Abstract
BACKGROUND
The accumulation of reactive oxygen species and subsequent oxidative DNA damage underlie the development of Barrett's esophagus (BE) and its progression to Barrett's dysplasia (BD) and adenocarcinoma (BAC).
METHODS
We systematically analyzed the promoter regions of 23 genes of the Glutathione S-transferase (GST) and Glutathione peroxidase (GPX) families. Quantitative bisulfite pyrosequencing, real-time RT-PCR (qRT-PCR), Western blot, and immunohistochemical (IHC) analysis methods were utilized in this study.
RESULTS
We identified 14 genes that have CpG islands around their transcription start sites; GSTs (M2-M5, A4, P1, Z1, T2, O1-O2) and GPXs (GPX1, GPX3, GPX4, GPX7). Analysis of an initial set of 20 primary samples demonstrated promoter DNA hypermethylation and mRNA down-regulation of GPX3, GPX7, GSTM2, GSTM3, and GSTM5 in more than half of the BACs samples. Further analysis of 159 primary human samples (37 normal, 11 BE, 11 BD, and 100 BACs) indicated frequent hypermethylation (≥10% methylation) of GPX3 (62%), GPX7 (67%), GSTM2 (69.1%), and GSTM3 (15%) in BACs. A significant inverse correlation between DNA methylation and mRNA expression level was shown for GPX3 (P<.0001), GPX7 (P=.002), GSTM2 (P<.0001), and GSTM5 (P=.01). Treatment of esophageal cancer cell lines with 5-Aza-2’-deoxycytidine and Trichostatin-A led to reversal of the methylation pattern and re-expression of these genes at the mRNA and protein levels. The IHC analysis of GPX3, GPX7, and GSTM2 on a tissue microarray that contained 75 BACs with normal squamous esophageal samples demonstrated an absent-to-weak staining in tumors (52% for GPX3, 57% for GPX7, and 45% for GSTM2) and a moderate-to-strong immunostaining in normal samples.
CONCLUSION
Epigenetic inactivation of members of the glutathione pathway can be an important mechanism in Barrett's tumorigenesis.
Keywords: DNA methylation, Glutathione-S-Transferases, Glutathione Peroxidases, Esophagus, Barrett's adenocarcinoma
Introduction
It is generally accepted that esophageal adenocarcinoma, a cancer with one of the fastest growing incidence rates among all tumors in the Western world, develops from a premalignant lesion of Barrett's esophagus [1, 2, 3]. Barrett's esophagus (BE) is an acquired condition in which the normal squamous cell epithelium of the esophagus is replaced by a metaplastic columnar epithelium [4, 5]. Chronic gastroesophageal reflux disease (GERD), with accumulation of reactive oxygen species (ROS) and subsequent oxidative DNA damage, are the main risk factors for the development of Barrett's esophagus (BE) and its progression to Barrett's adenocarcinoma (BAC) [4, 5]. Although several molecular changes have been demonstrated in tumor initiation and progression [6, 7, 8], the contribution of epigenetic mechanisms in Barrett's tumorigenesis is not well characterized.
There are increasing evidences demonstrating that oxidative injury due to H2O2 and other endogenous ROS are a major cause of DNA damage that correlate with multiple human diseases including cancer [9, 10, 11]. Recent studies have shown an increase in the production of mucosal ROS and subsequent oxidative DNA stress in Barrett's mucosa, suggesting that ROS and subsequent oxidative stress may play an important role in cell damage and the carcinogenesis of Barrett's esophagus [12, 13, 14, 15]. The rat esophago-duodenal anastomosis model has also provided a strong support for the concept that oxidative DNA damage is an important factor for the development of BE and progression to adenocarcinoma [16, 17].
Normal cells can handle the oxidative stress through intact antioxidative systems, among which the Glutathione S-transferase superfamily and Glutathione peroxidase family members play a crucial role. Glutathione S-transferases (GSTs) constitute a superfamily of ubiquitous, multifunctional enzymes, which play a key role in cellular detoxification, protecting macromolecules from attack of reactive electrophiles [18, 19, 20]. The GSTs catalyze the conjugation of the tripeptide glutathione to a wide variety of exogenous and endogenous chemicals with electrophilic functional groups (e.g. products of oxidative stress, environmental pollutants, and carcinogens), thereby neutralizing their electrophilic sites and rendering the products more water-soluble. Glutathione peroxidases (GPXs) catalyze the reduction of hydrogen peroxide, organic hydroperoxide, and lipid peroxides by reduced glutathione, thereby protecting cells against oxidative damage [21, 22, 23].
DNA methylation plays an important role in the regulation of gene expression. Aberrant DNA methylation, namely overall DNA hypomethylation and regional DNA hypermethylation has been linked to carcinogenesis of various organs [24, 25, 26]. Aberrant DNA methylation of CpG islands in the promoter region has been associated with gene silencing of several genes in cancer such as p16, hMLH1 and CDH1 genes [27, 28, 29]. Dysfunction of the GSTP1 gene through aberrant DNA methylation has been demonstrated in several human tumors, especially in prostate carcinoma [30, 31]. These reports suggest that aberrant DNA methylation may be a common and crucial mechanism for the dysfunction of antioxidative members. In this study, we have examined the DNA methylation status of the GSTs and GPXs in a systematic fashion using state-of-the-art quantitative bisulfite pyrosequencing technology (Biotage, Uppsala, Sweden) which allows quantification and comparison of the methylation levels of individual CpG sites in a large panel of samples.
Materials and Methods
Search for the CpG islands in the promoter DNA regions of the GSTs and GPXs
The DNA sequences around the transcription start sites (from −1000 to + 300 bp), that usually contain a promoter region, were obtained from the UCSC Genome Browser web site (http://genome.ucsc.edu/) and were confirmed identical to that from the DBTSS (Database of transcriptional start sites, http://dbtss.hgc.jp). The CpG islands were searched using a CpG island searcher online tool (http://www.uscnorris.com/cpgislands2/cpg.aspx). The criteria used for the definition of CpG islands as the regions of DNA larger than 500 bp with a G+C equal to or greater than 55% with an observed CpG/expected CpG of 0.65. Genes with a CpG island in their promoter regions were included in the present study; GSTA4, GSTM1, GSTM2, GSTM3, GSTM4, GSTM5, GSTO1, GSTO2, GSTP1, GSTT1, GSTT2, GSTZ1, GPX1, GPX3, GPX4, and GPX7.
Tissue Samples
All tissue samples were obtained from the archives of pathology at Vanderbilt University (Nashville, TN, USA), Otto-von-Guericke University (Magdeburg, Germany), and from the National Cancer Institute Cooperative Human Tissue Network (CHTN). The use of specimens from the tissue repository was approved by the Institutional Review Board protocol numbers 03-1078 and 33-2001. All patients provided a written consent and samples were collected after surgical resection. All tissue samples that were included in this study were collected from tissues that remained after completion of diagnosis, that are otherwise discarded, and were coded. All personal identifiers were removed prior to receiving samples. For DNA and mRNA analysis, 131 coded frozen tissue samples (91 BACs, 3 BE, 20 normal esophagus, and 17 normal stomach samples) were collected. In addition, 28 paraffin-embedded formalin-fixed tissue samples were obtained from 11 patients: 8 Barrett's esophagus without dysplasia, 11 Barrett's high-grade dysplasia, and 9 BACs. The normal esophagus and stomach samples were taken from tumor-free margins of resected tumors and were histologically normal. Histopathological diagnosis of the Barrett's esophagus, dysplasia and adenocarcinoma was verified on the basis of H & E-stained sections according to the Vienna classification of gastrointestinal epithelial neoplasia [32]. All tissue samples were dissected to obtain ≥70% cell purity. For immunohistochemical analysis of protein expression, a tissue microarray containing 93 samples of 75 tumors, 6 Barrett's esophagus, and 12 normal esophageal tissues was developed. All adenocarcinomas were classified according to the recent guidelines of the International Union Against Cancer (UICC) TNM classification system. All Barrett's adenocarcinomas were originated from the lower esophagus or gastroesophageal junction corresponding to AEG Type 1 adenocarcinoma [33]. The patients’ ages ranged from 34-84 years (median at 65 years). The adenocarcinomas ranged from well-differentiated to poorly-differentiated, stages I to IV, with a mix of intestinal- and diffuse-type tumors.
Quantitative real-time RT-PCR (qRT-PCR) analyses of GSTs and GPXs
mRNA expression of the GST and GPX family members that contain a CpG island within their promoter (GSTA4, GSTM2, GSTM3, GSTM4, GSTM5, GSTO1, GSTO2, GSTP1, GSTT2, GSTZ1, GPX1, GPX3, GPX4, and GPX7) were first evaluated in an initial set of 20 primary samples (10 tumors and 10 normal samples from the same patients). Following the results, mRNA was examined in 96 frozen primary human samples that included 30 normal mucosae of the esophagus and the stomach and 66 samples of Barrett's adenocarcinoma. These 96 samples were also included in the original 131 frozen samples that were used in the methylation assay below. Total RNA was isolated using the RNeasy mini kit (Qiagen, Valencia, CA), and single-stranded cDNA was subsequently synthesized using the Advantage™ RT-for-PCR Kit (Clontech, Palo Alto, CA). The primers were designed using the online software, Primer 3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). The forward and reverse primers were designed to span two different exons. The oligos were obtained from Integrated DNA Technologies (IDT, Coralville, IA) and the oligos’ sequences are shown in Supplementary Table 1. The qRT-PCR was performed using an iCycler (Bio-Rad, Hercules, CA), with the threshold cycle number determined by use of iCycler software version 3.0. Reactions were performed in triplicate, and the threshold numbers were averaged. The results of these genes were normalized to HPRT1, which had minimal variation in all normal and tumor samples tested [34]. Expression fold was calculated according to the formula 2(Rt-Et)/2(Rn-En) [34], where Rt is the threshold cycle number for the reference gene observed in the tumor, Et is the threshold cycle number for the experimental gene observed in the tumor, Rn is the threshold cycle number for the reference gene observed in the normal samples, and En is the threshold cycle number for the reference gene observed in the tumor. Rn and En values were calculated as an average of the 30 normal samples. For all the primary BACs, the gene was considered to be down-regulated if the mRNA expression fold was ≤0.5 in comparison with the normal samples.
DNA bisulfite treatment and pyrosequencing analysis
DNA was purified using a DNeasy tissue kit (Qiagen, Valencia, CA). The bisulfite modification of the DNA from cell lines and frozen tissues was performed using an EZ DNA Methylation-Gold Kit (ZYMO Research, Orange, CA), and DNA from paraffin-embedded formalin-fixed tissues was performed using CpGenome DNA Modification Kit (CHEMICON, Temecula, CA), according to the manufacturer's protocol. 20 ng of modified DNA was subjected to PCR amplification of the specific promoter region containing a CpG island, by use of a primer set designed to amplify both methylated and unmethylated sequences of selected genes. The primers were designed using PSQ assay design software (Biotage); where one of the primers was biotin-labeled. The primer sequences are provided in Supplementary Table 2. The Platinum PCR SurperMix High Fidelity (Invitrogen, Carlsbad, CA) was used to prepare the PCR reaction solution. The resultant PCR products were checked by gel-electrophoresis to confirm the size of the product and rule out the formation of primer dimers. The specific PCR products were then subjected to quantitative pyrosequencing analysis using a Biotage PyroMark MD system (Biotage) following the protocol provided by the manufacturer. The results were analyzed by Pyro Q-CpG 1.0.9 software (Biotage). Based on control normal samples and internal quality controls provided in the software analysis, we used 10% as a cut off for identification of DNA methylation for all of the genes. Statistical analysis was performed to detect significant changes in the frequencies of DNA methylation of CpG sites between tumor and normal samples.
5-Aza-2’ deoxycytidine (5-Aza) and Trichostatin A (TSA) treatment
For validation of the role of DNA methylation in transcriptional regulation in vitro, three esophageal cancer cell lines (TE7, SKGT4 and OE33) were maintained in DMEM media, supplemented with 10% fetal bovine serum (FBS) and antibiotics (Invitrogen, Carlsbad, CA). Cells were seeded at low density for 24 hours, and then treated with 2 μM 5-Aza (Sigma-Aldrich, St. Louis, MO) for 72 hours and/or 100 nM TSA (Wako, Osaka, Japan) for 24 hours. Total RNA and DNA were isolated and purified by QIAGEN RNeasy kit and DNeasy tissue kit (QIAGEN) as described above. DNA methylation levels of specific genes in the samples before and after treatments were determined by pyrosequencing and mRNA expression levels were determined by qRT-PCR as described above.
Western blotting analysis
Cell lysates from SKGT4 cancer cell line was obtained following the treatment of cells with DMSO (control), 5-Aza or 5-Aza/TSA as described above. Cell lysates were prepared in phosphate saline buffer (PBS) containing 1x protease cocktail inhibitor (Pierce, Rockford, IL), centrifuged at 3500 rpm for 10 minutes at 4°C. Protein concentration was measured using a Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). Protein (15μg) from each sample was subjected to SDS-PAGE and transferred onto nitro-cellulose membrane. Target proteins were detected following standard protocols by using specific antibodies against GPX3 (mouse anti-GPX3 monoclonal antibody, Clone 23B1, Abcam Inc, Cambridge, MA), GPX7 (mouse anti-GPX7 monoclonal antibody, Clone 2704, GeneTeX, San Antonio, TX), GSTM2 (Goat anti-GSTM2/M1 polyclonal antibody, Imagenex, San Diego, CA), and □-actin (rabbit anti-□-actin, clone 13E5, Cell Signaling, Beverly, MA).
Immunohistochemical analysis
For immunohistochemical analysis of protein expression, a tissue microarray containing 93 samples that included 75 tumors, 6 Barrett's esophagus, and 12 normal esophageal tissues was developed. All tissue samples were histologically verified and representative regions were selected for inclusion in the tissue microarray. All of the adenocarcinoma samples were collected from either the gastroesophageal junction or lower esophagus and ranged from well-differentiated to poorly-differentiated, stages I to IV, with a mix of intestinal- and diffuse-type tumors. Tissue cores with a diameter of 0.5 mm were retrieved from the selected regions of the donor blocks and punched to the recipient block using a manual tissue array instrument (Beecher Instruments, Silver Spring, MD, USA). Each tissue sample was represented by four tissue cores on the tissue microarray. Sections (5 μm) were transferred to polylysine-coated slides (SuperFrostPlus, Menzel-Gläser, Braunschweig, Germany) and incubated at 37°C for 2 hours. The resulting microarray was used for immunohistochemical analysis utilizing antibodies against GPX3 (mouse anti-GPX3 monoclonal antibody, Clone 23B1, 1:100, Abcam Inc, Cambridge, MA), GPX7 (mouse anti-GPX7 monoclonal antibody, Clone 2704, 1:500,GeneTeX, San Antonio, TX), and GSTM2 (Goat anti-GSTM2/M1 polyclonal antibody,1:200, Imagenex, San Diego, CA). De-waxing and rehydration by descending concentrations of ethanol was followed by antigen retrieval (20 minutes in a microwave, 450 W, 10 mM EDTA, pH 8.0). Blocking was performed with 10% goat serum in PBS for 5 minutes. All sections were incubated overnight with above primary antibodies, followed by washing in PBS and incubation with anti-goat or anti-mouse secondary antibody for 1 hour at room temperature, and then washed in PBS. The Vectastain ABC-AP KIT (vector; Alexis, Gruenberg, Germany) was used as the chromogen substrate, and the specimens were counterstained with hematoxylin. Specificity of immunostaining was checked by omitting a single step in the protocol and replacing the primary antibody with non-immune serum. Protein expression level was estimated semi-quantitatively [35]. Cores with torn tissues were excluded from the analyses. Tissues with no evidence of staining, or only rare scattered positive cells, less than 3%, were recorded as negative. The immunohistochemical results were evaluated for intensity and frequency of staining. The intensity of staining was graded as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The frequency was graded from 0 to 4 by the percentage of positive cells as follows: grade 0, <3%; grade 1, 3-25%; grade 2, 25-50%; grade 3, 50-75%; grade 4, more than 75%. The index score was the product of multiplication of the intensity and frequency grades, which was then classified into a 4 point scale: index score 0 = product of 0, index score 1 = products 1 and 2, index score 2 = products 3 and 4, index score 3 = products 6 through 12.
Statistical analysis
The Wilcoxon rank-sum test was used; (1) to compare the DNA methylation level between normal, BE, BD, and BACs, (2) to compare the mRNA expression fold between normal and tumor samples and between unmethylated and methylated BACs, and (3) to analyze the association between DNA methylation and the clinicopathological factors. The correlations between DNA methylation level and mRNA expression fold and between methylation level and age were determined by Spearman Correlation. The comparison of immunohistochemistry scores among normal, BE, BD, and BACs and association between immunohistochemistry score and clinicopathological factors were carried out by Chi-square test or Fisher's exact test. All P-values were based on two-sided tests and differences were considered statistically significant when P-value ≤0.05.
Results
Identification of CpG islands in the promoters of GST and GPX family members
Analysis of the promoter regions of GST and GPX members indicated the presence of CpG islands within -1000 bp to +300 bp, relative to the transcription start site, in 16 genes; GSTA4, GSTM1, GSTM2, GSTM3, GSTM4, GSTM5, GSTO1, GSTO2, GSTP1, GSTT1, GSTT2, GSTZ1, GPX1, GPX3, GPX4, and GPX7. The essential information about the GSTs and GPXs is summarized in Table 1. GSTM1 and GSTT1, which have a null-type polymorphism in nearly 50% of healthy populations [36], were excluded from this study. The remaining 14 genes with CpG islands were analyzed by qRT-PCR and quantitative bisulfite pyrosequencing analysis of promoter DNA methylation.
Table 1.
Genomic information of GST and GPX family members
| Class | Gene | Cytoband | Promoter CpG island* | Representative mRNA ACC | Representative protein ACC | Subcellular location |
|---|---|---|---|---|---|---|
|
GSTs
|
||||||
| Alpha (a) | GSTA1 | 6p12 | no | NM_145740 | NP_665683 | cytoplasm |
| GSTA2 | 6p12.1 | no | NM_000846 | NP_000837 | cytoplasm | |
| GSTA3 | 6p12 | no | NM_000847 | NP_000838 | cytoplasm | |
| GSTA4 | 6p12 | yes | NM_001512 | NP_001503 | cytoplasm | |
| GSTA5 | 6p12.1 | no | NM_153699 | NP_714543 | cytoplasm (by similarity). | |
| Mu (m) | GSTM1 | 1p13.3 | yes | NM_000561 | NP_666533 | cytoplasm |
| GSTM2 | 1p13 | yes | NM_000848 | NP_000839 | cytoplasm | |
| GSTM3 | 1p13.3 | yes | NM_000849 | NP_000840 | cytoplasm | |
| GSTM4 | 1p13.4 | yes | NM_000850 | NP_671490 | cytoplasm | |
| GSTM5 | 1p13.5 | yes | NM_000851 | NP_000842 | cytoplasm | |
| Omega (w) | GSTO1 | 10q25.1 | yes | NM_004832 | NP_004823 | cytoplasm |
| GSTO2 | 10q25.2 | yes | NM_183239 | NP_899062 | cytoplasm | |
| Pi (p) | GSTP1 | 11q13-qter | yes | NM_000852 | NP_000843 | cytoplasm |
| Theta (q) | GSTT1 | 22q11.2 | yes | NM_000853 | NP_000844 | cytoplasm |
| GSTT2 | 22q11.3 | yes | NM_000854 | NP_000845 | cytoplasm | |
| Zeta (z) | GSTZ1 | 14q24.3 | yes | NM_145870 | NP_665878 | cytoplasm (by similarity) |
|
GPXs
|
||||||
| GPX1 | 3p21.31 | yes | NM_000581 | NP_958799 | cytoplasm | |
| GPX2 | 14q23.3 | no | NM_002083 | NP_002074 | mainly cytoplasmic | |
| GPX3 | 5q33.1 | yes | NM_002084 | NP_002075 | secreted protein | |
| GPX4 | 19p13.3 | yes | NM_002085 | NP_002076 | mitochondrion. Cytoplasm | |
| GPX5 | 6p22.1 | no | NM_001509 | NP_003987 | secreted protein | |
| GPX6 | 6p22.1 | no | NM_182701 | NP_874360 | secreted protein (by similarity) | |
| GPX7 | 1p32.3 | yes | NM_015696 | NP_056511 | periplasm (probable) |
The GSTM1 and GSTT1, which have a null-type polymorphism in nearly 50% of healthy populations were excluded from further analysis
the details of CpG islands are shown in supplemntary figure 1
Frequent methylation of promoter DNA of GSTs and GPXs in BACs
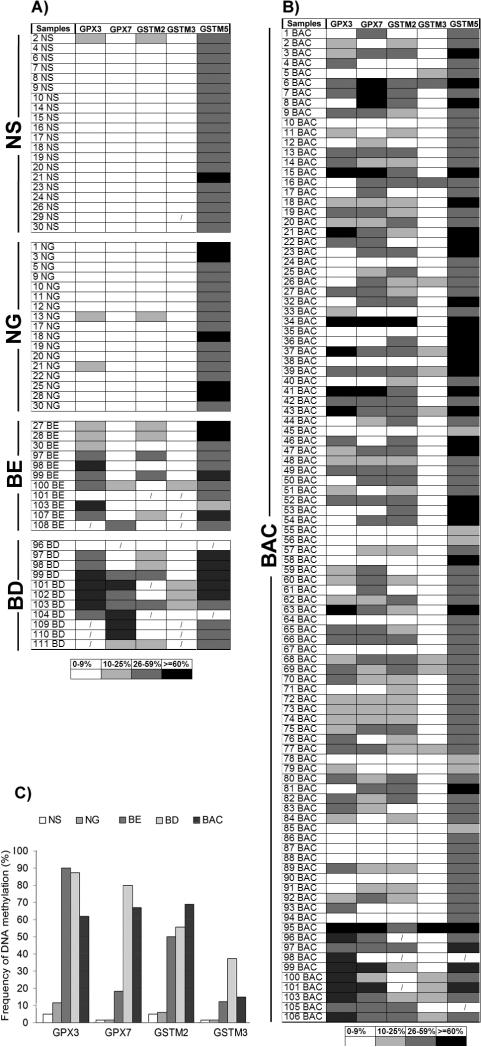
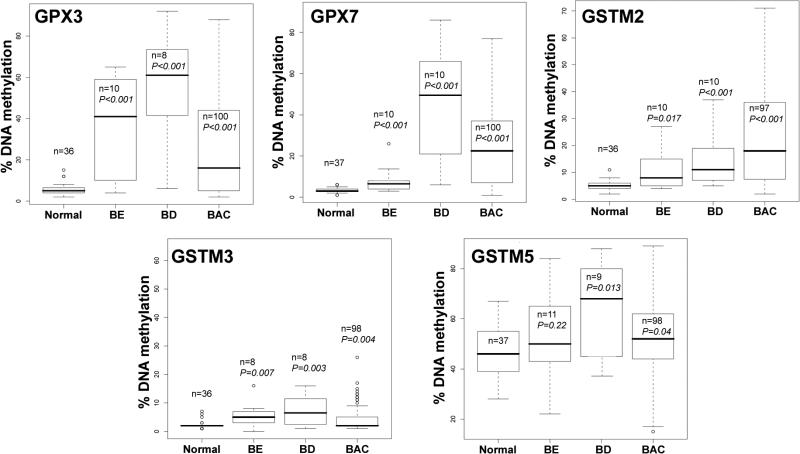
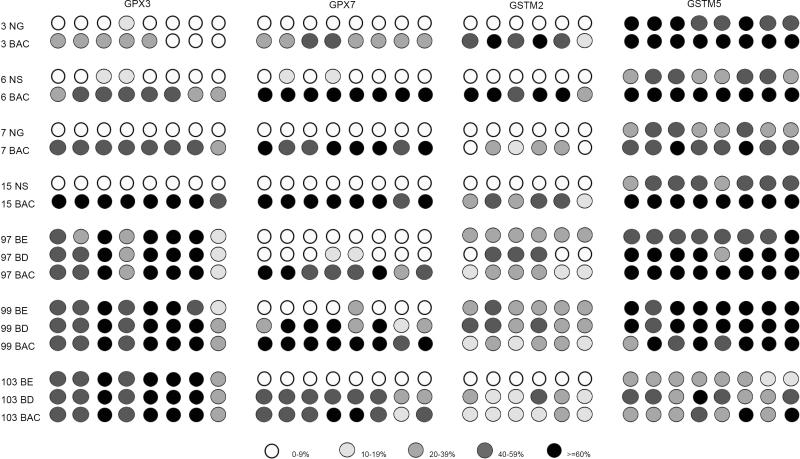
Gene expression and DNA methylation levels were investigated for 14 genes (GSTA4, GSTM2, GSTM3, GSTM4, GSTM5, GSTO1, GSTO2, GSTP1, GSTT2, GSTZ1, GPX1, GPX3, GPX4, and GPX7) using an initial set of 20 primary samples, 10 tumors with matched normal samples from the same patients (Table 2). The GSTO2 gene was not expressed in any normal or tumor sample suggesting that the expression of this gene is possibly tissue specific. In addition, we did not detect a significant down-regulation of GSTO1, GPX1 and GPX4 in tumor samples as compared to normal samples. These four genes were; therefore excluded from further analysis. We continued our analysis of DNA methylation for the remaining 10 genes using quantitative bisulfite pyrosequencing technology on a subset of 77 primary samples (40 tumors, 37 normals). A schematic diagram showing the CpG sites of the promoter regions of these 10 genes and the pyrosequencing assays is given in a supplementary Figure 1. We observed low frequency of DNA methylation (<10% of tumors) for GSTA4, GSTM4, GSTZ1, GSTT2 and GSTP1 (Supplementary Figure 2). These results suggested that DNA methylation of these five genes is not a feature of Barrett's adenocarcinomas. Therefore, further analysis of these five genes was discontinued. We proceeded with our analysis for the remaining five genes (GPX3, GPX7, GSTM2, GSTM3, and GSTM5) on 159 primary samples (100 primary BAC samples, 11 Barrett's esophagus, 11 Barrett's dysplasia, 20 normal esophageal mucosae, and 17 normal gastric mucosae samples). A schematic diagram showing the methylation profile of each sample for GPX3, GPX7, GSTM2, GSTM3, and GSTM5 is shown in Figure 1. We detected absent to low methylation (0-9% methylation level) in normal samples and frequent hypermethylation (≥10% methylation level) in tumor samples for GPX3 (62%, P<.001), GPX7 (67%, P<.001), GSTM2 (69.1%, P<.001), and GSTM3 (15%, P=.004) (Figure 1C and Figure 2). Interestingly, the average DNA methylation level for GSTM5 in normal samples was 25-50%; however, the methylation levels were significantly higher in tumor samples where 29 samples (30%) showed ≥60% methylation levels (P=.04) (Figures 1 and 2). We detected significantly higher levels of DNA methylation in premalignant BE and BD samples as compared to normal samples for GPX3, GPX7, GSTM2, and GSTM3 (P<.01) (Figure 2). These samples included 21 pairs of normal and tumor samples that included 6 matched samples of tumor, BE and/or BD samples from the same patients. A representative summary of quantitative DNA methylation analysis of individual CpG sites in matched samples is illustrated in Figure 3.
Table 2.
mRNA expression and DNA methylation of GSTs and GPXs in Barrett's adenocarcinomas
| Sample code | mRNA expression fold (% DNA methylation) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GSTA4 | GSTM2 | GSTM3 | GSTM4 | GSTM5 | GSTO1 | GSTO2 | GSTP1 | GSTT2 | GSTZ1 | GPX1 | GPX3 | GPX4 | GPX7 | |
| 1 N | 1 (2) | 1 (3) | 1 (1) | 1 (2) | 1 (60) | 1 (/) | 0 (/) | 1 (3) | 1 (4) | 1 (2) | 1 (/) | 1 (6) | 1 (/) | 1 (1) |
| 1 T | 0.15 (3) | 1.1 (6) | 0.8 (1) | 0.22 (2) | 4.3 (40) | 1.58 (/) | 0 (/) | 0.5 (3) | 0.05 (3) | 0.05 (1) | 0.6 (/) | 1.8 (3) | 0.2 (/) | 0.2 (36) |
| 2 N | 1 (4) | 1 (2) | 1 (2) | 1 (9) | 1 (63) | 1 (/) | 0 (/) | 1 (1) | 1 (3) | 1 (2) | 1 (/) | 1 (7) | 1 (/) | 1 (1) |
| 2 T | 0.02 (4) | 0.01 (48) | 0.06 (9) | 0.02 (2) | 0.07 (82) | 0.35 (/) | 0 (/) | 1.4 (5) | 0.03 (3) | 0.06 (3) | 0.72 (/) | 0.01 (18) | 0.27 (/) | 0.09 (36) |
| 3 N | 1 (3) | 1 (6) | 1 (1) | 1 (6) | 1 (45) | 1 (/) | 0 (/) | 1 (7) | 1 (4) | 1 (2) | 1 (/) | 1 (3) | 1 (/) | 1 (5) |
| 3 T | 0.51 (2) | 0.1 (4) | 0.09 (12) | 1.4 (4) | 0.1 (59) | 0.27 (/) | 0 (/) | 10.8 (6) | 0.28 (2) | 0.38 (3) | 0.92 (/) | 0.11 (4) | 2.6 (/) | 1.3 (4) |
| 4 N | 1 (1) | 1 (2) | 1 (1) | 1 (3) | 1 (35) | 1 (/) | 0 (/) | 1 (5) | 1 (6) | 1 (2) | 1 (/) | 1 (5) | 1 (/) | 1 (4) |
| 4 T | 0.04 (3) | 0.06 (19) | 0.55 (6) | 0.45 (2) | 0.02 (54) | 1.1 (/) | 0 (/) | 1.7 (3) | 0.07 (5) | 0.04 (3) | 0.37 (/) | 0.03 (45) | 0.8 (/) | 0.7 (61) |
| 5 N | 1 (2) | 1 (8) | 1 (6) | 1 (3) | 1 (48) | 1 (/) | 0 (/) | 1 (8) | 1 (5) | 1 (3) | 1 (/) | 1 (1) | 1 (/) | 1 (3) |
| 5 T | 0.22 (3) | 1.1 (15) | 0.96 (5) | 0.69 (3) | 0.01 (38) | 0.21 (/) | 0 (/) | 0.89 (10) | 0.2 (4) | 0.17 (3) | 1.1 (/) | 4.5 (12) | 1.1 (/) | 2.7 (7) |
| 6 N | 1 (5) | 1 (4) | 1 (2) | 1 (3) | 1 (51) | 1 (/) | 0 (/) | 1 (7) | 1 (4) | 1 (4) | 1 (/) | 1 (4) | 1 (/) | 1 (3) |
| 6 T | 0.23 (4) | 0.1 (5) | 1.17 (2) | 0.32 (3) | 0.2 (49) | 0.97 (/) | 0 (/) | 12.6 (10) | 0.03 (5) | 0.48 (5) | 3.2 (/) | 2.7 (6) | 2.0 (/) | 0.7 (15) |
| 7 N | 1 (4) | 1 (11) | 1 (5) | 1 (4) | 1 (36) | 1 (/) | 0 (/) | 1 (8) | / (8) | 1 (4) | 1 (/) | 1 (15) | 1 (/) | 1 (5) |
| 7 T | 0.55 (2) | 0.02 (51) | 0.34 (2) | 0.04 (3) | 0.01 (51) | 1.01 (/) | 0 (/) | 0.25 (38) | / (8) | 0.84 (2) | 1.06 (/) | 0.04 (53) | 1.77 (/) | 0.24 (58) |
| 8 N | 1 (2) | 1 (5) | 1 (2) | 1 (3) | 1 (62) | 1 (/) | 0 (/) | 1 (5) | 1 (4) | 1 (2) | 1 (/) | 1 (5) | 1 (/) | 1 (3) |
| 8 T | 0.6 (2) | 0.78 (26) | 2.0 (7) | 0.93 (4) | 4.3 (58) | 1.2 (/) | 0 (/) | 4.9 (6) | 7.3 (3) | 0.81 (3) | 2.5 (/) | / (5) | 5.5 (/) | / (10) |
| 9 N | 1 (/) | 1 (/) | 1 (/) | 1 (/) | 1 (/) | 1 (/) | 0 (/) | 1 (/) | 1 (/) | 1 (/) | 1 (/) | 1 (/) | 1 (/) | 1 (/) |
| 9 T | 0.14 (3) | 0.04 (5) | 0 (2) | 0.12 (2) | 0.01 (48) | 0.93 (/) | 0 (/) | 0.14 (2) | 0.05 (11) | 0.55 (1) | 1.6 (/) | 0.1 (11) | 2.2 (/) | 2.0 (3) |
| 10 N | 1 (/) | 1 (/) | 1 (/) | 1 (/) | 1 (/) | 1 (/) | 0 (/) | 1 (/) | 1 (/) | 1 (/) | 1 (/) | 1 (/) | 1 (/) | 1(/) |
| 10 T | 0.11 (3) | 0.73 (34) | 1.42 (15) | 0.01 (2) | 0.03 (65) | 1.2 (/) | 0 (/) | 1.5 (5) | 0.13 (2) | 0.71 (4) | 0.53 (/) | 0.18 (32) | 0.55 (/) | 0.07 (34) |
N, normal; T, tumor; /, missing. Gene expression fold for each primary tumor was compared to its paired normal sample from the same patient, thus the expression fold of each normal sample was set to 1.0. GSTO1, GSTO2, GPX1, and GPX4 were excluded from methylation analysis since they were either not expressed in any sample (GSTO2) or showed no downregulation in tumor samples (GSTO1, GPX1 and GPX4).
Figure 1. Summary of DNA methylation profiles of GPX3, GPX7, GSTM2, GSTM3, and GSTM5 in 195 samples from 110 patients.
A) DNA methylation profiles of 20 normal esophagus mucosae (NS), 17 normal gastric mucosae (NG), 11 Barrett's esophagus (BE) and 11 Barrett's dysplasia (BD). B) DNA methylation profiles of 100 Barrett's adenocarcinomas (BACs). The DNA methylation was quantified using pyrosequencing technology. The data are presented as an average methylation percentage of the analyzed CpG nucleotides for each gene. The details of the primers and the relationship between the transcription start site and the regions that were analyzed are given in supplementary Table 2 and supplementary Figure 1. The grid and the color codes are given at the bottom of the figure. C) A summary graph showing the frequencies of DNA methylation. GSTM5 is not shown since it showed more than 10% methylation in all normal and tumor samples.
Figure 2. Comparison of the DNA methylation levels.
The percentages of DNA methylation of the GPX3, GPX7, GSTM2, GSTM3, and GSTM5 genes were determined by quantitative bisulfite pyrosequencing. The horizontal bars locate the median of DNA methylation level. The DNA methylation levels were analyzed using the Wilcoxon Rank Sum test to determine the statistical significance. The tumor and premalignant (BE and BD) samples were compared to normal samples (NS + NG). A P value of ≤.05 was considered statistically significant.
Figure 3. A representative DNA methylation profile of an individual CpG site in matched normal, BE, BD, and BAC samples of 7 patients.
This figure demonstrates the methylation levels of individual CpG nucleotides in a representative set of matched tumor, normal, BE, and BD samples from the same patients. Each circle represents one CpG site in the promoter region. The number on the left side represents the samples’ code, as shown in Figure 1. The GSTM3 genes is not shown since only a few samples showed DNA methylation, see Figure 1.
DNA methylation of GSTs and GPXs correlated with reduced gene expression
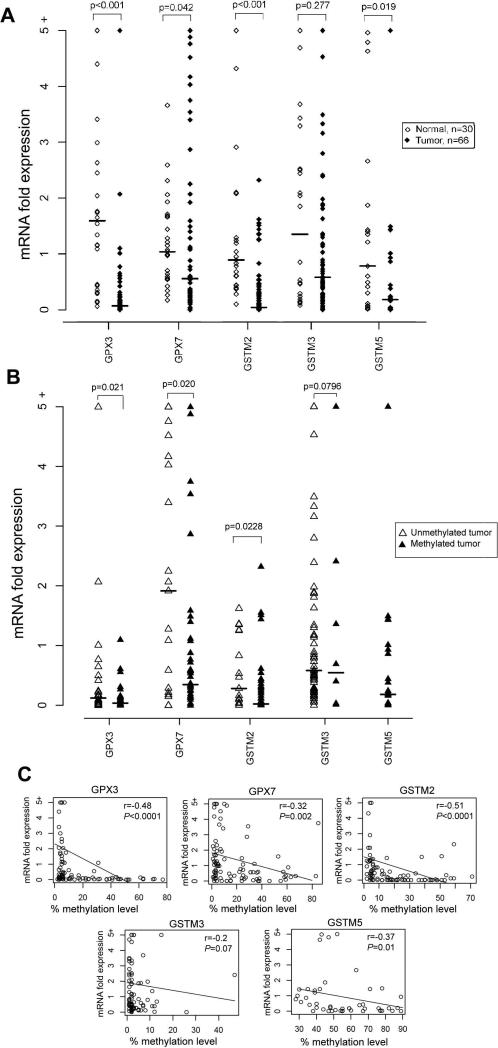
In order to determine the role of DNA methylation in regulating the expression of GSTs and GPXs, mRNA was available from 96 samples (66 BACs and 30 normal samples) of the 159 samples that were evaluated for DNA methylation. Using ≤0.5 fold as a cutoff threshold for gene down-regulation, we detected frequent and significant down-regulation of GPX3 (58/66, 89.2%, P<.001), GPX7 (40/66, 60.6%, P=.042), GSTM2 (55/66, 83.3%, P<0.001) (Figure 4A) in BACs in comparison with normal samples. Because of the high methylation level of GSTM5 in normal samples, we analyzed its mRNA expression in only 21 cases where we have matched tumor and normal samples from the same patients. GSTM5 mRNA expression was significantly down-regulated in tumor samples, as compared to normal samples (15/21, 71.4%, P=.019) (Figure 4A).
Figure 4. A correlation analysis between DNA methylation and gene expression levels.
A) mRNA expression of the GPX3, GPX7, GSTM2, GSTM3, and GSTM5 genes in normal (n=30) and primary Barrett's adenocarcinoma (n=66). The mRNA expression fold was determined by real-time RT-PCR and normalized to the average value of all the normal samples as described in materials and methods. Each open and black diamond represents a normal and tumor sample respectively. B) The mRNA expression fold is shown for methylated (black triangle) and unmethylated (open triangle) tumors. The horizontal bars in A and B locate the median expression fold. The statistical significance was determined by Wilcoxon Rank Sum test. C) The Spearman correlation analysis between DNA methylation level and mRNA expression fold in all 96 samples in A is depicted for each gene. Significant correlations were found for GPX3 (P<.0001), GPX7 (P=.002), GSTM2 (P=.0001), and GSTM5 (P=.01).
We further analyzed the promoter DNA methylation against mRNA expression levels in BACs and found that tumors with hypermethylation (≥10%) had significant down-regulation for GPX3 (P=.021), GPX7 (P=.02), and GSTM2 (P=.0228) as compared to tumors with absent to low promoter DNA methylation level (0 – 9%) (Figure 4B). The Spearman correlation analysis of all 96 samples that were analyzed for promoter DNA methylation and mRNA expression, demonstrated a significant inverse correlation between promoter DNA methylation level and gene expression fold for GPX3 (r=−0.48, P<.0001), GPX7 (r=−0.32, P=.002), GSTM2 (r=−0.51, P=<.0001), GSTM5 (r=−0.37, P =.01) and a correlation trend for GSTM3 (r=−0.2, P=.07) (Figure 4C). The statistical analysis did not identify any significant correlation between promoter DNA methylation level or gene expression fold and clinical (age, sex, survival) or histopathological (histological subtype, tumor grade, TNM staging) parameters. This finding may be explained by the early onset of these changes during Barrett's tumorigenesis, irrespective of these parameters, which is partially supported by the presence of these changes in premalignant lesions as shown in Figure 1.
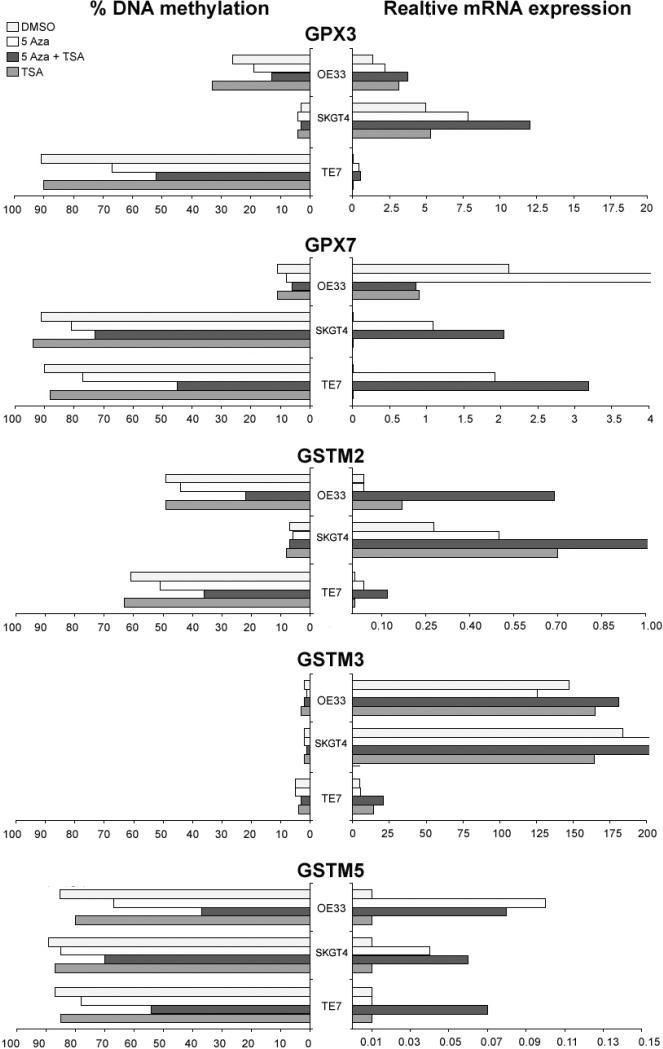
Treatment with 5-Aza restores gene expression in cell lines
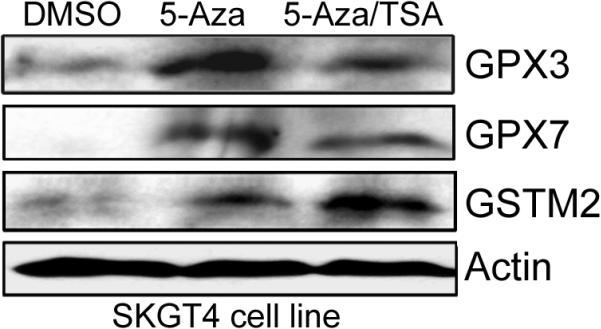
As shown in Figure 5, the 5-Aza treatment restored gene expression for GSTM2, GSTM3, GSTM5, GPX3 and GPX7 in the methylated cell lines and this restoration of gene expression was associated with promoter DNA demethylation. In addition, TSA treatment alone was effective in restoring the gene expression of GSTM2 in SKGT4 and OE33 (Figure 5), suggesting that histone modifications may also be involved in regulating the expression of GSTM2. TSA treatment alone did not change the methylation levels. However, administration of TSA following 5-Aza had an additive effect in restoring gene expression. Furthermore, TSA treatment following the 5-Aza led to a further decrease in the methylation level of hypermethylated genes with mRNA re-expression. These results are in agreement with recent studies that suggested that TSA can have a demethylation effect in a gene-specific manner [37, 38]. The Western blot analysis using the SKGT4 cancer cell line, as a model, confirmed the up-regulation of GPX3, GPX7, and GSTM2 proteins following the 5-Aza and 5-Aza/TSA treatments (Figure 6).
Figure 5. Transcriptional re-expression of the methylated genes following the 5-Aza and TSA treatments.
Three esophageal cancer cell lines (OE33, SKGT4, and TE7) were treated with 2 μM 5-Aza for 72 hours and/or 100 nM TSA for 24 hours. The methylation levels were determined by pyrosequencing. The mRNA expression was calculated as the relative expression as compared to reference gene (HPRT) using the formula 2(Et-Rt) where Et is the threshold cycle number experimental gene in the test sample and Rt is the threshold cycle number for the reference gene in the test sample. The results were multiplied by 100 for a better visualization. The average percentage of DNA methylation of each gene is shown on the left side; whereas the relative mRNA expression of each gene is shown on the right side.
Figure 6. Expression of GPX3, GPX7, and GSTM2 at the protein level following 5-Aza and TSA treatments.
Western blot analysis of SKGT4 cells following treatment with DMSO (control), 5-Aza, or 5-Aza/TSA for 72 hours demonstrate up-regulation of the GPX3, GPX7, and GSTM2 proteins in treated cells as compared to control (DMSO). The □-actin is shown as a loading control.
Down-regulation of protein expression of GPX3, GPX7, and GSTM2 in primary BACs
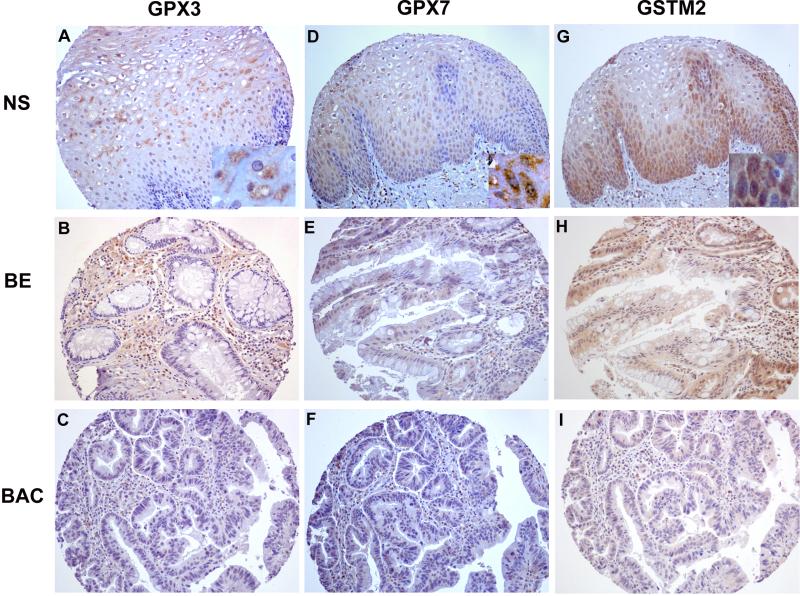
The aforementioned data have strongly indicated the frequent and significant promoter DNA hypermethylation and mRNA down-regulation of GPX3, GPX7, and GSTM2 in BACs. Because protein expression is the ultimate mediator of the biological processes, we followed on our findings by using immunohistochemistry in primary tumor samples. The immunohistochemical analysis demonstrated a moderate to strong immunostaining (scores 2 and 3) of these proteins in normal esophageal samples. On the other hand, we observed absent to weak immunostaining (scores 0 and 1) in BACs (52% for GPX3, 57% for GPX7, and 45% for GSTM2). The details are shown in Table 3 and Figure 7. We could not find any significant statistical correlation between immunohistochemical results and clinical or histopathological parameters.
Table 3.
Immunohistochemistry analysis of GPX3, GPX7 and GSTM2
| IHC score | NS (%) | BE (%) | BAC (%) | P value* | |
|---|---|---|---|---|---|
| GPX3 | <0.001 | ||||
| 0 | 0 | 2 (40) | 19 (30.6) | ||
| 1 | 0 | 0 | 13 (21.0) | ||
| 2 | 2 (18.2) | 3 (60) | 18 (29.0) | ||
| 3 | 9 (81.8) | 0 | 12 (19.4) | ||
| GPX7 | <0.001 | ||||
| 0 | 0 | 1 (16.7) | 20 (30.8) | ||
| 1 | 0 | 2 (33.3) | 17 (26.2) | ||
| 2 | 4 (33.3) | 3 (50) | 17 (26.2) | ||
| 3 | 8 (66.7) | 0 | 11 (16.9) | ||
| GSTM2 | <0.001 | ||||
| 0 | 1 (8.3) | 1 (20) | 25 (39.1) | ||
| 1 | 0 | 2 (40) | 4 (6.3) | ||
| 2 | 4 (33.3) | 1 (20) | 12 (18.8) | ||
| 3 | 7 (58.3) | 1 (20) | 23 (35.9) |
IHC, Immunohistochemistry; NS, normal esophagus squmaous epithelia; BE, Barrett's esophagus; BAC, Barrett's adenocarcinoma.
Fisher's Exact Probability Test.
Figure 7. Immunohistochemical analyses of GPX3, GPX7 and GSTM2 proteins in primary BACs.
As shown, normal esophageal mucosa demonstrate moderate to strong immunostaining (+2 to +3) for GPX3, GPX7, and GSTM2 (A, D, and G, respectively). The tumor samples showed absent to weak immunostaining (0 to +1) for GPX3, GPX7, and GSTM2 (C, F, and I, respectively). The Barrett's esophagus displayed reduced immunostaining for GPX3, GPX7, and GSTM2 (B, E and H, respectively) compared to normal esophagus. All images are shown at original magnification of x200 with insertions at the lower right quadrants of A, D and G shown at 400x magnification.
Discussion
Chronic GERD is the main risk factor for the development of BE and its progression to Barrett's adenocarcinoma [4, 5]. Elevated levels of mucosal ROS have been reported in Barrett's mucosa, suggesting that ROS and subsequent oxidative stress may play an important role in cell damage and carcinogenesis of Barrett's esophagus [12, 13, 14]. Failure to correct the exogenous and endogenous oxidative stresses such as ROS can occur if the oxidative stress exceeds a defendable level or due to a dysfunction of the cellular anti-oxidative system. In this case, the accumulation of ROS will produce oxidative DNA damage that plays an important role in DNA mutagenesis and cell death [9, 10, 11]. Normal cells with an intact anti-oxidative system with expression of the GST and GPX family members can deal with the oxidative stress and prevent cells from further oxidative damage.
The aberrant DNA hypermethylation of gene promoter regions is an important epigenetic mechanism that regulates gene expression leading to down-regulation and silencing of several tumor suppressor genes such as p16, APC, and MGMT [6, 39, 40]. Several recent reports have demonstrated a link between inflammation and the epigenetic inactivation of genes [41, 42]. Aberrant DNA methylation of several tumor-related genes has been reported in cancer-related inflammatory diseases such as gastritis [43], chronic colitis [44] and chronic pancreatitis [45]. It is, therefore, plausible to hypothesize that the persistent inflammation associated with chronic GERD may lead to a field defect where DNA methylation and silencing of expression of several genes that regulate the oxidative DNA damage could occur. This process can trigger the carcinogenesis cascade by allowing a cumulative increase in the mutation load in the cell and leading to progression towards cancer.
In this study, we applied a systematic approach and investigated the role of DNA methylation as an epigenetic mechanism regulating the levels of gene expression of members of the GSTs and GPXs. We have implemented a state-of-the-art quantitative bisulfite pyrosequencing technology (Biotage, Sweden) for the analysis of DNA methylation levels of individual CpG sites and combined this approach with qRT-PCR for the evaluation of mRNA gene expression. We identified a subset of genes that contain promoter CpG islands. Interestingly, we detected frequent down-regulation and promoter DNA hypermethylation of GPX3, GPX7, GSTM2, GSTM3, and GSTM5 in BACs, as compared to normal samples. A significant increase in DNA methylation of GPX3, GPX7, GSTM2, and GSTM3 was also detected in BE and BD as compared with normal samples, suggesting that DNA hypermethylation of these genes may be an early event in the Barrett's esophagus-dysplasia-adenocarcinoma sequence.
Glutathione peroxidases are a major antioxidative damage enzyme family that catalyzes the reduction of hydrogen peroxide, organic hydroperoxide, and lipid peroxides by reduced glutathione [21, 22, 23]. We observed frequent and significant promoter DNA hypermethylation of two members of the GPXs family in BACs; GPX3 and GPX7. The DNA methylation levels of GPX3 and GPX7 had a significant inverse correlation with mRNA expression. The immunohistochemical analysis on tissue microarray confirmed the down-regulation of GPX3 and GPX7 in more than half of the tumors, providing additional confirmation of the dysfunction of these genes in BACs. Based on the known functions of GPXs, it is likely that GPX3 and GPX7 could play an important role in neutralizing the damaging effect of ROS in chronic GERD. In addition, GPX3 has recently been shown to be a potential tumor suppressor gene in prostate carcinoma [46]. Whether GPX3 and GPX7 may have tumor suppressor functions in BACs remain to be elucidated in further studies.
The Mu-class of GST subfamily is encoded by a 100kb gene cluster at 1p13.3 arranged as 5’-GSTM4-GSTM2-GSTM1-GSTM5-GSTM3-3’ [36]. These genes share more than 60 to 80% amino acid sequence identity. However, based on this study, the promoter methylation revealed unique patterns among these genes. While no DNA methylation was observed in the GSTM4 promoter, we detected variable levels of DNA methylation in GSTM2, GSTM3, and GSTM5 in BACs. Analysis of GSTM2 demonstrated a frequent hypermethylation of its promoter DNA with a significant inverse correlation with mRNA expression level. The immunohistochemical analysis demonstrated moderate to strong immunostaining in normal esophageal samples with absent to low intensity in approximately half of the BACs. The GSTM2 has been shown to protect cells from oxidative stress in various animal models and human cells [47, 48, 49]. Although down-regulation of GSTM3 mRNA expression was detected in about 50% of BACs, the overall expression level in tumors did not reach a statistical significance in comparison with that in normal samples (P=.277, Figure 4A). The DNA hypermethylation was observed in few tumors (Figure 4B) suggesting the involvement of other genetic and/or epigenetic mechanisms in its transcription regulation in Barrett's carcinogenesis. The mutated GSTM3 gene has been linked to an increased susceptibility for the development of bladder cancer [50]. The mutations and allelic loss of GSTM3 can lead to lack of detoxification by glutathione conjugation and predispose to bladder cancer [50]. Interestingly, we observed DNA methylation of GSTM5 in normal samples. However, the methylation levels were significantly higher in BACs than that in normal samples (P=.04). In matched tumor and normal samples, we found that GSTM5 expression had a significant inverse correlation with DNA methylation levels (P=.01). Therefore, the high levels of DNA methylation in normal samples could reflect a tissue specific cellular mechanism for regulating its levels in normal esophageal mucosa. Nevertheless, our findings indicate that DNA hypermethylation of tumor samples plays a role in regulating GSTM5 mRNA expression and is possibly involved in Barrett's carcinogenesis. The possible role of GSTM5 in the development of cancer remains to be explored.
We have confirmed our results and have shown that the 5-Aza treatment demethylated the DNA as compared to the DMSO-treated control cells and restored the expression of GPX3, GPX7, GSTM2, GSTM3, and GSTM5. While the TSA treatment alone did not have any demethylation effect, it restored the expression of GSTM2. On the other hand, combined treatment of TSA following 5-Aza showed a possible synergistic effect and restored the expression of the down-regulated genes at higher levels. This finding confirms the epigenetic regulation of these genes and supports earlier reports that a combination of 5-Aza and TSA can have synergistic effects in the reactivation of epigenetically silenced tumor suppressor genes [51]. Interestingly, we observed that TSA administration following 5-Aza treatment led to further DNA demethylation of hypermethylated genes in these cell lines. These results are in agreement with recent studies that suggested that TSA can have a demethylation effect in a gene-specific manner [37, 38]. This effect could be mediated by down-regulation of DNA methyltransferase 3b (DNMT3b) [52] or DNMT1[53]. However, the detailed mechanism by which TSA increases the demethylation effect of 5-Aza remains unclear and requires further investigation.
In conclusion, this study demonstrates the importance of promoter DNA hypermethylation in regulating gene expression of several members of the Mu-class Glutathione-S-Transferases and Glutathione Peroxidases. The epigenetic inactivation of members of the glutathione pathway can be an important mechanism in the development of Barrett's adenocarcinoma.
Supplementary Material
Supplementary Table1. Primer sequences used in quantitative real-time RT-PCR
Supplementary Table 2. Primers for Pyro-sequencing analyses
Acknowledgments
We thank Dr. Christopher C. Moskaluk at the Department of Pathology, University of Virginia for his excellent assistance in the preparation of this manuscript. This study was supported by the National Cancer Institute Grant CA106176 (WER). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or University of Vanderbilt.
Abbreviations
- GERD
gastroesophageal reflux disease
- ROS
reactive oxygen species
- NS
normal esophageal squamous epithelia
- NG
normal gastric epithelia
- BE
Barrett's esophagus
- BD
Barrett's dysplasia
- BAC
Barrett's adenocarcinoma
- GST
Glutathione S-transferase
- GPX
Glutathione peroxidase
- 5-Aza
5-Aza-2’-deoxycytidine
- TSA
Trichostatin A
- qRT-PCR
quantitative real-time reverse transcriptase polymerase chain reaction
Footnotes
Licence for Publication:
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Gut editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence (http://gut.bmjjournals.com/ifora/licence.pdf).
Statement of Competing Interest: None to declare.
REFERENCES
- 1.Altorki NK, Skinner DB. Adenocarcinoma in Barrett's esophagus. Semin Surg Oncol. 1990;6:274–8. doi: 10.1002/ssu.2980060509. [DOI] [PubMed] [Google Scholar]
- 2.Hesketh PJ, Clapp RW, Doos WG, et al. The increasing frequency of adenocarcinoma of the esophagus. Cancer. 1989;64:526–30. doi: 10.1002/1097-0142(19890715)64:2<526::aid-cncr2820640228>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Blot WJ, Devesa SS, Fraumeni JF., Jr Continuing climb in rates of esophageal adenocarcinoma: an update. Jama. 1993;270:1320. [PubMed] [Google Scholar]
- 4.Bonino JA, Sharma P. Barrett esophagus. Curr Opin Gastroenterol. 2004;20:375–80. doi: 10.1097/00001574-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Falk GW. Gastroesophageal reflux disease and Barrett's esophagus. Endoscopy. 2001;33:109–18. doi: 10.1055/s-2001-11669. [DOI] [PubMed] [Google Scholar]
- 6.Eads CA, Lord RV, Wickramasinghe K, et al. Epigenetic patterns in the progression of esophageal adenocarcinoma. Cancer Res. 2001;61:3410–8. [PubMed] [Google Scholar]
- 7.El-Rifai W, Frierson HF, Jr., Moskaluk CA, et al. Genetic differences between adenocarcinomas arising in Barrett's esophagus and gastric mucosa. Gastroenterology. 2001;121:592–8. doi: 10.1053/gast.2001.27215. [DOI] [PubMed] [Google Scholar]
- 8.Metzger R, Schneider PM, Warnecke-Eberz U, et al. Molecular biology of esophageal cancer. Onkologie. 2004;27:200–6. doi: 10.1159/000076913. [DOI] [PubMed] [Google Scholar]
- 9.Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001;477:7–21. doi: 10.1016/s0027-5107(01)00091-4. [DOI] [PubMed] [Google Scholar]
- 10.Loft S, Poulsen HE. Cancer risk and oxidative DNA damage in man. J Mol Med. 1996;74:297–312. doi: 10.1007/BF00207507. [DOI] [PubMed] [Google Scholar]
- 11.Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–96. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 12.Lee JS, Oh TY, Ahn BO, et al. Involvement of oxidative stress in experimentally induced reflux esophagitis and Barrett's esophagus: clue for the chemoprevention of esophageal carcinoma by antioxidants. Mutat Res. 2001;480-481:189–200. doi: 10.1016/s0027-5107(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 13.Olyaee M, Sontag S, Salman W, et al. Mucosal reactive oxygen species production in oesophagitis and Barrett's oesophagus. Gut. 1995;37:168–73. doi: 10.1136/gut.37.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wetscher GJ, Hinder RA, Klingler P, et al. Reflux esophagitis in humans is a free radical event. Dis Esophagus. 1997;10:29–32. doi: 10.1093/dote/10.1.29. discussion 3. [DOI] [PubMed] [Google Scholar]
- 15.Dvorak K, Payne CM, Chavarria M, et al. Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett's oesophagus. Gut. 2007;56:763–71. doi: 10.1136/gut.2006.103697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein SR, Yang GY, Curtis SK, et al. Development of esophageal metaplasia and adenocarcinoma in a rat surgical model without the use of a carcinogen. Carcinogenesis. 1997;18:2265–70. doi: 10.1093/carcin/18.11.2265. [DOI] [PubMed] [Google Scholar]
- 17.Sato T, Miwa K, Sahara H, et al. The sequential model of Barrett's esophagus and adenocarcinoma induced by duodeno-esophageal reflux without exogenous carcinogens. Anticancer Res. 2002;22:39–44. [PubMed] [Google Scholar]
- 18.Salinas AE, Wong MG. Glutathione S-transferases--a review. Curr Med Chem. 1999;6:279–309. [PubMed] [Google Scholar]
- 19.Strange RC, Spiteri MA, Ramachandran S, et al. Glutathione-S-transferase family of enzymes. Mutat Res. 2001;482:21–6. doi: 10.1016/s0027-5107(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 20.Whalen R, Boyer TD. Human glutathione S-transferases. Semin Liver Dis. 1998;18:345–58. doi: 10.1055/s-2007-1007169. [DOI] [PubMed] [Google Scholar]
- 21.Halliwell B. Antioxidant defence mechanisms: from the beginning to the end (of the beginning). Free Radic Res. 1999;31:261–72. doi: 10.1080/10715769900300841. [DOI] [PubMed] [Google Scholar]
- 22.Miyamoto Y, Koh YH, Park YS, et al. Oxidative stress caused by inactivation of glutathione peroxidase and adaptive responses. Biol Chem. 2003;384:567–74. doi: 10.1515/BC.2003.064. [DOI] [PubMed] [Google Scholar]
- 23.Ray G, Husain SA. Oxidants, antioxidants and carcinogenesis. Indian J Exp Biol. 2002;40:1213–32. [PubMed] [Google Scholar]
- 24.Jones PA. DNA methylation and cancer. Oncogene. 2002;21:5358–60. doi: 10.1038/sj.onc.1205597. [DOI] [PubMed] [Google Scholar]
- 25.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 26.Wilson AS, Power BE, Molloy PL. DNA hypomethylation and human diseases. Biochim Biophys Acta. 2007;1775:138–62. doi: 10.1016/j.bbcan.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–16. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 28.Chan AO, Rashid A. CpG island methylation in precursors of gastrointestinal malignancies. Curr Mol Med. 2006;6:401–8. doi: 10.2174/156652406777435417. [DOI] [PubMed] [Google Scholar]
- 29.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–54. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 30.Esteller M, Corn PG, Baylin SB, et al. A gene hypermethylation profile of human cancer. Cancer Res. 2001;61:3225–9. [PubMed] [Google Scholar]
- 31.Hopkins TG, Burns PA, Routledge MN. DNA methylation of GSTP1 as biomarker in diagnosis of prostate cancer. Urology. 2007;69:11–6. doi: 10.1016/j.urology.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Schlemper RJ, Kato Y, Stolte M. Diagnostic criteria for gastrointestinal carcinomas in Japan and Western countries: proposal for a new classification system of gastrointestinal epithelial neoplasia. J Gastroenterol Hepatol. 2000;15(Suppl):G49–57. doi: 10.1046/j.1440-1746.2000.02266.x. [DOI] [PubMed] [Google Scholar]
- 33.Siewert JR, Feith M, Stein HJ. Biologic and clinical variations of adenocarcinoma at the esophago-gastric junction: relevance of a topographic-anatomic subclassification. J Surg Oncol. 2005;90:139–46. doi: 10.1002/jso.20218. discussion 46. [DOI] [PubMed] [Google Scholar]
- 34.El-Rifai W, Moskaluk CA, Abdrabbo M, et al. Gastric Cancers Overexpress S100A calcium binding proteins. Cancer Res. 2002;62:6823–6. [PubMed] [Google Scholar]
- 35.Lee OJ, Hong SM, Razvi MH, et al. Expression of calcium-binding proteins S100A2 and S100A4 in Barrett's adenocarcinomas. Neoplasia. 2006;8:843–50. doi: 10.1593/neo.06481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parl FF. Glutathione S-transferase genotypes and cancer risk. Cancer Lett. 2005;221:123–9. doi: 10.1016/j.canlet.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Ou JN, Torrisani J, Unterberger A, et al. Histone deacetylase inhibitor Trichostatin A induces global and gene-specific DNA demethylation in human cancer cell lines. Biochem Pharmacol. 2007;73:1297–307. doi: 10.1016/j.bcp.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 38.Wu LP, Wang X, Li L, et al. HDAC inhibitor depsipeptide activates silenced genes through decreasing both CpG and H3K9 methylation on the promoter. Mol Cell Biol. 2008 doi: 10.1128/MCB.01516-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulmann K, Sterian A, Berki A, et al. Inactivation of p16, RUNX3, and HPP1 occurs early in Barrett's-associated neoplastic progression and predicts progression risk. Oncogene. 2005;24:4138–48. doi: 10.1038/sj.onc.1208598. [DOI] [PubMed] [Google Scholar]
- 40.Clement G, Braunschweig R, Pasquier N, et al. Methylation of APC, TIMP3, and TERT: a new predictive marker to distinguish Barrett's oesophagus patients at risk for malignant transformation. J Pathol. 2006;208:100–7. doi: 10.1002/path.1884. [DOI] [PubMed] [Google Scholar]
- 41.Valinluck V, Sowers LC. Inflammation-mediated cytosine damage: a mechanistic link between inflammation and the epigenetic alterations in human cancers. Cancer Res. 2007;67:5583–6. doi: 10.1158/0008-5472.CAN-07-0846. [DOI] [PubMed] [Google Scholar]
- 42.Wehbe H, Henson R, Meng F, et al. Interleukin-6 contributes to growth in cholangiocarcinoma cells by aberrant promoter methylation and gene expression. Cancer Res. 2006;66:10517–24. doi: 10.1158/0008-5472.CAN-06-2130. [DOI] [PubMed] [Google Scholar]
- 43.Kang GH, Lee HJ, Hwang KS, et al. Aberrant CpG island hypermethylation of chronic gastritis, in relation to aging, gender, intestinal metaplasia, and chronic inflammation. Am J Pathol. 2003;163:1551–6. doi: 10.1016/S0002-9440(10)63511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Issa JP, Ahuja N, Toyota M, et al. Accelerated age-related CpG island methylation in ulcerative colitis. Cancer Res. 2001;61:3573–7. [PubMed] [Google Scholar]
- 45.Peng DF, Kanai Y, Sawada M, et al. DNA methylation of multiple tumor-related genes in association with overexpression of DNA methyltransferase 1 (DNMT1) during multistage carcinogenesis of the pancreas. Carcinogenesis. 2006;27:1160–8. doi: 10.1093/carcin/bgi361. [DOI] [PubMed] [Google Scholar]
- 46.Yu YP, Yu G, Tseng G, et al. Glutathione peroxidase 3, deleted or methylated in prostate cancer, suppresses prostate cancer growth and metastasis. Cancer Res. 2007;67:8043–50. doi: 10.1158/0008-5472.CAN-07-0648. [DOI] [PubMed] [Google Scholar]
- 47.Nishimura J, Dewa Y, Okamura T, et al. Possible involvement of oxidative stress in fenofibrate-induced hepatocarcinogenesis in rats. Arch Toxicol. 2008 doi: 10.1007/s00204-007-0278-2. [DOI] [PubMed] [Google Scholar]
- 48.Zhou SG, Wang P, Pi RB, et al. Reduced expression of GSTM2 and increased oxidative stress in spontaneously hypertensive rat. Mol Cell Biochem. 2008;309:99–107. doi: 10.1007/s11010-007-9647-7. [DOI] [PubMed] [Google Scholar]
- 49.Ebert MN, Klinder A, Peters WH, et al. Expression of glutathione S-transferases (GSTs) in human colon cells and inducibility of GSTM2 by butyrate. Carcinogenesis. 2003;24:1637–44. doi: 10.1093/carcin/bgg122. [DOI] [PubMed] [Google Scholar]
- 50.Schnakenberg E, Breuer R, Werdin R, et al. Susceptibility genes: GSTM1 and GSTM3 as genetic risk factors in bladder cancer. Cytogenet Cell Genet. 2000;91:234–8. doi: 10.1159/000056851. [DOI] [PubMed] [Google Scholar]
- 51.Cameron EE, Bachman KE, Myohanen S, et al. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–7. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 52.Xiong Y, Dowdy SC, Podratz KC, et al. Histone deacetylase inhibitors decrease DNA methyltransferase-3B messenger RNA stability and down-regulate de novo DNA methyltransferase activity in human endometrial cells. Cancer Res. 2005;65:2684–9. doi: 10.1158/0008-5472.CAN-04-2843. [DOI] [PubMed] [Google Scholar]
- 53.Yang X, Phillips DL, Ferguson AT, et al. Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res. 2001;61:7025–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table1. Primer sequences used in quantitative real-time RT-PCR
Supplementary Table 2. Primers for Pyro-sequencing analyses