Abstract
The purpose of this work was to determine platelet and myeloid cell-specific requirements for β3-containing integrins in hemostasis, bone resorption, and tumor growth. LoxP-flanked mice were generated to study the conditional deletion of β3-integrin in platelets [knockout in platelets (KOP)] and myeloid cells [knockout in myeloid (KOM)]. Using the β3KOP and β3KOM strains of mice, we studied the role of β3-integrin in hemostasis, bone resorption, and subcutaneous tumor growth. Tissue-specific deletion of platelet β3-integrins in β3KOP mice did not affect bone mass but resulted in a severe bleeding phenotype. No growth difference of tumor xenografts or in neoangiogenesis were found in β3KOP mice, in contrast to the defects observed in germline β3−/− mice. Conditional deletion of myeloid β3-integrins in β3KOM mice resulted in osteopetrosis but had no effect on hemostasis or mortality. Tumor growth in β3KOM mice was increased and accompanied by decreased macrophage infiltration, without increase in blood vessel number. Platelet β3-integrin deficiency was sufficient to disrupt hemostasis but had no effect on bone mass or tumor growth. Myeloid-specific β3-integrin deletion was sufficient to perturb bone mass and enhance tumor growth due to reduced macrophage infiltration in the tumors. These results suggest that β3-integrins have cell-specific roles in complex biological processes.—Morgan, E. A., Schneider, J. G., Baroni, T. E., Uluçkan, Ö., Heller, E., Hurchla, M. A., Deng, H., Floyd, D., Berdy, A., Prior, J. L., Piwnica-Worms, D., Teitelbaum, S. L., Ross, F. P., Weilbaecher, K. N. Dissection of platelet and myeloid cell defects by conditional targeting of the β3-integrin subunit.
Keywords: Pf4-Cre, LysM-Cre, conditional knockout
The β3-integrin monomer forms two heterodimeric transmembrane glycoproteins: αIIbβ3 (glycoprotein IIb/IIIa) and ανβ3 (the vitronectin receptor). αIIbβ3 is expressed on platelets and megakaryocytes and plays a critical role in platelet aggregation (1). ανβ3 is expressed in multiple cell types (osteoclasts, platelets, activated monocytes/macrophages, activated B and T cells, smooth muscle cells, and certain tumor cells) (2, 3) and regulates adhesion, migration, proliferation, and survival (4,5,6,7,8).
Mice with targeted deletion of the β3 integrin (β3−/−) are viable and fertile but have significant and diverse embryonic and postnatal phenotypes (9). The β3−/− mice have severe defects in platelet aggregation, resulting in bleeding diatheses and spontaneous peri- and postnatal bleeding events (9); these mice develop osteopetrosis (high bone mass) due to dysfunctional osteoclasts (OCs) (10). We and others have demonstrated that β3−/− mice also have increased inflammation and accelerated atherosclerosis (11, 12). Interestingly, β3−/− mice have intact embryonic blood vessel development (9). However, β3−/− mice have enhanced tumor-associated angiogenesis in subcutaneous tumor xenografts (13, 14). Hyper-responsiveness to VEGF and increased activation and expression of Flk-1 in endothelial cells have been identified as mechanisms for the increased tumor-associated angiogenesis in β3−/− mice (13, 15). However, bone marrow transplantation studies have shown that the absence of β3-integrin expression in bone marrow-derived cells can also contribute to enhanced tumor growth without a relevant role of bone marrow-derived endothelial cells in the tumor vasculature (16). Interestingly, β3-integrin blockade using antibodies, Arg-Gly-Asp (RGD) peptide antagonists, or signaling-defective mutants results in reduced (not enhanced) tumor growth and angiogenesis (17,18,19,20).
We have previously demonstrated that β3−/− mice are protected from bone metastasis and osteolytic bone destruction (21). Binding of ανβ3 to bone matrix proteins containing the RGD amino acid motif has been identified as critical to OC function and bone resorption (8, 10, 22). The defective OC function of the β3−/− mice likely results in significant protection from tumor-associated bone loss. Specific pharmacological inhibition of platelet αIIbβ3 also protected mice from bone metastasis (21). OC function and platelet aggregation have been shown to play important roles in regulation of bone mass, hemostasis, and metastasis (10, 21). However, endothelial and immune cell functions are also strongly implicated in these complex biological process, and β3-integrins are involved in modulating platelet, osteoclast, endothelial, and immune cell functions.
We sought to understand more precisely the mechanisms by which β3-integrins contribute to the specific cellular processes involved in tumor biology. To study the complex phenotype of the β3-integrin deficiency in a cell-autonomous fashion, we engineered mice with platelet and myeloid/monocytic inactivation of β3-integrins using Cre/loxP technology. β3KOP [knockout in platelets (KOP)] or β3KOM [knockout in myeloid (KOM)] animals were viable and fertile, and they allowed us to study the role of the β3-integrin selectively in platelets or myeloid cells. The β3KOP mice largely reproduced the bleeding phenotype of β3−/− mice with little effect on bone mass or pathological angiogenesis. The loss of β3-integrin in myeloid cells (β3KOM mice) resulted in osteopetrosis due to dysfunctional OCs with little effect on hemostasis. Finally, we found that myeloid cell β3-integrins were critical to the enhanced subcutaneous tumor growth. Thus, β3-integrin-floxed mice (β3flox/flox) will be useful in dissecting the roles of specific cell types in the complex process of hemostasis, metastasis, tumor-associated angiogenesis, and inflammatory responses.
MATERIALS AND METHODS
Animals
All animal protocols were approved by the Washington University Animal Studies Committee. A combination of conventional cloning and long-distance PCR was used to generate the targeting vector shown in Supplemental Fig. 1A. This construct was electroporated into B6/Blu-1 embryonic stem (ES) cells, derived from a C57Bl/6 transgenic cell line that contains a LacZ reporter for chimeric determination. The LacZ gene is fused to β-globin and is expressed exclusively in peripheral blood cells (23). Out of 30 correctly targeted clones, one was chosen for Cre-mediated removal of the phosphoglycerin kinase (PGK)-neo-selection marker. Two of 152 screened clones showed the desired deletion of the PGK-neo-selection marker and were microinjected into male C57Bl/6 blastocysts to generate chimeric mice. The chimeras were selected by LacZ expression assessment, and germline transmission was confirmed by genomic (tail) DNA Southern blot (Supplemental Fig. 1D).
β3KOP mice were produced by mating the β3flox/flox mice with platelet factor 4-(Pf4)-Cre transgenic mice (33). Lysozyme M-(LysM)-Cre knock-in mice that express the Cre recombinase in the myelomonocytic and osteoclast lineages (24) were utilized for the generation of β3KOM mice. The Cre-transgenic/knock-in mice were bred to β3−/− mice that were rederived on a pure C57Bl/6 background (12) to yield TS-Cre+/−-β3+/− mice (TS-Cre: tissue-specific Cre). These mice were crossed with β3flox/flox mice to generate TS-Cre−/−-β3flox/+ [wild type (WT)], TS-Cre+/−-β3flox/+ [tissue-specific heterozygous (TS-HET)], TS-Cre−/−-β3flox/− [heterozygous (HET)], and TS-Cre+/−-β3flox/− (tissue-specific null-KOP or KOM) mice (Supplemental Fig. 1E). β3+/− mice already have a 50% reduction of the cell surface β3-integrin without demonstrating a phenotype (9). Combining one β3-integrin null allele and a floxed allele in a mouse (TS-Cre+/−-β3flox/−) facilitates full tissue-specific gene deletion by Cre recombinase (25).
Southern blot analysis
ES cell or genomic DNA was digested overnight with StuI and/or EcoRI and detected with probes generated from genomic DNA isolated from a C57BL/6NTac mouse (Taconic, Germantown, NY, USA). 32P-labeled probes were applied to the membrane at 65°C and exposed to film (Kodak, Rochester, NY, USA) at −80°C with an intensifier screen.
RNA extraction and RT-PCR
RNA extraction was performed using the RNeasy Mini kit (Qiagen, Valencia, CA, USA). For RT-PCR, total RNA was treated with DNase and reverse transcribed using the Superscript II kit (Invitrogen, Carlsbad, CA, USA). A negative control using RNA not subjected to reverse transcription was included in each assay. Gene amplification was performed in a conventional thermal cycler or a GeneAmp 7000 Sequence Detection System using the Sybr Green Universal Master Mix reagent kit (Applied Biosystems, Foster City, CA, USA). All assays were run in triplicate. Primer sequences are available on request. GAPDH or L32 levels were not affected by genotype.
Immunoblotting
Twenty-five micrograms of protein was resolved on either 8 or 10% SDS-polyacrylamide gels and electrotransferred onto PVDF membrane (Hybond-P; Amersham Biosciences, Piscataway, NJ, USA). Membranes were incubated with a rabbit integrin β3 antibody (Cell Signaling Technology, Danvers, MA, USA), followed by horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody (Amersham Biosciences). Specific bands were developed for HRP activity by enhanced chemiluminescence. β-Actin (clone AC15; Sigma, St. Louis, MO, USA) was used as a loading control.
Platelet isolation and functional assays
Platelets were separated from pooled whole blood by sequential centrifugation (9, 26). The resulting platelet-rich plasma/platelet-rich buffer (PRP/PRB) mixture was used for platelet aggregation and clot retraction. Platelet lysates were prepared as described previously (9, 27). The slide platelet aggregation test (SPAT) platelet aggregation assay using PRP was performed according to the manufacturer’s instruction (Analytical Control Systems, Fishers, IN, USA). Clot retraction and bleeding times were performed as described previously (9, 28). Screening for fecal occult blood in mouse feces was performed according to the manufacturer’s instructions (Physician Sales and Services, Jacksonville, FL, USA).
Endothelial cell isolation and functional assays
Endothelial cells were isolated from pooled lung tissue employing a 2-step magnetic bead (Dynabead M-450; Invitrogen) purification with rat anti-mouse CD31 antibody. This method has been shown to produce typically >92% pure endothelial cells as assessed by flow cytometry (29). To assess neoangiogenesis, matrigel (BD Biosciences, San Jose, CA, USA) mixed with VEGF and heparin was implanted by subcutanenous injection, and the tissue was harvested and processed after 10 d, as described previously (13, 20). Frozen sections were then stained with rabbit anti-mouse polyclonal CD31 antibody (Abcam, Cambridge, MA, USA) and hamster anti-mouse monoclonal antibody against β3-integrin (BD Pharmingen, San Jose, CA, USA) and visualized by immunofluorescence. Mouse aortic rings were dissected from 6- to 12-wk-old mice from each genotype and sliced into 0.5-cm rings. Each ring was transferred to 50 μl of polymerized matrigel in a 96-well plate. The rings were embedded with an additional 150 μl of matrigel before addition of culture medium containing 30 ng/ml VEGF. Aortic rings were cultured and pictures were taken at baseline and after 10 d using phase contrast microscopy. Angiogenesis was quantitated by counting the numbers of capillary vessel sprouts per ring.
Blood and serum assays
Whole blood was analyzed on a Hemavet Automated Coulter Counter (CBC Tech, Oxford, CT, USA). Differential blood counts were also performed by manual counting of blood smears. Fragments of type I collagen in mouse serum were measured by the ALPHA-CTX ELISA (Nordic Bioscience, Herlev, Denmark) following the manufacturer’s recommendations.
Subcutaneous tumor formation and imaging
Mice were anesthetized, and 5 × 105 B16-F10 melanoma cells stably expressing firefly luciferase (B16-FL) (30) mixed in 200 μl matrigel were injected subcutaneously. In vivo bioluminescence imaging (BLI) was performed as described previously (30, 31). After the final BLI, mice were sacrificed. Dissected tumors were weighed and processed for staining with the CD31 antibody or with rat anti-mouse F4/80 or anti-Mac3 monoclonal antibodies (Abcam). Sections were counterstained with hematoxylin and eosin after colorization with 3,3′-diaminobenzidine (DAB). Sections not incubated with primary antibody were negative controls. DAB staining of macrophages was quantitated using a modified method of color deconvolution according to a previously published protocol employing Image J (National Institutes of Health, Bethesda, MD, USA) (32). The number of blood vessels was counted manually in 5 high-power fields for each tumor.
Osteoclast generation
Bone marrow-derived macrophages were harvested from mice and cultured with 10% fetal bovine serum and 100 ng/ml M-CSF. Three days later, cells were induced to undergo osteoclast differentiation by supplementing the medium with 100 ng/ml RANKL and 50 ng/ml M-CSF as described previously (30). Cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP), using the leukocyte acid phosphatase kit (Sigma) to identify multinucleated OCs.
Bone histomorphometry and bone mineral density (BMD)
Trabecular bone over total bone volume was measured using a standard protocol with specialized software (Bioquant, Nashville, TN, USA), as described previously (30). BMD was measured by dual-energy X-ray absorptiometry (DEXA) according to the manufacturer’s protocol (Lunar Corporation, Madison, WI, USA).
Flow cytometry analysis
Flow cytometry for F4/80 and CD11b on peripheral blood and spleen was performed as previously described (30).
Statistical analysis
Data are presented as means ± se. Statistical significance of differences was calculated using the Student’s unpaired t test for parametric data involving 2 groups, and ANOVA for >2 groups. The χ2 test was used to detect differences in group numbers. Statistical significance was accepted at a value of P < 0.05.
RESULTS
Generation of β3flox/flox mice
We generated an Itgb3 (β3-integrin) gene allele with exon 1 and a PGK-neo selectable marker flanked by loxP recombination sites (Supplemental Fig. 1A). Exon 1 encodes the signal peptide required for expression and processing of the β3-integrin (9). After electroporation of the construct into a pure C57Bl/6 ES cell, we tested the correct orientation and genomic localization by PCR (data not shown) and Southern blot (Supplemental Fig. 1B). Removal of the PGK-neo marker by Cre-mediated excision was confirmed by PCR analysis (Supplemental Fig. 1C, lanes 1 and 2). Microinjection of the correctly targeted ES cell clone into C57Bl/6 blastocysts resulted in chimeric mice. Germline transmission potential was assessed by breeding of chimeric male mice from 2 separate lines to WT C57Bl/6 female mice. Proper genomic targeting was confirmed by Southern blot analysis of tail genomic DNA (Supplemental Fig. 1D) and confirmed by PCR analysis (Supplemental Fig. 1C, lanes 3–5). Offspring floxed mice were viable and fertile. Intercrossing β3flox/+ mice yielded offspring consistent with Mendelian inheritance (data not shown). The 3 genotypes (β3flox/+, β3flox/flox, β3+/+) were phenotypically indistinguishable, demonstrating that the introduction/presence of the loxP sites was a “silent” event (data not shown).
Successful deletion of β3-integrin in platelets (β3KOP) and myeloid cells (β3KOM)
In contrast to the germline β3−/− mice (9), β3KOP (β3flox/−, Pf4cre/+) mice were born at expected frequencies; average genotype frequencies were 1.88 ± 1.33 β3KOP mice, 2.08 ± 1.11 HET (heterozygous-β3flox/−) mice, 1.96 ± 1.13 TS-HET (β3flox/+, Pf4cre/+) mice, and 1.96 ± 1.33 WT mice/litter at weaning; n = 47, 52, 49, and 49, respectively. Postnatally, 15% of β3KOP mice expired because of spontaneous bleeding events, comparable to the reported 18% loss of β3−/− mice (9).
β3-Integrin protein was absent in platelets from β3KOP mice (Fig. 1A). In the β3KOM mice, β3-integrin mRNA was absent during in vitro OC differentiation, and the expression level of β3-integrin mRNA was induced in WT littermate OCs (Supplemental Fig. 2). Correspondingly, β3-integrin protein was not detected in OCs from β3KOM mice but was abundant in WT OCs (Fig. 1B).
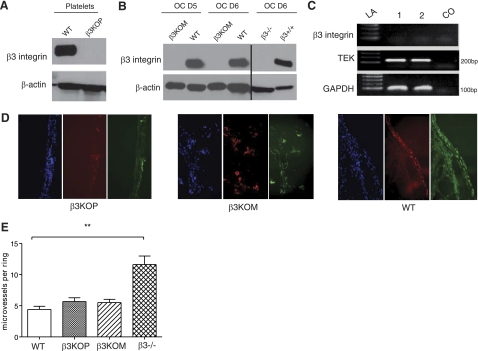
Figure 1.
Successful disruption of the β3-integrin gene selectively in mouse platelets or OCs. A) β3-Integrin protein expression was absent in β3KOP mice. Representative Western blots of total β3-integrin protein in platelet lysates from 4- to 6-wk-old WT (first lane) and β3KOP mice (second lane). Total β-actin protein as loading control. B) β3-Integrin protein expression was absent during OC differentiation in β3KOM mice. Representative Western blots of total β3-integrin protein in OC lysates from WT and β3KOM mice at d 5 and 6 of OC differentiation. Lysates from d 6 β3−/− (lane 5) and β3+/+ (last lane) OCs were used as a control. Total β-actin protein as loading control. C) Absence of β3-integrin mRNA in lung endothelial cells (ECs) of WT mice. Endpoint RT-PCR was performed after EC isolation from pooled lung tissue (n=5) after a CD31 magnetic bead-mediated selection procedure. GAPDH was used as a housekeeping control gene. TEK, EC-specific receptor tyrosine kinase (∼200 bp); CO, control (no RT); LA, ladder. D) Detection of CD31 (red) and neighboring β3-integrin (green) in blood vessels recruited into a matrigel plug 10 d after subcutaneous placement of matrigel mixed with VEGF and heparin in β3KOP, β3KOM, and WT mice. Visualization by immunofluorescence. Nuclei are stained with DAPI. E) Number of vessel sprouts from aortic rings explanted from WT, β3KOP, β3KOM, and β3−/− mice and cultured in matrigel in the presence of VEGF. **P < 0.01 for β3−/− vs. WT mice. Data are means ± se.
We were not able to detect the β3-integrin transcript at all in normal, resting, primary lung endothelial cells isolated from β3KOP, β3KOM, or WT mice (Fig. 1C and data not shown), but β3-integrin expressed by the endothelium has been reported to play an important role during neovascularization and pathological angiogenesis (5, 15). In line with these observations, we found β3-integrin expression on endothelial cells recruited to VEGF matrigel plugs in β3KOP, β3KOM, and WT mice that was colocalized with the endothelial cell marker CD31 (Fig. 1D). These data did not reveal a difference in β3 expression on endothelial cells in WT, β3KOM, and β3KOP mice during neoangiogenesis (Fig. 1D). To further confirm that β3KOM and β3KOP endothelial cells have intact β3 expression during neoangiogenesis that is not different from WT endothelium, we performed an ex vivo aortic ring assay with aortic ring segments from the different genotypes including germline β3−/−, embedded in matrigel and stimulated with VEGF. Angiogenesis was quantified by counting the number of microvessels that sprouted directly from the rings. As expected (13), VEGF-mediated microvessel sprouting was enhanced in β3−/− rings as compared to rings from β3KOM, β3KOP, and WT mice (Fig. 1E).
Targeted deletion of platelet β3KOP recapitulates the phenotype of Glanzmann thrombasthenia
β3KOP mice that survived weaning developed normally, but were subject to both acute and chronic gastrointestinal and intracranial bleeding events as previously seen in germline β3−/− mice (9). There were no differences in red or white blood cell counts (Fig. 2A) and differential count (not shown) between the β3KOP mice and littermate controls, whereas germline β3−/− mice showed a significant increase in monocytes/macrophages (11). Although there was a trend toward decreased platelet numbers in the β3KOP mice, this was not significantly different from the control groups (Fig. 2A). We assessed the occurrence of chronic (gastrointestinal) bleeding using a fecal occult blood test. Here 85% of 8–13-wk-old β3KOP mice had a positive test, strongly suggesting gastrointestinal bleeding events, compared with 9.5% of WT mice (Fig. 2B). In support of this finding, β3KOP mice had significantly decreased hemoglobin levels as compared to their littermate controls mice (12.9±1.2 vs. 14.9±1.2 and 14.8±2 g/dl for β3KOP, WT, and HET, respectively; P < 0.05). β3KOP mice showed splenomegaly at necropsy (cumulative spleen weight from n=3 β3KOP mice 0.4 vs. 0.1 g for WT controls), likely because of chronic blood loss and extramedullary hematopoiesis (Supplemental Fig. 3). Standardized bleeding times were performed to assess in vivo platelet function in β3KOP, TS-HET, and WT mice. β3KOP mice had significantly prolonged bleeding times compared to the littermate controls, requiring early termination of the experiment (Fig. 2C). Clot retraction assays were performed by adding 50 mM CaCl2 to PRP of β3KOP and HET mice. A clot formed within 1 h in PRP from HET mice. In contrast, clot retraction did not occur in PRP from β3KOP mice (Fig. 2D). Likewise, pooled PRP from β3KOP mice did not undergo ADP-induced platelet aggregation, whereas platelets from WT mice did aggregate (Fig. 2E). These results demonstrate that the in vivo and ex vivo bleeding complications in β3KOP mice were essentially identical to those observed in the germline β3−/− mice, suggesting that cell autonomous defects in platelet β3-integrins are the dominant cause for hemostasis problems in Glanzmann thrombasthenia. Thus, the β3KOP mice represent a new model to study the role of platelet β3-integrins in human Glanzmann thrombasthenia.
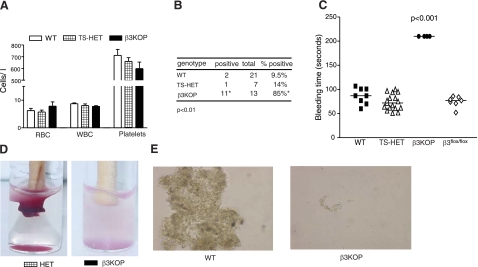
Figure 2.
Disruption of β3-integrin in platelets and megakaryocytes results in a bleeding phenotype. A) No differences observed in red blood cell (RBC), white blood cell (WBC), or platelet counts in β3KOP (solid bars) vs. TS-HET (hatched bars) and WT mice (open bars). B) Increased gastrointestinal bleeding events in β3KOP mice. Fecal occult blood tests were performed on feces samples from β3KOP and littermate controls and demonstrated a significantly higher number of positive tests in β3KOP mice. *P < 0.01 vs. controls; χ2 test. C) Increased bleeding times in β3KOP mice. Each symbol represents bleeding time on a single mouse according to genotype. In each case. bleeding in β3KOP mice had to be stopped by the examiner when bleeding did not stop spontaneously. P < 0.001; ANOVA with appropriate post hoc test. D, E) Platelet aggregation was dysfunctional in β3KOP platelets. D) In vivo clot retraction was assessed using pooled PRP from β3KOP mice and HET control mice. Red blood cells were added to enhance the color contrast for the photograph. E) Platelet aggregation assays on a slide (SPAT) were performed using PRP. No significant platelet aggregation was observed in PRP isolated from β3KOP mice; each test performed ≥3 times/genotype.
Platelet-targeted β3KOP mice have normal bone mass and osteoclast function
We next evaluated effects of platelet-specific loss of β3-integrins on bone mass, because platelets secrete a variety of factors, such as VEGF, ADP, TSP1, and thromboxane, that could affect osteoclast, osteoblast, and bone marrow endothelial cell function. Osteoclast function and expression of β3-integrin were evaluated in β3KOP mice to confirm that Cre recombinase driven by the Pf4 promoter (33) did not delete β3-integrin in the OC lineage. Although Pf4 was previously considered to be a specific marker for the megakaryocyte cell lineages, expression has recently been shown in monocytes (34), and we found that Pf4 is also expressed by WT OCs (Supplemental Fig. 4A). OCs harvested from mice transgenic for the Cre recombinase driven by the Pf4 promoter did not show abnormalities in their differentiation (Supplemental Fig. 4B). OCs cultured from β3KOP mice had a reduction but no loss of expression of β3-integrin mRNA (Fig. 3A) and protein (Fig. 3B) compared to WT littermate mice. Despite the decrease in β3-integrin expression, OCs cultured from β3KOP mice differentiated normally (Fig. 3C), and the size of multinucleated OCs at d 6 was not significantly different between β3KOP, WT, and HET mice (72.12±14, 63.5±10.06, and 63.53±25%, respectively; n=3/genotype). BMD as measured by DEXA was not significantly different in β3KOP mice compared with WT and HET mice (Fig. 3D). In vivo bone resorption as measured by serum levels of CTX (a marker of OC-mediated degradation of bone collagen I; ref. 35) was not significantly different in β3KOP mice compared with WT and HET mice (Fig. 3E). Histomorphometric analysis of tibial and femoral bones from 6-mo-old β3KOP mice showed no significant differences in trabecular bone volume compared to WT and HET littermates (Fig. 3F, G). Thus, despite having significant bleeding diatheses, β3KOP mice did not have significant impairment of OC function (in vitro or in vivo) corresponding with intact β3-integrin function in OCs. These data suggest that the OC defects and high bone mass observed in the germline β3−/− mice were not significantly influenced by abnormal platelet aggregation and bleeding complications.
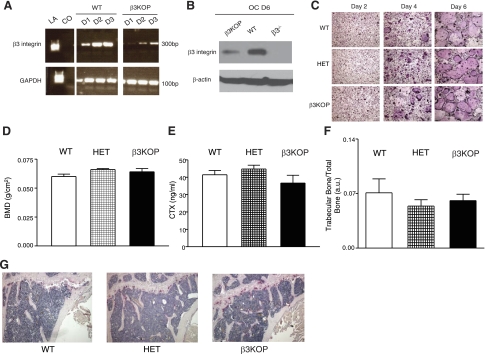
Figure 3.
β3KOP mice have normal bone mass and osteoclast function. A) Expression of Pf4 in OC results in a reduction of β3-integrin mRNA in OCs from β3KOP mice compared to OC from WT mice. RT-PCR was performed using mRNA harvested from OCs of β3KOP and WT littermates at d 1–3 of OC differentiation. A ∼300 bp band shows abundance of β3-integrin message in WT OCs and a distinct level of β3-integrin expression in OCs from β3KOP mice. LA, marker; CO, negative control. GAPDH was used as a housekeeping control gene. B) β3-Integrin protein expression is reduced, but not absent, in β3KOP OCs at d 6 of OC differentiation compared to WT OCs. β3-Integrin protein from d 6 OC from β3−/− mice served as negative control and β-actin as loading control. C) OCs from WT, HET, and β3KOP mice demonstrated equivalent differentiation from bone marrow macrophages at d 2, 4, and 6 of OC differentiation. Representative TRAP staining of cultured macrophages and in vitro differentiated OCs from β3KOP mice and controls. D–F) BMD (D; n=6/group), serum CTX levels (E; n=10/group), and trabecular bone volume (F; n≥6/group) were equivalent in 6-mo-old WT (open bars), HET (hatched bars), and β3KOP mice (solid bars). G) Representative histology of femurs analyzed for trabecular bone volume.
Myeloid-targeted β3KOM mice have intact platelet function but dysfunctional osteoclasts and high bone mass
The β3−/− mice are osteopetrotic and have dysfunctional OCs (8, 10, 22). β3KOM mice were used to investigate whether specific inactivation of β3-integrin in myeloid lineage cells would also result in osteopetrosis independent of β3-specific effects on cell lineages known to affect bone physiology such as platelets and endothelial cells. We used the LysM-Cre knock-in mice, which have been previously used to target gene loss in OCs (36, 37). For some experiments TS-HET and HET mice were omitted, because they consistently showed the same phenotype as WT mice. Hematological profiles of blood collected from randomly selected 8- to 12-wk-old WT and β3KOM mice showed no significant differences in red blood cell, white blood cell, and platelet counts, respectively (Fig. 4A). Despite the significant increase in monocytes/macrophages in the germline β3−/− mice (11), no differences in differential blood counts, spleen size, and spleen monocyte/macrophage numbers between β3KOM and WT control mice were observed (data not shown). No significant differences in fecal occult blood testing (Fig. 4B), bleeding times (Fig. 4C), or in vitro platelet aggregation using PRP from β3KOM and WT mice (Fig. 4D) were detected.
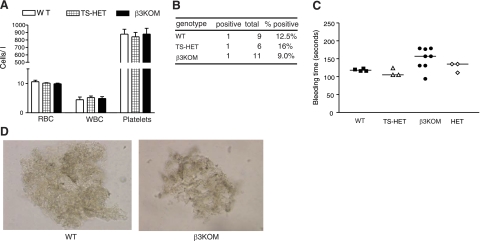
Figure 4.
Myeloid-targeted β3KOM mice have intact platelet function. A) No significant difference in red blood cell (RBC), white blood cell (WBC), and platelet counts was detected in blood from β3KOM (solid bars), TS-HET (hatched bars), and WT mice (open bars). B) There was no significant difference in fecal occult blood testing in β3KOM, TS-HET, and WT mice. C) No significant difference in bleeding times of β3KOM, HET, TS-HET, and WT mice was measured. Each symbol represents bleeding time on a single mouse of appropriate genotype. D) Platelet aggregation was equivalent in β3KOM and WT mice. Platelet aggregation assays on a slide (SPAT) were performed using PRP; each test performed ≥3 times/genotype.
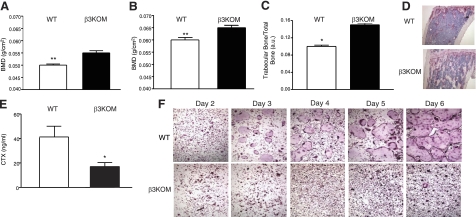
BMD, as measured by DEXA, was significantly increased in 2-mo-old (Fig. 5A) and 6-mo-old β3KOM mice (Fig. 5B) compared to WT littermate controls. Histomorphometric analyses of femoral and tibial bones demonstrated significant increases in trabecular bone volume in β3KOM mice compared to WT littermate controls (Fig. 5C, D). Serum CTX levels were significantly decreased in β3KOM mice compared to WT control mice (Fig. 5E). As observed in the germline β3−/− mice, OCs cultured from β3KOM BMD macrophages demonstrated markedly decreased fusion and were decreased in number during differentiation compared to WT littermate controls (Fig. 5F). The size of multinucleated OCs was significantly lower for the β3KOM mice compared with WT mice (10.8±1.3 vs. 81.75±3.71%, respectively; n=3/genotype; P<0.001). OC differentiation of macrophages harvested from LysM-Cre knock-in mice was not different from WT mice (Supplemental Fig. 5), suggesting that one allele of lysozyme M is sufficient to support normal osteoclastogenesis. These data demonstrate that β3KOM mice develop osteopetrosis as observed in the germline β3−/− mice, without having platelet aggregation defects.
Figure 5.
Myeloid-specific ablation of the β3-integrin gene recapitulates the osteopetrotic phenotype described in β3−/− mice. A, B) β3KOM mice (solid bars) demonstrated increased BMD compared to WT mice (open bars) at 2 mo (A) and at 6 mo of age (B). C) Trabecular bone volume was significantly increased in β3KOM mice vs. controls. D) Representative histology of femurs analyzed for trabecular bone volume (measured in C). Note the pronounced increase in trabecular bone in the β3KOM sample. E) CTX was significantly decreased in the serum of β3KOM mice vs. WT mice. F) Macrophages harvested and cultured from β3KOM mice demonstrated markedly dysfunctional differentiation into OCs on d 2–6 of OC differentiation compared to WT macrophages, as shown by representative in vitro TRAP staining. n ≥ 6/group. *P < 0.05; **P < 0.01.
No increase in subcutaneous tumor growth in β3KOP mice
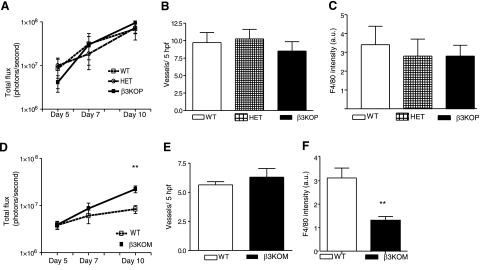
β3−/− mice are protected from bone metastasis (21), but they also have enhanced tumor-associated angiogenesis and increased subcutaneous tumor growth (13, 16). Increased Flk-1 expression on β3−/− tumor-associated endothelial cells (14), as well as loss of β3-integrin on hematopoietic cells (16), has been shown to contribute to the increased tumor angiogenesis observed in β3−/− mice. Platelets are of hematopoeitic origin, and their activation via β3-integrin ligation and consecutive integrin-like kinase activation leads to secretion of α-granules and the release of both pro- and antiangiogenic factors such as VEGF and thrombospondin-1 (38, 39). It has recently been shown that angiogenic factors such as VEGF accumulate in platelets of tumor-bearing mice (40). Therefore, we evaluated subcutaneous tumor growth in the platelet-targeted β3KOP mice. The live cell mass of syngeneic B16-FL melanoma cells implanted subcutaneously as measured by bioluminescence imaging was not significantly different between β3KOP mice and littermate controls (Fig. 6A). Immunohistochemical staining of tumor sections for blood vessel formation (CD31) and macrophage infiltration (F4/80) demonstrated no significant differences in vessel number (Fig. 6B) or the number of tumor-associated macrophages (Fig. 6C) between β3KOP and control mice. These data indicate that the enhanced subcutaneous tumor growth and associated angiogenesis observed in the germline β3−/− mice was likely not related to the lack of β3-integrin in platelets and its associated bleeding phenotype.
Figure 6.
Subcutaneous tumor growth is enhanced in β3KOM, but not β3KOP, mice. A) In vivo bioluminescence at d 5, 7, and 10 after subcutaneous injection of B16-FL cells as assessed by photon flux in contoured ROI was not different between β3KOP mice (solid line), HET mice (hatched line), and WT mice (pointed line), n ≥ 5 tumors/group. Data are presented on a log-scaled y axis. B) Tumor-associated angiogenesis, as measured by immunohistochemical staining for CD31-positive blood vessels in 5-μm paraffin-embedded flank tumor sections counterstained with H&E and visualized by DAB, was equivalent in β3KOP mice (solid bar), HET controls (hatched bar), and WT mice (open bar); n ≥ 5/group. Data are presented as means ± sd. C) Macrophage infiltration, as measured by immunohistochemical staining for F4/80 in 5-μm paraffin-embedded flank tumor sections counterstained with H&E was equivalent in β3KOP, HET controls, and WT mice; n ≥ 5 tumors/group. D) In vivo bioluminescence at d 5, 7, and 10 after subcutaneous injection of B16-FL cells as assessed by photon flux in contoured region of interest (ROI) demonstrated increased bioluminescence in tumors from β3KOM mice (solid line) vs. WT mice (open line) at d 10; n ≥ 5/group. E) No difference in tumor-associated angiogenesis between β3KOM vs. WT mice, as measured by immunohistochemical staining for CD31-positive blood vessels in 5-μm paraffin-embedded flank tumor sections counterstained with H&E and visualized by DAB; n ≥ 5/group. F) Macrophage infiltration, as measured by immunohistochemical staining, for F4/80 or Mac-3 in 5-μm paraffin-embedded flank tumor sections counterstained with H&E, was decreased in tumors from β3KOM vs. WT mice; n ≥ 5/group. **P < 0.01.
Subcutaneous tumor growth is enhanced in β3KOM mice
Macrophages can exert tumoricidal activity or support tumor progression and metastasis (41, 42). Bone marrow transplant data show that loss of β3-integrins on hematopoeitic cells (which includes platelets and myeloid cells) contributes to increased subcutaneous tumor growth in xenografts (16). We evaluated subcutaneous tumor growth in the myelomonocytic-targeted β3KOM mice. Tumors from syngeneic B16-FL melanoma cells implanted subcutaneously had significantly higher live cell mass as measured by bioluminescence imaging (Fig. 6D) and were larger by volume as measured by caliper and weight on dissection in β3KOM mice compared to littermate controls (data not shown). We found that endothelial β3-integrin expression during neoangiogenesis was present and not deleted in β3KOM mice (Fig. 1D, E). Immunohistochemical staining of tumor sections for blood vessel formation (CD31) demonstrated that the absence of β3-integrins on myelomonocytic cells did not impair vessel development, which was slightly, but not statistically, increased, compared to the WT mice (Fig. 6E). Staining for macrophages (F4/80) resulted in significantly lower numbers of infiltrating macrophages in tumors of β3KOM mice compared to control mice (Fig. 6F), which has been described in the germline β3−/− mice (16). These data show that myeloid β3-integrins can influence tumor growth in the setting of endothelial cells with intact β3-integrin expression.
DISCUSSION
β3−/− mice have a complex phenotype, including a severe bleeding disorder, high bone mass (osteopetrosis), enhanced pathological angiogenesis, and inflammation and increased intrauterine mortality (9). However, the integration of diverse cell type-specific functions of the β3-integrin makes it difficult to distinguish cell-autonomous effects of β3-integrins in complex biological processes. Thus, we generated mice with conditional deletion of the β3 gene using loxP technology (β3flox/flox, Supplemental Fig. 1A–D). Crossing our β3flox/flox mice with Pf4-Cre mice (33) efficiently deleted β3-integrin in platelets (Fig. 1A). β3KOP mice replicated the platelet aggregation defects and bleeding complications observed in the germline β3−/− mice (Fig. 2B–E) (9). In contrast with germline β3−/− mice, the β3KOP mice had normal bone mass and did not display enhanced tumor-associated angiogenesis. These results suggest that platelet β3-integrins and the associated severe platelet aggregation defects do not play a critical role in the regulation of bone mass or tumor-associated angiogenesis. Based on these data we believe that the β3KOP mice represent an appropriate model of the human Glanzmann thrombasthenia, as they only display the striking bleeding phenotype due to specific deletion of the β3-integrin in platelets.
Pf4 has been shown to have low-level expression in monocytes and in in vitro differentiated macrophages (34). We found that Pf4 was also present in OCs at different stages of differentiation (Supplemental Fig. 4A). This Pf4 expression in OCs was accompanied with a reduction of β3-integrin mRNA and protein in OC of the β3KOP mice (Fig. 3A, B). However, we demonstrated that OCs derived from Pf4-Cre and β3KOP mice differentiated normally in vitro, and there were no significant effects on BMD or markers of OC function in vivo (Fig. 3C–G and Supplemental Fig. 4B). β3-Integrins in platelets play important roles in tumor cell metastasis (21) and inflammation. The β3KOP mice will be useful to delineate the specific contributions of platelet β3-integrins during these processes.
To investigate whether the tissue specific deletions of β3-integrin in myeloid lineage cells would reproduce the osteopetrosis phenotype observed in the germline β3−/− mice, we created β3KOM mice by mating β3flox/flox mice with the myeloid cell-lineage-specific LysM-Cre mice (24). The LysM-Cre mice have been successfully used to obtain a tissue-specific gene deletion in OCs (37). We first ruled out any functional consequences for lysozyme M haploinsufficiency in OC differentiation by culturing preosteoclasts from LysM-Cre and WT mice (Supplemental Fig. 5). Peripheral blood cell counts (Fig. 4A) and differential cell counts (data not shown) were normal in the β3KOM mice, and no bleeding abnormalities in vitro or in vivo in the β3KOM mice were detected (Fig. 4B–D). The observations of increased BMD at different ages of the β3KOM mice (Fig. 5A, B) and increased trabecular bone and decreased CTX levels (Fig. 5C–E) were accompanied by severe dysfunction of the OCs in the β3KOM mice. There have been reports that LysM-Cre mice may not only target Cre expression to myeloid lineage cells (43); however, we did not observe effects on platelet or endothelial cell function. The β3KOM mice accurately recapitulate the bone phenotype of the β3−/− mice (10), demonstrating that the conditional deletion of β3-integrin using the β3flox/flox mice will be a valuable tool to study the physiological and pathophysiologic cell autonomous roles of β3-integrin in myeloid tissues.
β3-Integrins play an important role in angiogenesis under pathological conditions (17, 18). There are no distinct abnormalities found in developmental angiogenesis in the β3−/− mice (13). We did not detect β3-integrin expression in normal adult endothelial cells (noncultured) derived from the lung of WT mice (Fig. 1C). However, we did observe β3-integrin expression in endothelial cells during neoangiogenesis (Fig. 1D) in β3KOM, β3KOP, and WT mice, suggesting that endothelial β3 expression is intact in these mouse strains. Indeed, ex vivo angiogenesis aortic ring assays demonstrated that the germline β3−/− mice, and not the β3KOM, β3KOP, and WT mice, showed significant increases in vessel sprouts (Fig. 1E). These data suggest that endothelial cell β3-integrin expression may be induced during postnatal vessel formation (44). The study of the specific role of β3-integrin in endothelial cells using β3flox/flox mice may be important to define whether endothelial cell β3-integrins play a causative role during pathological angiogenesis.
Pathological VEGF-mediated angiogenesis enhances subcutaneous tumor growth in β3−/− mice (14). The suspected direct involvement of bone marrow-derived cells in tumor growth in the β3−/− mice (16) prompted us to evaluate subcutaneous tumor growth in the β3KOP and β3KOM mice. Platelets represent a significant source of proangiogenic (VEGF) and antiangiogenic factors (TSP1) and are recruited to sites of tumor where their aggregation could affect local tumor growth (38, 39, 40). Interestingly, we found that subcutaneous melanoma tumor growth and vessel number was not significantly enhanced in β3KOP mice (Fig. 6A, B) as has been observed in the germline β3−/− mice, suggesting that platelet-expressed β3-integrin does not have a direct effect on tumor growth or neovascularization.
Inspired by previous studies on the isolated role of VEGF in myeloid cells in accelerated tumorigenesis (45), we evaluated the effect of deletion of β3-integrin in myeloid cells on tumor growth. The growth rate of tumor xenografts was accelerated in β3KOM mice with fewer macrophages observed within the tumor (Fig. 6D, F). Interestingly, tumors in β3KOM mice did not have significantly increased vascular density, suggesting that the lack of myeloid β3-integrin was sufficient to promote larger growth of tumor xenografts despite intact endothelial β3-integrin function. Defective macrophage tumor infiltration has previously been seen in the β3−/− mice (16) and in the signaling-defective DiYF β3 knock-in mice (knock-in mice with 2 mutated tyrosine residues) (46, 47). Previous studies (48) and our own results (11) suggest that defective migration observed in β3−/− cells could be due to a failure in efficient cytoskeletal (re)organization. β3-Integrin-mediated enhanced tumor elimination by macrophages was seen in a glioblastoma model, which leaves room to speculate that the lack of appropriately polarized macrophages within the tumor may at least partly be responsible for the increased tumor growth in β3KOM mice (49, 50).
Using the β3-integrin-signaling-defective DiYF mice, Feng et al. (47) found that DiYF bone marrow-derived cells had altered recruitment and retention of macrophages to sites of neoangiogenesis. However, the lack of functional β3-integrin phosphorylation in germline DiYF mice resulted in impaired pathological angiogenesis. In β3KOM mice, we found little effect on blood vessel number, but a decrease in macrophage tumor infiltration and an increase in tumor growth. The divergent results on tumor growth between mice with germline β3-integrin deletion, signaling-defective DiYF mice, and mice treated with β3-integrin inhibitors have been the subject of significant debate.
In summary, we have successfully generated a mouse with a loxP-flanked β3-integrin gene and achieved tissue-specific gene inactivation in platelets and myeloid cells. The phenotypic characterization of these mice showed that β3KOP mice represent an ideal model for human Glanzmann disease. The β3KOM mice had a severe OC dysfunction similar to the β3−/− mice but no bleeding diatheses. Myeloid-targeted β3-integrin loss resulted in increased subcutaneous tumor growth with decreased macrophage infiltration in the tumor, but no increase in blood vessel number. Platelet-derived β3-integrin was dispensable for subcutaneous tumor growth, angiogenesis, and tumor infiltration by macrophages. The β3flox/flox mice are therefore a useful tool to dissect the complex phenotypes of β3−/− mice in a cell-autonomous fashion.
Supplementary Material
Acknowledgments
The authors thank Michael Tomasson, Timothy Ley, Anthony Muslin, Evan Sadler, Clay F. Semenkovich, Barry Sleckman, Mike White, Deborah Novack, and Haibo Zhao for their help and guidance. This work was supported by U.S. National Institutes of Health (NIH) grant NIHR0152152 (to K.N.W., J.G.S., T.B., O.U, H.D., D.F., E.H., and M.A.H); Medical Scientist Training Program grant NIH T32 GM7200 (E.A.M.), and NIH grants AR046852 (F.P.R.), AR032788, and AR046523 (S.L.T.); and by the Barnes-Jewish Foundation (J.S., O.U.) and the St. Louis Men’s Club against Cancer (A.B.). The Molecular Imaging Center (grant P50 CA94056) supported D.P.W., J.L.P., and O.U. The authors acknowledge the assistance of Kimberly Carter and the DDRCC Morphology Core Facility at Washington University (DDRCC P30 DK52574) and Elaine Ross from the Embryonic Stem Cell Core, Washington University. The authors also thank Trey Coleman and the Center for Nutrition Research Unit for assistance with DEXA scanning, supported by grant DK56341.
References
- Phillips D R, Charo I F, Scarborough R M. GPIIb-IIIa: the responsive integrin. Cell. 1991;65:359–362. doi: 10.1016/0092-8674(91)90451-4. [DOI] [PubMed] [Google Scholar]
- Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Schwartz M A, Schaller M D, Ginsberg M H. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Hynes R O. Integrins: a family of cell surface receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Brooks P C, Clark R A, Cheresh D A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Jones J I, Prevette T, Gockerman A, Clemmons D R. Ligand occupancy of the alpha-V-beta3 integrin is necessary for smooth muscle cells to migrate in response to insulin-like growth factor. Proc Natl Acad Sci U S A. 1996;93:2482–2487. doi: 10.1073/pnas.93.6.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero C A, Mendez E, Zarate S, Isa P, Lopez S, Arias C F. Integrin alpha (v) beta(3) mediates rotavirus cell entry. Proc Natl Acad Sci U S A. 2000;97:14644–14649. doi: 10.1073/pnas.250299897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccio R, Takeshita S, Zallone A, Ross F P, Teitelbaum S L. c-Fms and the alphavbeta3 integrin collaborate during osteoclast differentiation. J Clin Invest. 2003;111:749–758. doi: 10.1172/JCI16924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodivala-Dilke K M, McHugh K P, Tsakiris D A, Rayburn H, Crowley D, Ullman-Cullere M, Ross F P, Coller B S, Teitelbaum S, Hynes R O. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh K P, Hodivala-Dilke K, Zheng M H, Namba N, Lam J, Novack D, Feng X, Ross F P, Hynes R O, Teitelbaum S L. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105:433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Avery J, Zhao H, Schneider J G, Ross F P, Muslin A J. Beta3 integrin deficiency promotes cardiac hypertrophy and inflammation. J Mol Cell Cardiol. 2007;42:367–377. doi: 10.1016/j.yjmcc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Schneider J G, Zhu Y, Coleman T, Semenkovich C F. Macrophage beta3 integrin suppresses hyperlipidemia-induced inflammation by modulating TNFalpha expression. Arterioscler Thromb Vasc Biol. 2007;27:2699–2706. doi: 10.1161/ATVBAHA.107.153650. [DOI] [PubMed] [Google Scholar]
- Reynolds L E, Wyder L, Lively J C, Taverna D, Robinson S D, Huang X, Sheppard D, Hynes R O, Hodivala-Dilke K M. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- Reynolds A R, Reynolds L E, Nagel T E, Lively J C, Robinson S D, Hicklin D J, Bodary S C, Hodivala-Dilke K M. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta3-integrin-deficient mice. Cancer Res. 2004;64:8643–8650. doi: 10.1158/0008-5472.CAN-04-2760. [DOI] [PubMed] [Google Scholar]
- Reynolds L E, Conti F J, Lucas M, Grose R, Robinson S, Stone M, Saunders G, Dickson C, Hynes R O, Lacy-Hulbert A, Hodivala-Dilke K. Accelerated re-epithelialization in beta3-integrin-deficient mice is associated with enhanced TGF-beta1 signaling. Nat Med. 2005;11:167–174. doi: 10.1038/nm1165. [DOI] [PubMed] [Google Scholar]
- Taverna D, Moher H, Crowley D, Borsig L, Varki A, Hynes R O. Increased primary tumor growth in mice null for beta3- or beta3/beta5-integrins or selectins. Proc Natl Acad Sci U S A. 2004;101:763–768. doi: 10.1073/pnas.0307289101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks P C, Am M, Rosenfeld M, Reisfeld R A, Hu T, Klier G, Cheresh D A. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Brooks P C, Stromblad S, Klemke R, Visscher D, Sarkar F H, Cheresh D A. Antiintegrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Investig. 1995;96:1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivatsa S S, La F, Tsao P W, Reilly T M, Holmes, Schwartz R S, Mousa S A. Selective alpha v beta 3 integrin blockade potently limits neointimal hyperplasia and lumen stenosis following deep coronary arterial stent injury: evidence for the functional importance of integrin alpha v beta 3 and osteopontin expression during neointima formation. Cardiovasc Res. 1997;36:408–428. doi: 10.1016/s0008-6363(97)00184-3. [DOI] [PubMed] [Google Scholar]
- Mahabeleshwar G H, Feng W, Phillips D R, Byzova T V. Integrin signaling is critical for pathological angiogenesis. J Exp Med. 2006;203:2495–2507. doi: 10.1084/jem.20060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakewell S J, Nestor P, Prasad S, Tomasson M H, Dowland N, Mehrotra M, Scarborough R, Kanter J, Abe K, Phillips D, Weilbaecher K N. Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proc Natl Acad Sci U S A. 2003;100:14205–14210. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross F P, Chappel J, Alvarez J I, Sander D, Butler W T, Farach C, Mintz K A, Robey P G, Teitelbaum S L, Cheresh D A. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J Biol Chem. 1993;268:9901–9907. [PubMed] [Google Scholar]
- Graubert T A, Hug B A, Wesselschmidt R, Hsieh C L, Ryan T M, Townes T M, Ley T J. Stochastic, stage-specific mechanisms account for the variegation of a human globin transgene. Nucleic Acids Res. 1998;26:2849–2858. doi: 10.1093/nar/26.12.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen B E, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- Castro C H, Stains J P, Sheikh S, Szejnfeld V L, Willecke K, Theis M, Civitelli R. Development of mice with osteoblast-specific connexin43 gene deletion. Cell Commun Adhes. 2003;10:445–450. doi: 10.1080/cac.10.4-6.445.450. [DOI] [PubMed] [Google Scholar]
- Frenette P S, Johnson R C, Hynes R O, Wagner D D. Platelets roll on stimulated endothelium in vivo: an interaction mediated by endothelial P-selectin. Proc Natl Acad Sci U S A. 1995;92:7450–7454. doi: 10.1073/pnas.92.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer J, Coller B S. Evidence that platelet glycoprotein IIIa has a large disulfide-bonded loop that is susceptible to proteolytic cleavage. J Biol Chem. 1989;264:17564–17573. [PubMed] [Google Scholar]
- Dejana E, Quintana A, Callioni A, de Gaetano G. Bleeding time in laboratory animals. Thromb Res. 1979;15:199–207. doi: 10.1016/0049-3848(79)90065-3. [DOI] [PubMed] [Google Scholar]
- Friedrich E B, Liu E, Sinha S, Cook S, Milstone D S, MacRae C A, Mariotti M, Kuhlencordt P J, Force T, Rosenzweig A, St Arnaud R, Dedhar S, Gerszten R E. Integrin-linked kinase regulates endothelial cell survival and vascular development. Mol Cell Biol. 2004;24:8134–8144. doi: 10.1128/MCB.24.18.8134-8144.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirbe A C, Uluckan O, Morgan E A, Eagleton M C, Prior J L, Piwnica-Worms D, Trinkaus K, Apicelli A, Weilbaecher K. Granulocyte colony-stimulating factor enhances bone tumor growth in mice in an osteoclast-dependent manner. Blood. 2007;109:3424–3431. doi: 10.1182/blood-2006-09-048686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uluckan O, Eagleton M C, Floyd D H, Morgan E A, Hirbe A C, Kramer M, Dowland N, Prior J L, Piwnica-Worms D, Jeong S S, Chen R, Weilbaecher K. APT102, a novel adpase, cooperates with aspirin to disrupt bone metastasis in mice. J Cell Biochem. 2008;104:1311–1323. doi: 10.1002/jcb.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruifrok A C, Johnston D A. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001;23:291–299. [PubMed] [Google Scholar]
- Tiedt R, Schomber T, Hao-Shen H, Skoda R C. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109:1503–1506. doi: 10.1182/blood-2006-04-020362. [DOI] [PubMed] [Google Scholar]
- Schaffner A, Rhyn P, Schoedon G, Schaer D J. Regulated expression of platelet factor 4 in human monocytes–role of PARs as a quantitatively important monocyte activation pathway. J Leukoc Biol. 2005;78:202–209. doi: 10.1189/jlb.0105024. [DOI] [PubMed] [Google Scholar]
- Rosen H N, Moses A C, Garber J, Iloputaife I D, Ross D S, Lee S L, Greenspan S L. Serum CTX: a new marker of bone resorption that shows treatment effect more often than other markers because of low coefficient of variability and large changes with bisphosphonate therapy. Calcif Tissue Int. 2000;66:100–103. doi: 10.1007/pl00005830. [DOI] [PubMed] [Google Scholar]
- Hilliard T J, Meadows G, Kahn A J. Lysozyme synthesis in osteoclasts. J Bone Mineral Res. 1990;5:1217–1222. doi: 10.1002/jbmr.5650051205. [DOI] [PubMed] [Google Scholar]
- Kim H J, Zhao H, Kitaura H, Bhattacharyya S, Brewer J A, Muglia L J, Ross F P, Teitelbaum S L. Glucocorticoids suppress bone formation via the osteoclast. J Clin Invest. 2006;116:2152–2160. doi: 10.1172/JCI28084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii D C, Psaila B, Butler J, Jin D K, Lyden D. Regulation of vasculogenesis by platelet-mediated recruitment of bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2008;28:217–222. doi: 10.1161/ATVBAHA.107.151159. [DOI] [PubMed] [Google Scholar]
- Tucker K L, Sage T, Stevens J M, Jordan P A, Jones S, Barrett N E, St Arnaud R, Frampton J, Dedhar S, Gibbins J M. A dual role for integrin-linked kinase in platelets: regulating integrin function and α-granule secretion. Blood. 2008;112:4523–4531. doi: 10.1182/blood-2008-03-148502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italiano J E, Jr, Richardson J L, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement G L. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J, Pollard J W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Hagemann T, Lawrence T, McNeish I, Charles K A, Kulbe H, Thompson R G, Robinson S C, Balkwill F R. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Iwasaki H, Laiosa C V, Stadtfeld M, Xie H, Heck S, Clausen B, Akashi K, Graf T. Hematopoietic stem cells expressing the myeloid lysozyme gene retain long-term, multilineage repopulation potential. Immunity. 2003;19:689–699. doi: 10.1016/s1074-7613(03)00299-1. [DOI] [PubMed] [Google Scholar]
- Robinson S D, Reynolds L E, Wyder L, Hicklin D J, Hodivala-Dilke K M. Beta3-integrin regulates vascular endothelial growth factor-A-dependent permeability. Arterioscler Thromb Vasc Biol. 2004;24:2108–2114. doi: 10.1161/01.ATV.0000143857.27408.de. [DOI] [PubMed] [Google Scholar]
- Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg J I, Cheresh D A, Johnson R S. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law D A, DeGuzman F R, Heiser P, Ministri-Madrid K, Killeen N, Phillips D R. Integrin cytoplasmic tyrosine motif is required for outside-in alphaIIbbeta3 signalling and platelet function. Nature. 1999;401:808–811. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- Feng W, McCabe N P, Mahabeleshwar G H, Somanath P R, Phillips D R, Byzova T V. The angiogenic response is dictated by beta3 integrin on bone marrow-derived cells. J Cell Biol. 2008;183:1145–1157. doi: 10.1083/jcb.200802179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerasinghe D, McHugh K P, Ross F P, Brown E J, Gisler R H, Imhof B A. A role for the alphavbeta3 integrin in the transmigration of monocytes. J Cell Biol. 1998;142:595–607. doi: 10.1083/jcb.142.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori M, Kawaguchi T, Berger M S, Pieper R O. Intracranial microenvironment reveals independent opposing functions of host alphaVbeta3 expression on glioma growth and angiogenesis. J Biol Chem. 2006;281:37256–37264. doi: 10.1074/jbc.M605344200. [DOI] [PubMed] [Google Scholar]
- Galarneau H, Villeneuve J, Gowing G, Julien J P, Vallieres L. Increased glioma growth in mice depleted of macrophages. Cancer Res. 2007;67:8874–8881. doi: 10.1158/0008-5472.CAN-07-0177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.