinase translocation. Diabetes. 2006;55:865–874. doi: 10.2337/diabetes.55.04.06.db05-1178. [DOI] [PubMed] [Google Scholar]
- 12.Goransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, Viollet B, Hardie DG, Sakamoto K. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J. Biol. Chem. 2007;282:32549–32560. doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 14.Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr. Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- 15.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J. Biol. Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 16.Caligiuri A, Bertolani C, Guerra CT, Aleffi S, Galastri S, Trap-poliere M, Vizzutti F, Gelmini S, Laffi G, Pinzani M, Marra F. Adenosine monophosphate-activated protein kinase modulates the activated phenotype of hepatic stellate cells. Hepatology. 2008;47:668–676. doi: 10.1002/hep.21995. [DOI] [PubMed] [Google Scholar]
- 17.Jorgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, Richter EA, Wojtaszewski JF. The alpha2-5’AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes. 2004;53:3074–3081. doi: 10.2337/diabetes.53.12.3074. [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto K, Holman GDBP. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am. J. Physiol. Endocrinol. Metab. 2008;295:E29–E37. doi: 10.1152/ajpendo.90331.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes. 2006;55:2067–2076. doi: 10.2337/db06-0150. [DOI] [PubMed] [Google Scholar]
- 20.Daval M, Diot-Dupuy F, Bazin R, Hainault I, Viollet B, Vaulont S, Hajduch E, Ferre P, Foufelle F. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J. Biol. Chem. 2005;280:25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan JE, Carey F, Carling D, Beri RK. Characterisation of 5’-AMP-activated protein kinase in human liver using specific peptide substrates and the effects of 5’-AMP analogues on enzyme activity. Biochem. Biophys. Res. Commun. 1994;200:1551–1556. doi: 10.1006/bbrc.1994.1627. [DOI] [PubMed] [Google Scholar]
- 22.Gaidhu MP, Fediuc S, Ceddia RB. 5-Aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside-induced AMP-activated protein kinase phosphorylation inhibits basal and insulin-stimulated glucose uptake, lipid synthesis, and fatty acid oxidation in isolated rat adipocytes. J. Biol. Chem. 2006;281:25956–25964. doi: 10.1074/jbc.M602992200. [DOI] [PubMed] [Google Scholar]
- 23.Lihn AS, Jessen N, Pedersen SB, Lund S, Richelsen B. AICAR stimulates adiponectin and inhibits cytokines in adipose tissue. Biochem. Biophys. Res. Commun. 2004;316:853–858. doi: 10.1016/j.bbrc.2004.02.139. [DOI] [PubMed] [Google Scholar]
- 24.Sell H, Dietze-Schroeder D, Eckardt K, Eckel J. Cytokine secretion by human adipocytes is differentially regulated by adiponectin, AICAR, and troglitazone. Biochem. Biophys. Res. Commun. 2006;343:700–706. doi: 10.1016/j.bbrc.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Mullane K. Acadesine: the prototype adenosine regulating agent for reducing myocardial ischaemic injury. Cardiovasc. Res. 1993;27:43–47. doi: 10.1093/cvr/27.1.43. [DOI] [PubMed] [Google Scholar]
- 26.Vincent MF, Erion MD, Gruber HE, Van den Berghe G. Hypoglycaemic effect of AICAriboside in mice. Diabetologia. 1996;39:1148–1155. doi: 10.1007/BF02658500. [DOI] [PubMed] [Google Scholar]
- 27.Bergeron R, Previs SF, Cline GW, Perret P, Russell RR, III, Young LH, Shulman GI. Effect of 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes. 2001;50:1076–1082. doi: 10.2337/diabetes.50.5.1076. [DOI] [PubMed] [Google Scholar]
- 28.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction-and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 29.Viollet B, Athea Y, Mounier R, Guigas B, Zarrinpashneh E, Horman S, Lantier S, Devin-Leclerc J, Beauloye C, Foretz M, Andreelli F, Ventura-Clapier R, Bertrand L. AMPK: lessons from transgenic and knockout animals. Front. Biosci. doi: 10.2741/3229. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buhl ES, Jessen N, Pold R, Ledet T, Flyvbjerg A, Pedersen SB, Pedersen O, Schmitz O, Lund S. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes. 2002;51:2199–2206. doi: 10.2337/diabetes.51.7.2199. [DOI] [PubMed] [Google Scholar]
- 31.Suwa M, Nakano H, Kumagai S. Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles. J. Appl. Physiol. 2003;95:960–968. doi: 10.1152/japplphysiol.00349.2003. [DOI] [PubMed] [Google Scholar]
- 32.Fiedler M, Zierath JR, Selen G, Wallberg-Henriksson H, Liang Y, Sakariassen KS. 5-aminoimidazole-4-carboxy-amide-1-beta-d-ribofuranoside treatment ameliorates hyperglycaemia and hyper-insulinaemia but not dyslipidaemia in KKAy-CETP mice. Diabetologia. 2001;44:2180–2186. doi: 10.1007/s001250100027. [DOI] [PubMed] [Google Scholar]
- 33.Halseth AE, Ensor NJ, White TA, Ross SA, Gulve EA. Acute and chronic treatment of ob/ob and db/db mice with AICAR decreases blood glucose concentrations. Biochem. Biophys. Res. Commun. 2002;294:798–805. doi: 10.1016/S0006-291X(02)00557-0. [DOI] [PubMed] [Google Scholar]
- 34.Iglesias MA, Ye JM, Frangioudakis G, Saha AK, Tomas E, Ruderman NB, Cooney GJ, Kraegen EW. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002;51:2886–2894. doi: 10.2337/diabetes.51.10.2886. [DOI] [PubMed] [Google Scholar]
- 35.Pold R, Jensen LS, Jessen N, Buhl ES, Schmitz O, Flyvbjerg A, Fujii N, Goodyear LJ, Gotfredsen CF, Brand CL, Lund S. Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes. 2005;54:928–934. doi: 10.2337/diabetes.54.4.928. [DOI] [PubMed] [Google Scholar]
- 36.Cuthbertson DJ, Babraj JA, Mustard KJ, Towler MC, Green KA, Wackerhage H, Leese GP, Baar K, Thomason-Hughes M, Sutherland C, Hardie DG, Rennie MJ. 5-aminoimidazole-4-carboxamide 1-beta-d-ribofuranoside acutely stimulates skeletal muscle 2-deoxyglucose uptake in healthy men. Diabetes. 2007;56:2078–2084. doi: 10.2337/db06-1716. [DOI] [PubMed] [Google Scholar]
- 37.Guigas B, Taleux N, Foretz M, Detaille D, Andreelli F, Viollet B, Hue L. AMP-activated protein kinase-independent inhibition of hepatic mitochondrial oxidative phosphorylation by AICA riboside. Biochem. J. 2007;404:499–507. doi: 10.1042/BJ20070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vincent MF, Bontemps F, Van den Berghe G. Inhibition of glycolysis by 5-amino-4-imidazolecarboxamide riboside in isolated rat hepatocytes. Biochem. J. 1992;281(Pt 1):267–272. doi: 10.1042/bj2810267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobs RL, Lingrell S, Dyck JR, Vance DE. Inhibition of hepatic phosphatidylcholine synthesis by 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAr) is independent of AMP-activated protein kinase (AMPK) activation. J. Biol. Chem. 2006;19:M605702200. doi: 10.1074/jbc.M605702200. [DOI] [PubMed] [Google Scholar]
- 40.Meley D, Bauvy C, Houben-Weerts JH, Dubbelhuis PF, Helmond MT, Codogno P, Meijer AJ. AMP-activated protein kinase and the regulation of autophagic proteolysis. J. Biol. Chem. 2006;281:34870–34879. doi: 10.1074/jbc.M605488200. [DOI] [PubMed] [Google Scholar]
- 41.Longnus SL, Wambolt RB, Parsons HL, Brownsey RW, Allard MF. 5-Aminoimidazole-4-carboxamide 1-beta-d-ribofuranoside (AICAR) stimulates myocardial glycogenolysis by allosteric mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R936–R944. doi: 10.1152/ajpregu.00319.2002. [DOI] [PubMed] [Google Scholar]
- 42.Shang J, Lehrman MA. Activation of glycogen phosphorylase with 5-aminoimidazole-4-carboxamide riboside (AICAR). Assessment of glycogen as a precursor of mannosyl residues in glycoconjugates. J. Biol. Chem. 2004;279:12076–12080. doi: 10.1074/jbc.M400431200. [DOI] [PubMed] [Google Scholar]
- 43.Vincent MF, Marangos PJ, Gruber HE, Van den Berghe G. Inhibition by AICA riboside of gluconeogenesis in isolated rat hepatocytes. Diabetes. 1991;40:1259–1266. doi: 10.2337/diab.40.10.1259. [DOI] [PubMed] [Google Scholar]
- 44.Fryer LG, Parbu-Patel A, Carling D. Protein kinase inhibitors block the stimulation of the AMP-activated protein kinase by 5-amino-4-imidazolecarboxamide riboside. FEBS Lett. 2002;531:189–192. doi: 10.1016/s0014-5793(02)03501-9. [DOI] [PubMed] [Google Scholar]
- 45.Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, Zhao G, Marsh K, Kym P, Jung P, Camp HS, Frevert E. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Sanders MJ, Ali ZS, Hegarty BD, Heath R, Snowden MA, Carling D. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J. Biol. Chem. 2007;282:32539–32548. doi: 10.1074/jbc.M706543200. [DOI] [PubMed] [Google Scholar]
- 47.Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. Embo J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bazuine M, Carlotti F, Rabelink MJ, Vellinga J, Hoeben RC, Maassen JA. The p38 mitogen-activated protein kinase inhibitor SB203580 reduces glucose turnover by the glucose transporter-4 of 3T3-L1 adipocytes in the insulin-stimulated state. Endocrinology. 2005;146:1818–1824. doi: 10.1210/en.2004-1347. [DOI] [PubMed] [Google Scholar]
- 49.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 50.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 51.Huang X, Wullschleger S, Shpiro N, McGuire VA, Sakamoto K, Woods YL, McBurnie W, Fleming S, Alessi DR. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem. J. 2008;412:211–221. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- 52.Pang T, Zhang ZS, Gu M, Qiu BY, Yu LF, Cao PR, Shao W, Su MB, Li JY, Nan FJ, Li J. Small molecule antagonizes autoinhibition and activates AMP-activated protein kinase in cells. J. Biol. Chem. 2008;283:16051–16060. doi: 10.1074/jbc.M710114200. [DOI] [PMC free article] [PubMed] [Google Scholar]