Abstract
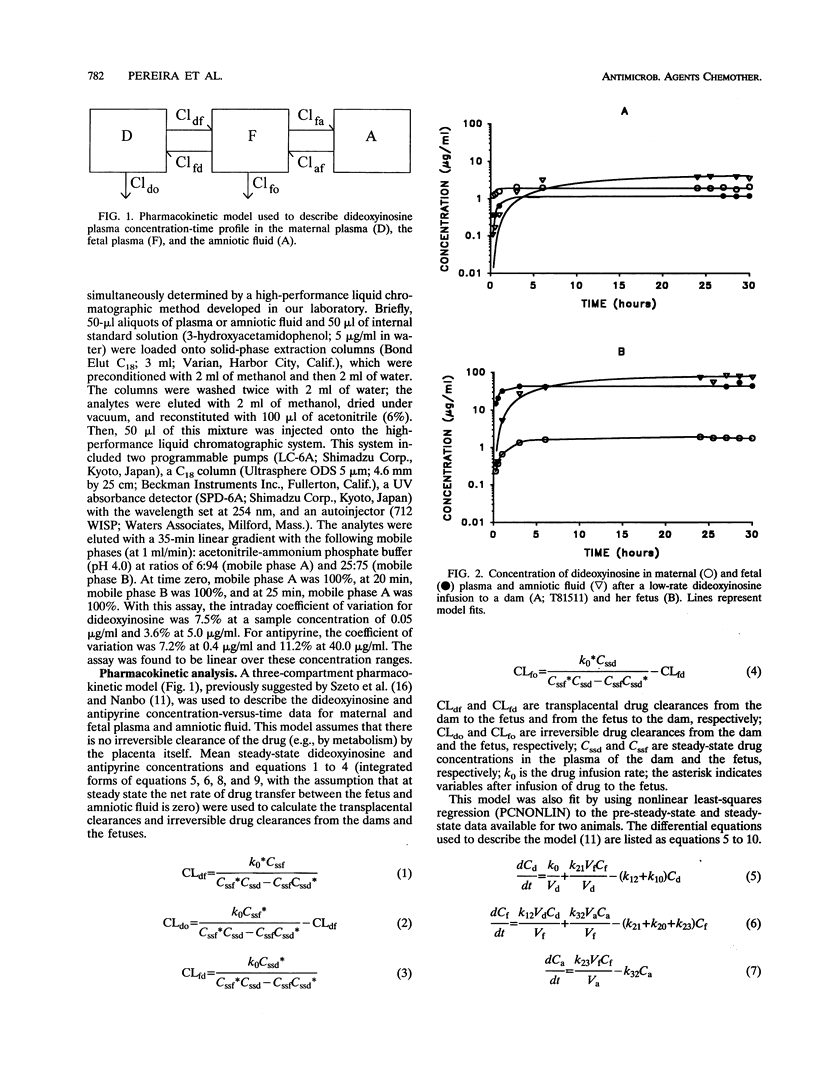
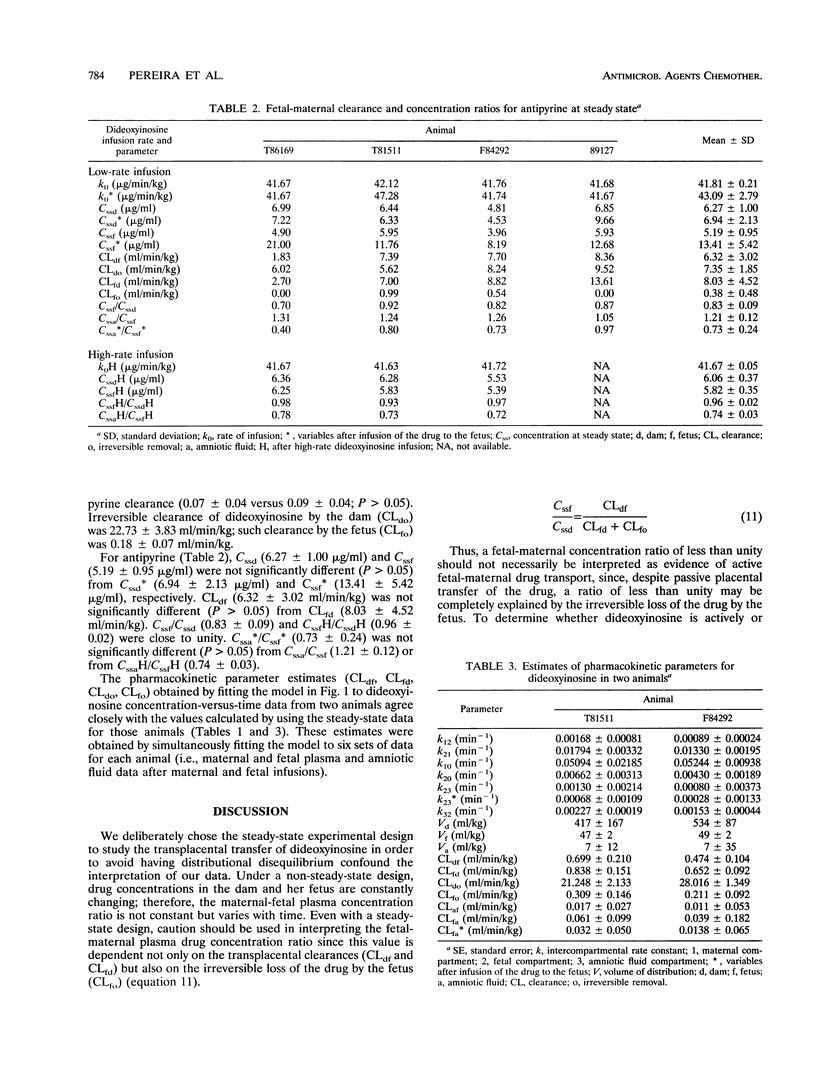
To determine whether dideoxyinosine is actively transported across the placenta, four pregnant macques (Macaca nemestrina) near term and their fetuses were infused intravenously in random order with simultaneous doses of dideoxyinosine (42.5 micrograms/min/kg of body weight) and antipyrine (41.7 micrograms/min/kg) for 30 h. The infusions took place after the dams had been chronically catheterized at 128 +/- 0.8 days of gestation. In a third infusion, the dams alone received a higher dosage of dideoxyinosine (425 micrograms/min/kg) and the same dosage of antipyrine (41.7 micrograms/min/kg). Samples of maternal and fetal blood and amniotic fluid were collected at intervals for up to 30 h. The concentrations of dideoxyinosine and antipyrine were determined by high-performance liquid chromatography. The transplacental maternal-fetal drug clearances were compared by the paired Student's t test. The ratio (mean +/- standard deviation) of the steady-state plasma dideoxyinosine concentration in the fetus to that in the dam was 0.49 +/- 0.10 at the low dideoxyinosine infusion rate and 0.51 +/- 0.00 at the high dideoxyinosine infusion rate. The clearance associated with maternal-fetal transfer of the drug, CLdf (0.38 +/- 0.21 ml/min/kg), was not significantly different (P > 0.05) from the clearance associated with fetal-maternal transfer of the drug, CLfd (0.56 +/- 0.27 ml/min/kg). Also, CLdf was not significantly different (P > 0.05) from CLfd when normalized with respect to the corresponding transplacental clearance of antipyrine (0.07 +/- 0.04 CLdf versus 0.09 +/- 0.04 CLfd). ur data indicate that passage of dideoxyinosine across the placenta in pregnant M. nemestrina near term is passive and constant over the dosage range studied.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agy M. B., Frumkin L. R., Corey L., Coombs R. W., Wolinsky S. M., Koehler J., Morton W. R., Katze M. G. Infection of Macaca nemestrina by human immunodeficiency virus type-1. Science. 1992 Jul 3;257(5066):103–106. doi: 10.1126/science.1621083. [DOI] [PubMed] [Google Scholar]
- Ahluwalia G., Cooney D. A., Mitsuya H., Fridland A., Flora K. P., Hao Z., Dalal M., Broder S., Johns D. G. Initial studies on the cellular pharmacology of 2',3'-dideoxyinosine, an inhibitor of HIV infectivity. Biochem Pharmacol. 1987 Nov 15;36(22):3797–3800. doi: 10.1016/0006-2952(87)90440-0. [DOI] [PubMed] [Google Scholar]
- Bawdon R. E., Sobhi S., Dax J. The transfer of anti-human immunodeficiency virus nucleoside compounds by the term human placenta. Am J Obstet Gynecol. 1992 Dec;167(6):1570–1574. doi: 10.1016/0002-9378(92)91742-s. [DOI] [PubMed] [Google Scholar]
- Chan T. C., Boon G. D., Shaffer L., Redmond R. Antiviral nucleoside toxicity in canine bone marrow progenitor cells and its relationship to drug permeation. Eur J Haematol. 1992 Aug;49(2):71–76. doi: 10.1111/j.1600-0609.1992.tb00034.x. [DOI] [PubMed] [Google Scholar]
- Dalton J. T., Au J. L. 2',3'-Dideoxyinosine is not metabolized in human placenta. Drug Metab Dispos. 1993 May-Jun;21(3):544–546. [PubMed] [Google Scholar]
- Dancis J., Lee J. D., Mendoza S., Liebes L. Transfer and metabolism of dideoxyinosine by the perfused human placenta. J Acquir Immune Defic Syndr. 1993 Jan;6(1):2–6. [PubMed] [Google Scholar]
- Knupp C. A., Shyu W. C., Dolin R., Valentine F. T., McLaren C., Martin R. R., Pittman K. A., Barbhaiya R. H. Pharmacokinetics of didanosine in patients with acquired immunodeficiency syndrome or acquired immunodeficiency syndrome-related complex. Clin Pharmacol Ther. 1991 May;49(5):523–535. doi: 10.1038/clpt.1991.63. [DOI] [PubMed] [Google Scholar]
- Lopez-Anaya A., Unadkat J. D., Schumann L. A., Smith A. L. Pharmacokinetics of zidovudine (azidothymidine). I. Transplacental transfer. J Acquir Immune Defic Syndr. 1990;3(10):959–964. [PubMed] [Google Scholar]
- Mitsuya H., Broder S. Inhibition of the in vitro infectivity and cytopathic effect of human T-lymphotrophic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) by 2',3'-dideoxynucleosides. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1911–1915. doi: 10.1073/pnas.83.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton W. R., Knitter G. H., Smith P. M., Susor T. G., Schmitt K. Alternatives to chronic restraint of nonhuman primates. J Am Vet Med Assoc. 1987 Nov 15;191(10):1282–1286. [PubMed] [Google Scholar]
- Nanbo T. Pharmacokinetics of clearance in the maternal-fetal amniotic fluid system of the rat. Toxicol Appl Pharmacol. 1988 Mar 15;92(3):381–389. doi: 10.1016/0041-008x(88)90178-0. [DOI] [PubMed] [Google Scholar]
- Ochs H. D., Morton W. R., Tsai C. C., Thouless M. E., Zhu Q., Kuller L. D., Wu Y. P., Benveniste R. E. Maternal-fetal transmission of SIV in macaques: disseminated adenovirus infection in an offspring with congenital SIV infection. J Med Primatol. 1991 Jun;20(4):193–200. [PubMed] [Google Scholar]
- Ravasco R. J., Unadkat J. D., Tsai C. C., Nosbisch C. Pharmacokinetics of dideoxyinosine in pigtailed macaques (Macaca nemestrina) after intravenous and subcutaneous administration. J Acquir Immune Defic Syndr. 1992 Oct;5(10):1016–1018. [PubMed] [Google Scholar]
- Shah M. V., Audus K. L., Borchardt R. T. The application of bovine brain microvessel endothelial-cell monolayers grown onto polycarbonate membranes in vitro to estimate the potential permeability of solutes through the blood-brain barrier. Pharm Res. 1989 Jul;6(7):624–627. doi: 10.1023/a:1015913817221. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H., Hunter J. J., Ruprecht R. M., Jaenisch R. Maternal transmission of retroviral disease: transgenic mice as a rapid test system for evaluating perinatal and transplacental antiretroviral therapy. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9792–9796. doi: 10.1073/pnas.85.24.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto H. H., Umans J. G., Rubinow S. I. The contribution of transplacental clearances and fetal clearance to drug disposition in the ovine maternal-fetal unit. Drug Metab Dispos. 1982 Jul-Aug;10(4):382–386. [PubMed] [Google Scholar]
- Zimmerman T. P., Mahony W. B., Prus K. L. 3'-azido-3'-deoxythymidine. An unusual nucleoside analogue that permeates the membrane of human erythrocytes and lymphocytes by nonfacilitated diffusion. J Biol Chem. 1987 Apr 25;262(12):5748–5754. [PubMed] [Google Scholar]
- Zimmerman T. P., Prus K. L., Mahony W. B., Domin B. A. 3'-azido-3'-deoxythymidine and acyclovir: antiviral nucleoside analogues with unusual cell membrane permeation properties. Adv Exp Med Biol. 1989;253B:399–406. doi: 10.1007/978-1-4684-5676-9_59. [DOI] [PubMed] [Google Scholar]


