Abstract
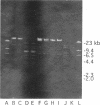
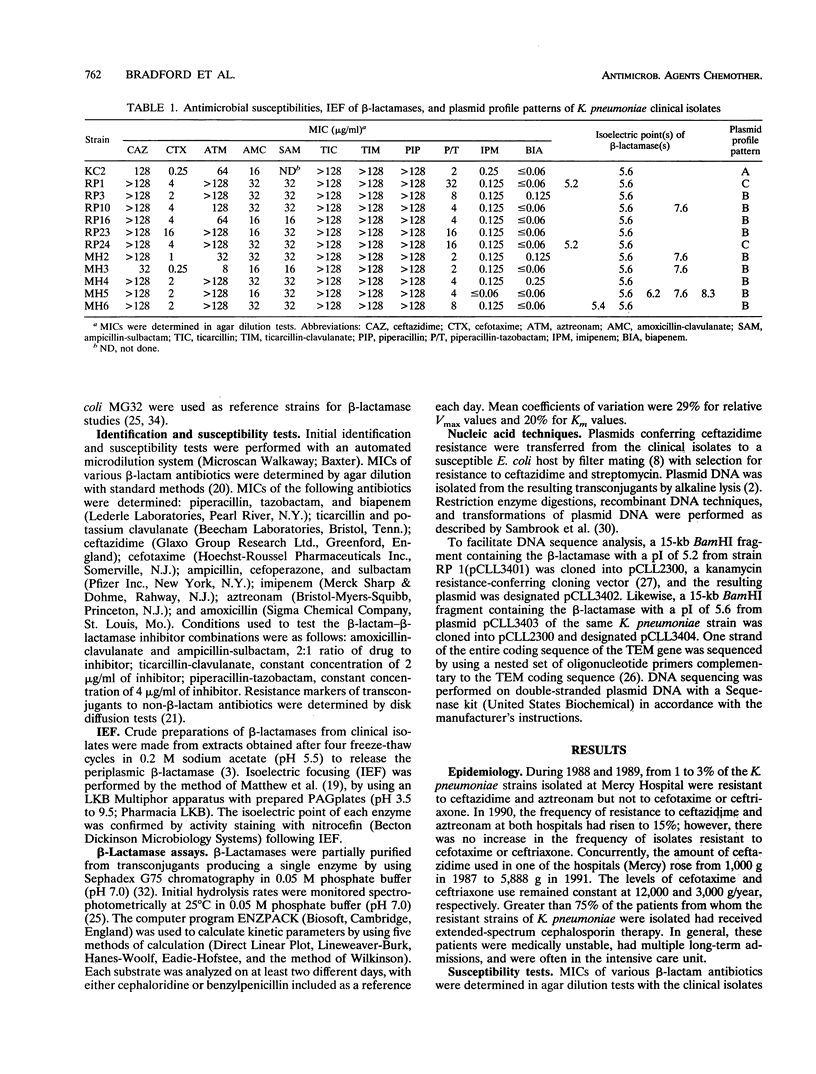
Ceftazidime-resistant Klebsiella pneumoniae strains began to appear when ceftazidime usage was increased in two unrelated Chicago hospitals. These strains produced a beta-lactamase with an isoelectric point of 5.6 (RP-5.6) and strong hydrolyzing activity against ceftazidime. Two different restriction digest profiles were associated with the ceftazidime resistance plasmids. A second beta-lactamase with a pI of 5.2 (RP-5.2) was coproduced in two representative strains. The second beta-lactamase hydrolyzed ceftazidime, cefotaxime, and aztreonam with relative hydrolysis rates of < 8% of that observed for benzylpenicillin. Both enzymes were inhibited by clavulanic acid and tazobactam. Nucleotide sequencing of the genes coding for RP-5.2 and RP-5.6 revealed sequences identical to those of the TEM-12 and TEM-10 beta-lactamase genes, respectively. Both genes were derived from a TEM-1 sequence related to that of the gene encoded on the Tn2 transposon. Single point mutations are required to progress from TEM-1 to TEM-12 and from TEM-12 to TEM-10. Extracts from broths grown from single cell isolates of the strain producing TEM-12 and TEM-10 were shown to contain both enzymes. Transconjugants producing either the TEM-12 or the TEM-10 beta-lactamase were obtained. A significant finding was that both enzymes were encoded by plasmids with identical restriction digest patterns. These studies show that mutations leading to extended-spectrum beta-lactamases can occur sequentially in the same organism, with the genes encoding both enzymes maintained stably.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Coulson A. F., Frère J. M., Ghuysen J. M., Joris B., Forsman M., Levesque R. C., Tiraby G., Waley S. G. A standard numbering scheme for the class A beta-lactamases. Biochem J. 1991 May 15;276(Pt 1):269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K., Singer S. B. Effective cooling allows sonication to be used for liberation of beta-lactamases from gram negative bacteria. J Antimicrob Chemother. 1989 Jul;24(1):82–84. doi: 10.1093/jac/24.1.82. [DOI] [PubMed] [Google Scholar]
- Chen S. T., Clowes R. C. Variations between the nucleotide sequences of Tn1, Tn2, and Tn3 and expression of beta-lactamase in Pseudomonas aeruginosa and Escherichia coli. J Bacteriol. 1987 Feb;169(2):913–916. doi: 10.1128/jb.169.2.913-916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Champs C., Sauvant M. P., Chanal C., Sirot D., Gazuy N., Malhuret R., Baguet J. C., Sirot J. Prospective survey of colonization and infection caused by expanded-spectrum-beta-lactamase-producing members of the family Enterobacteriaceae in an intensive care unit. J Clin Microbiol. 1989 Dec;27(12):2887–2890. doi: 10.1128/jcm.27.12.2887-2890.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goussard S., Courvalin P. Sequence of the genes blaT-1B and blaT-2. Gene. 1991 Jun 15;102(1):71–73. doi: 10.1016/0378-1119(91)90540-r. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Kontomichalou P., Smith J. T. Molecular specificities of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1974 Jan;117(1):56–62. doi: 10.1128/jb.117.1.56-62.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heritage J., Hawkey P. M., Todd N., Lewis I. J. Transposition of the gene encoding a TEM-12 extended-spectrum beta-lactamase. Antimicrob Agents Chemother. 1992 Sep;36(9):1981–1986. doi: 10.1128/aac.36.9.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A., Medeiros A. A. More extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1991 Sep;35(9):1697–1704. doi: 10.1128/aac.35.9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A., Medeiros A. A., O'Brien T. F., Pinto M. E., Jiang H. Broad-spectrum, transmissible beta-lactamases. N Engl J Med. 1988 Sep 15;319(11):723–724. doi: 10.1056/NEJM198809153191114. [DOI] [PubMed] [Google Scholar]
- Jarlier V., Nicolas M. H., Fournier G., Philippon A. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988 Jul-Aug;10(4):867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- Legrand P., Fournier G., Buré A., Jarlier V., Nicolas M. H., Decré D., Duval J., Philippon A. Detection of extended broad-spectrum beta-lactamases in Enterobacteriaceae in four French hospitals. Eur J Clin Microbiol Infect Dis. 1989 Jun;8(6):527–529. doi: 10.1007/BF01967473. [DOI] [PubMed] [Google Scholar]
- Liu P. Y., Gur D., Hall L. M., Livermore D. M. Survey of the prevalence of beta-lactamases amongst 1000 gram-negative bacilli isolated consecutively at the Royal London Hospital. J Antimicrob Chemother. 1992 Oct;30(4):429–447. doi: 10.1093/jac/30.4.429. [DOI] [PubMed] [Google Scholar]
- Mabilat C., Courvalin P. Development of "oligotyping" for characterization and molecular epidemiology of TEM beta-lactamases in members of the family Enterobacteriaceae. Antimicrob Agents Chemother. 1990 Nov;34(11):2210–2216. doi: 10.1128/aac.34.11.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Naumovski L., Quinn J. P., Miyashiro D., Patel M., Bush K., Singer S. B., Graves D., Palzkill T., Arvin A. M. Outbreak of ceftazidime resistance due to a novel extended-spectrum beta-lactamase in isolates from cancer patients. Antimicrob Agents Chemother. 1992 Sep;36(9):1991–1996. doi: 10.1128/aac.36.9.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippon A., Ben Redjeb S., Fournier G., Ben Hassen A. Epidemiology of extended spectrum beta-lactamases. Infection. 1989 Sep-Oct;17(5):347–354. doi: 10.1007/BF01650727. [DOI] [PubMed] [Google Scholar]
- Philippon A., Labia R., Jacoby G. Extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1989 Aug;33(8):1131–1136. doi: 10.1128/aac.33.8.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J. P., Miyashiro D., Sahm D., Flamm R., Bush K. Novel plasmid-mediated beta-lactamase (TEM-10) conferring selective resistance to ceftazidime and aztreonam in clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother. 1989 Sep;33(9):1451–1456. doi: 10.1128/aac.33.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen B. A., Bradford P. A., Quinn J. P., Wiener J., Weinstein R. A., Bush K. Genetically diverse ceftazidime-resistant isolates from a single center: biochemical and genetic characterization of TEM-10 beta-lactamases encoded by different nucleotide sequences. Antimicrob Agents Chemother. 1993 Sep;37(9):1989–1992. doi: 10.1128/aac.37.9.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen B. A., Gluzman Y., Tally F. P. Cloning and sequencing of the class B beta-lactamase gene (ccrA) from Bacteroides fragilis TAL3636. Antimicrob Agents Chemother. 1990 Aug;34(8):1590–1592. doi: 10.1128/aac.34.8.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice L. B., Marshall S. H., Carias L. L., Sutton L., Jacoby G. A. Sequences of MGH-1, YOU-1, and YOU-2 extended-spectrum beta-lactamase genes. Antimicrob Agents Chemother. 1993 Dec;37(12):2760–2761. doi: 10.1128/aac.37.12.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice L. B., Willey S. H., Papanicolaou G. A., Medeiros A. A., Eliopoulos G. M., Moellering R. C., Jr, Jacoby G. A. Outbreak of ceftazidime resistance caused by extended-spectrum beta-lactamases at a Massachusetts chronic-care facility. Antimicrob Agents Chemother. 1990 Nov;34(11):2193–2199. doi: 10.1128/aac.34.11.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Bonner D. P., Bush K., Georgopapadakou N. H. Azthreonam (SQ 26,776), a synthetic monobactam specifically active against aerobic gram-negative bacteria. Antimicrob Agents Chemother. 1982 Jan;21(1):85–92. doi: 10.1128/aac.21.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedel G., Mabilat C., Goussard S., Picard B., Fournier G., Gilly L., Paul G., Philippon A. Two variants of transferrable extended-spectrum TEM-beta-lactamase successively isolated from a clinical Escherichia coli isolate. FEMS Microbiol Lett. 1992 Jun 1;72(2):161–166. doi: 10.1016/0378-1097(92)90522-p. [DOI] [PubMed] [Google Scholar]
- Weber D. A., Sanders C. C., Bakken J. S., Quinn J. P. A novel chromosomal TEM derivative and alterations in outer membrane proteins together mediate selective ceftazidime resistance in Escherichia coli. J Infect Dis. 1990 Aug;162(2):460–465. doi: 10.1093/infdis/162.2.460. [DOI] [PubMed] [Google Scholar]
- de Champs C., Sirot D., Chanal C., Poupart M. C., Dumas M. P., Sirot J. Concomitant dissemination of three extended-spectrum beta-lactamases among different Enterobacteriaceae isolated in a French hospital. J Antimicrob Chemother. 1991 Apr;27(4):441–457. doi: 10.1093/jac/27.4.441. [DOI] [PubMed] [Google Scholar]
- el Solh N., Allignet J., Bismuth R., Buret B., Fouace J. M. Conjugative transfer of staphylococcal antibiotic resistance markers in the absence of detectable plasmid DNA. Antimicrob Agents Chemother. 1986 Jul;30(1):161–169. doi: 10.1128/aac.30.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]