Abstract
The purpose of the present study was to test the therapeutic efficiency and safety of compacted-DNA nanoparticle-mediated gene delivery into the subretinal space of a juvenile mouse model of retinitis pigmentosa. Nanoparticles containing the mouse opsin promoter and wild-type mouse Rds gene were injected subretinally into mice carrying a haploinsufficiency mutation in the retinal degeneration slow (rds+/−) gene at postnatal day (P)5 and 22. Control mice were either injected with saline, injected with uncompacted naked plasmid DNA carrying the Rds gene, or remained untreated. Rds mRNA levels peaked at postinjection day 2 to 7 (PI-2 to PI-7) for P5 injections, stabilized at levels 2-fold higher than in uninjected controls for both P5 and P22 injections, and remained elevated at the latest time point examined (PI-120). Rod function (measured by electroretinography) showed modest but statistically significant improvement compared with controls after both P5 and P22 injections. Cone function in nanoparticle-injected eyes reached wild-type levels for both ages of injections, indicating full prevention of cone degeneration. Ultrastructural examination at PI-120 revealed significant improvement in outer segment structures in P5 nanoparticle-injected eyes, while P22 injection had a modest structural improvement. There was no evidence of macrophage activation or induction of IL-6 or TNF-α mRNA in P5 or P22 nanoparticle-dosed eyes at either PI-2 or PI-30. Thus, compacted-DNA nanoparticles can efficiently and safely drive gene expression in both mitotic and postmitotic photoreceptors and retard degeneration in this model. These findings, using a clinically relevant treatment paradigm, illustrate the potential for application of nanoparticle-based gene replacement therapy for treatment of human retinal degenerations.—Cai, X., Conley, S. M., Nash, Z., Fliesler, S. J., Cooper, M. J., Naash, M. I. Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa.
Keywords: gene replacement therapy, retinal degeneration slow protein, mouse opsin promoter
Inherited retinal diseases, including retinitis pigmentosa (RP) and various forms of macular dystrophy/degeneration account for ∼80% of retinal dystrophies (1). One of the most common photoreceptor genes associated with inherited retinal degeneration is RDS, which encodes the retinal degeneration slow (RDS) protein (also called peripherin/rds and Prph2), a 36-kDa tetraspanin glycoprotein expressed on the rims of rod and cone outer segment (OS) discs and required for the initial formation and subsequent maintenance of their structure (2, 3). Loss-of-function mutations in this gene cause a haploinsufficiency phenotype in patients heterozygous for the mutation and account for 5% of dominant retinitis pigmentosa (RP) (4, 5). The monogenic nature of most RP cases linked to RDS makes them prime candidates for gene therapy-based treatments. Furthermore, the availability of a well-characterized animal model for RDS-associated RP (the rds+/− mouse) provides a valuable and readily accessible in vivo system for developing and testing such treatments. The RP phenotype of the rds+/− mouse much more closely resembles the phenotype seen in patients with RDS-associated RP (compared with the more severe rds−/−) and was therefore chosen for this study as the appropriate clinically relevant model (6, 7).
In the heterozygous rds+/− disease model, photoreceptor degeneration results from apoptotic cell death (common in many types of photoreceptor degeneration/dystrophy; refs. 8,9,10,11). The mouse exhibits early onset, slow, progressive rod degeneration (scotopic a-wave amplitudes are reduced by ∼50% by 1 mo of age; ref. 12) followed by late-onset cone degeneration (6, 13). RDS protein levels in rds+/− retinas are about one-fourth of those seen in the wild-type (WT) retina, significantly less than the 50% predicted by mRNA levels (14). The OSs of rds+/− animals are short, highly disordered, and contain membranous whorls instead of neatly stacked, well-aligned discs or lamellae (6, 15, 16).
The eye offers an excellent target for gene therapy studies: it is easily accessible and relatively immune privileged, and the contralateral eye provides an ideal internal control in the same animal. Historically, development of nonviral gene delivery systems has been hampered by limited cell uptake of the vector (thus causing low gene expression levels) or by transient expression (17), although substantial progress has been made recently in these areas (18). In the present study, we utilize a specific formulation of DNA nanoparticles consisting of single molecules of DNA compacted with polyethylene glycol (PEG)-substituted lysine 30-mer peptides (CK30PEG10K) (19,20,21,22,23). These nanoparticles can deliver a wide range of cargo sizes and have been effectively utilized with plasmids as big as 20 kb (24). We have recently characterized transgene expression profiles after delivery of these nanoparticles to the WT eye (20). When injected in the subretinal space, these nanoparticles drive high, sustainable levels of transgene expression and do not provoke any appreciable immune response, cytotoxic side effects, or disruption of retinal function (25). Furthermore, we have recently shown that delivery of these nanoparticles carrying a therapeutic gene (the normal mouse peripherin/Rds, herein called NMP) can mediate substantial improvements in a gene phenotype (19). When we delivered nanoparticles containing the NMP gene under the control of a ubiquitously expressed promoter (CBA) or a photoreceptor-specific promoter (rod/cone promoter-interphotoreceptor retinoid binding protein-IRBP) to the subretinal space of neonatal rds+/− mice, we observed long-term, persistent gene expression and improvement in the RP disease phenotype (19). In terms of structural and functional rescue, treatment with the IRBP-NMP nanoparticle led to cone functional improvement to WT levels. However, despite elevated gene expression levels in rods, scotopic electroretinography (ERG) amplitudes were only slightly improved. These preliminary studies demonstrated the striking potential for nanoparticles to be developed as a clinically relevant therapeutic option for the treatment of RP but also highlighted 3 points that warranted further investigation and prompted the current study.
First, RP is characterized by initial rod degeneration, and consequent night blindness, followed later by cone degeneration and tunnel vision. While our previous study demonstrated substantial improvement in cone-based retinal function, we wanted to focus on enhancing rod improvement. To that end, for this study, we chose the powerful mouse rod opsin promoter (MOP). The promoter fragment we have chosen has been successfully used in many gene therapy studies, and while it has traditionally been considered rod specific, it has also been shown to direct expression in cones (26).
Second, the safety profile of compacted DNA administered to the diseased retina needs to be evaluated. In the lung, compacted DNA has a benign safety profile, with minimal evidence of bronchoalveolar cytokine induction despite the administration of compacted DNA containing numerous CpG islands (23). Although our preliminary data suggest that there is no detectable nanoparticle-generated immune response in the WT eye (25), assessment of potential cytokine induction and macrophage activation has not been evaluated in the rds+/− eye or in response to a therapeutic gene. The degenerating retinal characteristic of RP and other ocular disorders may have enhanced sensitivity to any drug compound, and hence safety and toxicology studies in an RP model are warranted.
Third, one of the ongoing critical issues impacting the design and improvement of gene therapy regimens (both viral and nonviral) for RP and allied disorders is the determination of the optimal time window for treatment efficacy. The onset of rod degeneration in the rds+/− retina occurs at about the end of the first postnatal month, while cones subsequently start dying a few months later. Gene replacement therapy attempts in which the WT Rds gene [in AAV form (27) or in compacted nanoparticles (19)] was subretinally delivered to the developing neonatal [postnatal day (P)5] eye were successful and yielded better rescue of the disease phenotype than in AAV gene transfer studies where treatment was delivered later (27,28,29). However, patients afflicted with RP typically do not present in the clinic until the retina is fully developed and has begun to degenerate; hence, gene therapy before the onset of retinal degeneration is rarely, if at all, possible or practical. Therefore, the purpose of the present study was to assess the efficiency of compacted DNA nanoparticles in rescuing the rds+/− phenotype in both the developing (P5) and postmitotic (P22) retina. We report here that nanoparticles containing the full-length cDNA of the NMP gene driven by MOP promoter successfully target and result in the expression of NMP in the OSs of photoreceptor cells spaced throughout the retina, resulting in structural and functional improvement of the disease phenotype. Moreover, there was no evidence of immune system activation following subretinal DNA nanoparticle administration to the rds+/− mouse. The findings presented herein suggest that the use of CK30PEG10K nanoparticles may provide a feasible postnatal gene therapy modality for treating diseases caused by mutations in ocular genes.
MATERIALS AND METHODS
Plasmid construction and nanoparticle compaction
The MOP-NMP vector (6122 bp) was generated and contains the full-length mouse Rds cDNA (1.7 kb) driven by the mouse MOP promoter (221 bp) (30) and followed by an SV40 poly(A) tail. The C terminus of the NMP cDNA contains the P341Q modification, which permits differential, epitope-specific detection of the transgenic protein against an endogenous RDS background using the 3B6 anti-rds monoclonal antibody (31). Compaction of this vector into CK30PEG10K nanoparticles was performed as described previously (19). Nanoparticles were suspended in saline at a final concentration of 3.06 μg/μl. Eyes injected with an equal amount of naked (unpackaged) MOP-NMP vector suspended in saline served as injected controls, while contralateral uninjected eyes served as uninjected controls.
Subretinal injection
P5 (or P2 where indicated) rds+/− mice were anesthetized by placement on ice for 2 min. The right eyelid was cut, and the cornea was punctured using a 30-gauge needle. A 35-gauge blunt-end needle attached to a 10-μl Nanofil syringe (World Precision Instruments, Sarasota, FL, USA) was inserted into the puncture site with visualization aided by use of an operating microscope (Carl Zeiss Surgical, Inc., Thornwood, NY, USA). A solution (0.3 μl) containing fluorescein dye (Bio-Rad Laboratories, Hercules, CA, USA) and either nanoparticles, naked DNA (each at 3.06 μg/μl containing ∼1.5×1011 DNA molecules), or saline (vehicle) was delivered into the subretinal space, usually in the superior temporal quadrant. Since the retina is not fully developed at this age, some injected material is likely released into the vitreous, although the site of injection is subretinal. After injection, the needle was left in place for a few seconds to allow full treatment delivery before being withdrawn gently. Successful delivery of material was confirmed by observation of subretinal yellow-green fluorescence at the time of injection. The eyelid was returned to its original position, and the surface of the eye was gently blotted with a KimWipe (Kimberley-Clark, Irving, TX, USA). Mice were warmed on a 37°C bed until fully awake.
Subretinal injections in P22 mice were undertaken as described previously. Briefly, animals were anesthetized by intramuscular injection of ketamine (85 mg/kg) and xylazine (14 mg/kg) (Pharmaceutical Systems Inc., Tulsa, OK, USA). Eyes were dilated with topical 1% Cyclogyl (Alcon, Decatur, IL, USA) and fundus visualization was enhanced by a drop of 2.5% Gonak Hypromellose solution (Akorn, Lake Forest, IL, USA) on the cornea. A corneal puncture was made, and transvitreal subretinal injection of 1 μl of either compacted or naked plasmid (1 μl at 3.06 μg/μl, containing ∼4.5×1011 DNA molecules) or saline was achieved using a 33-gauge blunt-end needle. Subretinal injections were confirmed by the appearance of a small retinal detachment (bleb) at the site of injection (32). At scheduled time points ranging from 2 to 120 days postinjection (PI-2 to PI-120), the eyes were harvested for endpoint monitoring as described below.
All animal protocols used in these experiments were approved by the local Institutional Animal Care and Use Committee (Oklahoma City, OK, USA) and were in accordance with the guidelines on the care and use of animals in research as established by the Society for Neuroscience and the Association for Research in Vision and Ophthalmology.
Quantitative real-time PCR (qRT-PCR)
qRT-PCR was performed using the primers and protocols previously reported (20). In brief, total RNA from 3 to 6 individual eyes was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and was treated with RNase-free DNase (Promega, Madison, WI, USA) to remove any traces of nanoparticle DNA, naked plasmid DNA, or genomic DNA. Reverse transcription was carried out with an Oligo-dT primer and Superscript III reverse transcriptase (Invitrogen). qRT-PCR was performed in triplicate on each cDNA sample using a single-color detector MyIQ (Bio-Rad) and relative expression was calculated against the house-keeping gene hypoxanthine phosphoribosyltransferase (HPRT; ref. 20). PCR amplification from a noncoding region of the plasmid or from samples in which reverse transcriptase was omitted was also carried out to confirm the complete removal of nanoparticle/plasmid and genomic DNA. Agarose gel electrophoresis and melt curve analysis were performed after each real-time reaction to confirm amplification specificity. Primer sequences for photoreceptor genes were as reported previously (33) and for cytokines were as follows: IL-6, forward ATGAACAACGATGATGCACTTG and reverse TATCCAGTTTGGTAGCATCCAT; TNF-α, forward AGCCCCCAGTCTGTATCCTT and reverse CTCCCTTTGCAGAACTCAGG.
Immunocytochemistry
Eyes were enucleated, fixed, and cryosectioned as described previously (6). The entire eye was sectioned, and every sixth section was collected to enable examination of the entire eye. After blocking (5% BSA in PBS), sections were incubated in primary antibody [anti-peripherin mAb 3B6, 1:100, a generous gift from Dr. Robert Molday (University of British Columbia, Vancouver, BC, Canada); RDS-CT rabbit polyclonal, 1:100, generated in-house (33, 34); S-opsin rabbit polyclonal, 1:500, generated in-house (33, 34); and F4/80 rat polyclonal antibody (macrophage marker), 1:500, Serotec Inc. (Oxford, UK)]; and secondary antibodies (1:1000 dilution; FITC-goat anti-mouse IgG, Cy3-goat anti-rabbit IgG, and AlexaFluor 555-goat anti-Rat IgG). Slides were mounted in Vectashield mounting medium with DAPI (Vector Laboratory Inc., Burlingame, CA, USA). Immunostaining of nanoparticle-injected eyes and controls (uninjected eyes and saline and naked DNA injected eyes) was performed simultaneously, and sections on which primary or secondary antibodies were omitted were also included. Observation and imaging were performed using an epifluorescence microscope (Axiophot; Zeiss Ltd., Oberkochen, Germany) and a spinning disk confocal microscope (BX62; Olympus, Tokyo, Japan).
Western blotting
Protein extraction and Western blotting were performed as reported previously (12, 34). Dissected individual retinas from nanoparticle-injected and control animals were homogenized on ice and solubilized [50 mM Tris (pH 7.8), 100 mM NaCl, 5 mM EDTA, 0.05% SDS, 1 mM PMSF, 1% TX-100, and 2.5% (v/v) glycerol]. Protein concentration was determined by Bradford assay (Bio-Rad), and equivalent protein amounts were loaded in each lane. The primary antibodies used were as follows: RDS-CT (1:1000) and ROM-1-CT (1:1000) rabbit polyclonals were generated in-house; mAB 1D4 against rhodopsin (1:10,000) was generated by Dr. Robert Molday; and monoclonal β-actin-horseradish perioxidase (HRP; 1:5000) was bought from Sigma (St. Louis, MO, USA). Blots were developed by exposure to suitable HRP-conjugated secondary antibodies and HRP substrates, with chemiluminescent detection.
ERG
Full-field ERG was performed at PI-30, PI-60, and PI-120 on animals injected at P5 or P22, as previously reported (12, 13). For testing scotopic responses, a stimulus intensity of 1.89 log cd · s · m−2 was applied to the dark-adapted (overnight) eyes in a Ganzfield (UTAS-3000; LKC Corp., Gaithersburg, MD, USA). For testing photopic responses, mice were adapted to a 1.46 log cd · s · m−2 light for 5 min, and then a light intensity of 1.89 log cd · s · m−2 was applied to the eye. A minimum of 5 animals was tested in each group/time point.
Histology and electron microscopy (EM)
Eyes were enucleated, fixed, and sectioned along the vertical meridian as described previously (12, 35). Briefly, enucleated eyes were fixed in 0.1 M sodium cacodylate buffer containing 2% glutaraldehyde, 2% paraformaldehyde, and 0.025% CaCl2 at 4°C overnight after the superior cornea was marked for orientation. Following removal of the cornea and lens, the eyecups were postfixed in 1% OsO4 in the above buffer at room temperature for 1 h. Eyecups were rinsed, then embedded in Spurr’s resin embedding fixative (Ted Pella Inc., Redding, CA, USA). Semithin (0.75 μm) sections were stained with 1% toluidine blue in 1% sodium borate and imaged with an Olympus BH-2 microscope under an ×63 oil-immersion objective. Ultrathin EM sections were poststained with uranyl acetate and lead citrate and imaged using a Jeol 100CX electron microscope (Jeol, Tokyo, Japan).
Statistical analyses
For qRT-PCR data, values are expressed as ratios (to uninjected eye) of mean ± se relative expression. Since ratios have an inherently skewed (non-Gaussian) distribution, data were log transformed before undergoing 1-way ANOVA. For ERG data, nanoparticle-injected groups were compared with naked DNA-injected groups and age-matched WT uninjected controls. All groups passed the Kolmogorov-Smirnov test for normality, and P values are from 1-way ANOVA with Bonferroni’s post hoc test. Means ± sd are reported in Table 1 as indicated. To determine whether there was any significant change in ERG amplitude over time, 2-way ANOVA with Bonferroni’s post hoc test was used to compare treatment groups at different ages. To determine whether naked DNA had any effect on function, 1-way ANOVA with Bonferroni’s post hoc test was used to compare the amplitudes of naked DNA-injected with those from saline-injected groups.
TABLE 1.
Average full-field ERG values at various time points
| Parameter | Treatment | P5
|
P22
|
||||
|---|---|---|---|---|---|---|---|
| N | Amplitude | P | N | Amplitude | P | ||
| Scotopic a-wave | |||||||
| PI-30 | Nanoparticle | 9 | 187.27 ± 43.81 | 8 | 115.17 ± 49.85 | ||
| Naked DNA | 10 | 75.06 ± 33.36 | <0.001 | 18 | 84.32 ± 52.76 | NS | |
| Uninjected WT | 14 | 429.16 ± 70.88 | <0.001 | 14 | 426.99 ± 77.87 | <0.001 | |
| PI-60 | Nanoparticle | 10 | 134.47 ± 38.17 | 5 | 158.33 ± 32.45 | ||
| Naked DNA | 6 | 84.24 ± 30.92 | NS | 5 | 60.26 ± 31.61 | NS | |
| Uninjected WT | 14 | 437.27 ± 79.09 | <0.001 | 14 | 392.76 ± 79.54 | <0.001 | |
| PI-120 | Nanoparticle | 5 | 104.48 ± 11.05 | 5 | 108.40 ± 19.64 | ||
| Naked DNA | 5 | 53.03 ± 33.90 | NS | 5 | 46.90 ± 30.62 | NS | |
| Uninjected WT | 12 | 387.72 ± 78.34 | <0.001 | 10 | 371.80 ± 96.08 | <0.001 | |
| Scotopic b-wave | |||||||
| PI-30 | Nanoparticle | 9 | 535.89 ± 156.81 | 8 | 362.98 ± 58.14 | ||
| Naked DNA | 10 | 241.21 ± 77.73 | <0.001 | 18 | 273.57 ± 145.43 | NS | |
| Uninjected WT | 14 | 806.70 ± 146.46 | <0.001 | 14 | 769.98 ± 146.89 | <0.001 | |
| PI-60 | Nanoparticle | 10 | 384.88 ± 147.90 | 5 | 614.85 ± 156.46 | ||
| Naked DNA | 6 | 193.60 ± 67.17 | <0.05 | 5 | 259.92 ± 134.11 | <0.001 | |
| Uninjected WT | 14 | 786.91 ± 113.51 | <0.001 | 14 | 753.39 ± 134.28 | NS | |
| PI-120 | Nanoparticle | 5 | 297.38 ± 29.99 | 5 | 364.52 ± 71.55 | ||
| Naked DNA | 5 | 157.67 ± 78.75 | NS | 5 | 189.16 ± 124.21 | NS | |
| Uninjected WT | 12 | 683.40 ± 180.37 | <0.001 | 10 | 702.23 ± 147.42 | <0.001 | |
| Photopic b-wave | |||||||
| PI-30 | Nanoparticle | 9 | 174.74 ± 53.43 | 8 | 204.85 ± 55.26 | ||
| Naked DNA | 10 | 115.51 ± 60.14 | <0.05 | 18 | 97.68 ± 32.09 | <0.001 | |
| Uninjected WT | 14 | 175.92 ± 40.45 | NS | 14 | 170.91 ± 25.68 | NS | |
| PI-60 | Nanoparticle | 10 | 137.83 ± 53.68 | 5 | 221.37 ± 34.29 | ||
| Naked DNA | 6 | 81.20 ± 38.10 | NS | 5 | 117.58 ± 72.70 | <0.001 | |
| Uninjected WT | 14 | 179.94 ± 52.37 | NS | 14 | 193.60 ± 34.16 | NS | |
| PI-120 | Nanoparticle | 5 | 106.24 ± 36.13 | 5 | 175.76 ± 36.12 | ||
| Naked DNA | 5 | 78.75 ± 51.64 | NS | 5 | 81.92 ± 58.65 | 0.01 | |
| Uninjected WT | 12 | 193.51 ± 56.57 | <0.01 | 10 | 174.17 ± 54.63 | NS | |
Values are averages ± sd. P values are based on 1-way ANOVA and are for comparison against nanoparticle treatment. NS, nonsignificant.
RESULTS
Compacted MOP-NMP nanoparticles drive stable gene expression after neonatal and juvenile treatment
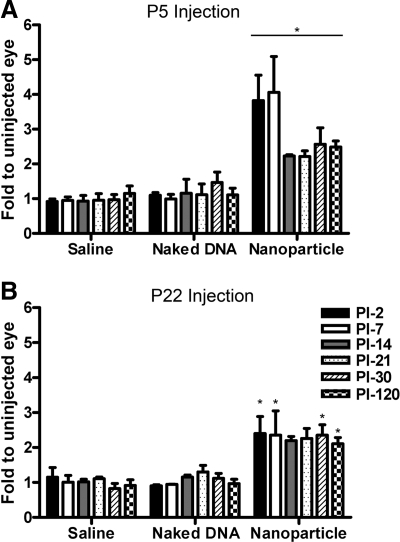
Rds gene expression levels were measured after treatment beginning at PI-2 and at various subsequent time points up to PI-120 (Fig. 1). Since the primers used amplify both endogenous and transgenic Rds message but do not amplify from the mutant Rds allele, values are expressed as fold change relative to the uninjected contralateral eye, enabling us to measure levels expressed by the transgene above endogenous levels. At early time points (PI-2 and PI-7), eyes injected neonatally (P5, just before initiation of OS formation) had Rds mRNA levels severalfold higher than the contralateral uninjected eyes (maximum 5.3-fold greater than controls, Fig. 1A). By PI-14, levels had decreased, but stabilized ∼2-fold higher than controls (statistically significant at P<0.01 at all time points) up to PI-120. Nanoparticle expression levels in P22-injected eyes were not significantly different from those in P5-injected eyes (∼2-fold elevation compared with controls, P<0.01); however, there was no early precipitous increase in mRNA levels after the P22 injection (Fig. 1B). Naked MOP-NMP DNA (unpackaged vector) and saline injection did not cause any significant alteration in Rds mRNA levels (Fig. 1).
Figure 1.
Injection of MOP-NMP nanoparticles into P5 and P22 rds+/− animals increases Rds mRNA levels. cDNA samples from eyes injected with saline, naked DNA, or nanoparticle DNA at P5 (A) or P22 (B) and from PI-2 through PI-120 were prepared and analyzed by qRT-PCR to determine relative Rds mRNA levels. Because Rds primers amplify both native (endogenous) and transgenic (exogenous) Rds genes, expression values are reported as fold change relative to the uninjected contralateral eye (control). Values are averages ± se (n=3–6 mice/group). Injection of nanoparticles at P5 (A) or P22 (B) led to statistically significant increases in Rds mRNA levels. Naked DNA and saline injection did not lead to any increase compared with the contralateral uninjected control eye. There was no significant difference between mRNA levels from eyes injected with nanoparticles at P5 and P22. *P < 0.01.
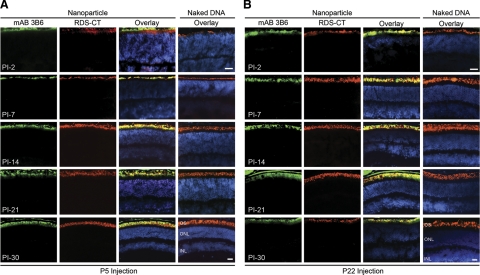
Next, the distribution and expression patterns of the transgenic (NMP) and endogenous RDS were studied throughout the eye by immunohistochemistry with mAB 3B6 and RDS-CT antibodies from PI-2 until PI-30 (Fig. 2; cf. Supplemental Figs. 1 and 2). We have previously shown that mAB 3B6 is specific for NMP (12, 36) and does not recognize endogenous mouse RDS. As confirmation, saline-injected eyes were also examined by immunohistochemistry, and no mAB 3B6 immunostaining was detected (37). At PI-2 after neonatal injection (corresponding to P7 in development), the OS is just starting to form (38) and endogenous RDS is being transported to the nascent OS blebs emanating from the distal tip of the photoreceptor cilium, projecting just above the outer nuclear layer (ONL). In these samples, transferred NMP was specifically localized to nascent OSs (Fig. 2A). As the OS developed (PI-7 to PI-30), mAB 3B6 immunoreactivity was localized to the growing OS (Fig. 2A); as evidenced by the colocalization of mAB 3B6 and RDS-CT immunofluorescence, no ectopic transgene expression was detected in any other photoreceptor organelle. The distribution pattern after injection in juvenile (P22) eyes was similar to that seen after P5 injection, i.e., NMP expression was detected in the photoreceptor OSs at all time points (Fig. 2B). Beginning around PI-21, transgene expression was occasionally detected in the RPE of nanoparticle-injected eyes (Fig. 2 and Supplemental Fig. 1) as evidenced by green signal in the RPE layer not overlapping with endogenous RDS-CT label, but RPE expression was extremely variable, and many injected eyes exhibited no RPE staining. No transgene expression was ever detected in cells of the inner retina or the anterior segment. Naked MOP-NMP did not drive any expression (Fig. 2; cf. Supplemental Fig. 2) after either P5 or P22 injection. To determine the extent to which the particles were distributed throughout the retina, the entire injected eye was sectioned and every sixth section was collected and examined. After both P5 and P22 injection, nanoparticle expression was not limited to the area of injection but was detected throughout the retina.
Figure 2.
Transgenic (nanoparticle-dependent) NMP colocalizes with endogenous RDS. Frozen retinal sections from eyes collected at multiple ages (PI-2 to PI-30) were immunostained for NMP (mAB 3B6, green) and total RDS (RDS-CT, red) with a nuclear counterstain (DAPI, blue). Transferred RDS from eyes injected with nanoparticles at P5 (A) and P22 (B) is detected beginning at PI-2. Expression remains strong through the latest time point analyzed (PI-30) and colocalizes with native RDS. Expression is limited to the OSs or nascent OSs and is not detected in any other retinal cell types, subcellular compartments, or layers. n = 3–5 mice/group. INL, inner nuclear layer. Scale bars = 20 μm.
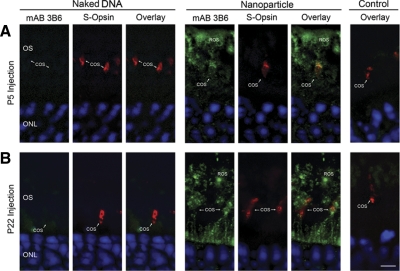
Since ∼95–97% of murine photoreceptors are rods, data in Fig. 2 clearly demonstrate that rods express the nanoparticle. However, since the MOP promoter has traditionally been considered to be rod specific, it was not clear whether cones would also express the transgene. Therefore, we performed coimmunolabeling at PI-30 with mAB 3B6 and anti-S-opsin (specific for short wavelength-sensitive cones). As shown in the confocal micrographs (single plane) in Fig. 3, after both P5 (Fig. 3A) and P22 (Fig. 3B) nanoparticle injection, cone photoreceptors were observed to express the transgenic NMP. In total, these results indicate that nanoparticle-delivered Rds is stably expressed (up to PI-120) at elevated levels in both rod and cone photoreceptors regardless of the stage of treatment.
Figure 3.
MOP-NMP nanoparticle injection drives gene expression in cone photoreceptors. Double immunolabeling for transferred RDS (3B6, green) and cone OSs (S-opsin, red) with nuclear counterstain (DAPI, blue) was performed at PI-30 from eyes injected at P5 (A) or P22 (B). Images are single planes from a spinning disk confocal image stack. Representative cones from nanoparticle-injected animals are shown for each treatment. Cones in eyes injected with nanoparticles express transgenic NMP. Naked DNA-injected eyes express no transgenic NMP. Controls (right) are from saline-injected (A) and uninjected (B) animals. n = 3–5 animals/group. ROS, segment; COS, cone outer segment. Scale bar = 5 μm.
Interestingly, these nanoparticles are also capable of driving transgene expression before expression of the corresponding endogenous protein begins. When subretinal injections were performed at P2, instead of P5, NMP expression was detected at PI-2 (P4), whereas endogenous RDS expression does not occur that early in development (see Supplemental Fig. 3). Future studies may take advantage of this finding to accelerate or improve the efficiency of rescue of disease phenotypes.
MOP-NMP nanoparticles do not induce acute immune response
Previously, we (20) have shown that nanoparticle injection, per se, does not compromise retinal function (as measured by ERG). In addition, our preliminary data have demonstrated that nanoparticles are not associated with infiltration of macrophages, neutrophils, or other immune cells in the eye and that there is no nanoparticle-associated increase in the expression of inflammatory chemokines, such as TNF-α, MCP, or KC (25). However, those studies were undertaken in the WT retina, and it is possible that the diseased rds+/− retina has a different immune response or is more sensitive than the WT to damage associated with subretinal injection or that delivery of a therapeutic gene (NMP) may cause a different immune response than delivery of the reporter gene we used previously.
We demonstrated that ∼60% of the retina is detached after subretinal injection of saline in adult WT mice and that the material injected is distributed throughout the entire eye (32). In these mice, the retina typically reattaches after 24 h, albeit with substantial folding that is mostly resolved by PI-6. Sixty percent functional recovery was recorded at PI-14 with complete recovery observed at the next time point, PI-60 (32). Our current results indicate that after P22 injection of saline into the subretinal space of rds+/− mice, the retina reattaches fairly quickly, between PI-2 and PI-7, although resolution of retinal folding is often delayed (compared with WT), with folds still observed at PI-21 throughout the eye. P5 injection of rds+/− also causes retinal detachment, with reattachment and resolution of folding occurring between PI-2 and PI-7. As with P22 injections, some eyes continue to exhibit retinal folding at PI-21 but only on the temporal (injected) side of the eye. Functionally, WT eyes injected with saline at P5 recover to levels seen in the contralateral control eye by PI-30 (Supplemental Fig. 4). On the other hand, rds+/− eyes exhibit delayed functional recovery, particularly in the scotopic a- and b-waves (69 and 73% of uninjected, respectively; Supplemental Fig. 4). This delay suggests that the degenerating rds+/− mice may be more affected by the subretinal injection procedure than WT and that any successful treatment would need to overcome both injection-related and disease-related defects. We do not detect any differences in detachment/reattachment between treatment groups (i.e., saline, naked DNA, or nanoparticle) but have observed that some animals heal faster than others, most likely due to variations in the injection procedure.
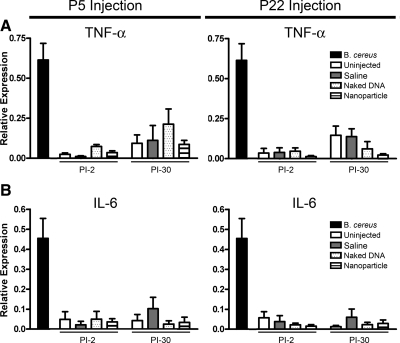
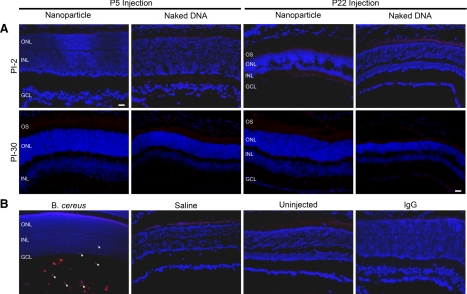
To determine whether the rds+/− retina might exhibit nanoparticle-associated immune responses, we used qRT-PCR to examine expression of inflammatory mediators TNF-α and IL-6 and immunofluorescence to examine expression of F4/80, a microglia/macrophage marker (39, 40) at PI-2 and PI-30 in rds+/− retinas that had been injected at P5 or P22 with nanoparticles (in comparison with naked DNA and saline controls). Eyes from an experimental endophthalmitis model (Bacillus cereus injection) with confirmed macrophage infiltration/immune response were used as positive controls.
Although induction of TNF-α and IL-6 is commonly observed in cases of immune system activation, no significant increase in message levels (compared with uninjected eyes) of either cytokine was detected in animals treated with nanoparticles, naked DNA, or saline (Fig. 4), although robust expression of these factors was detected in positive control samples. Induction of F4/80 in response to ischemia-induced retinopathy has been described in mouse retina (41) and has been implicated in experimental choroidal neovascularization (42). However, as shown in Fig. 5A, no F4/80 immunoreactivity was detected in nanoparticle-, naked DNA-, or saline-injected eyes. B. cereus-injected eyes were used as positive controls with uninjected eyes and nanoparticle-injected eyes without primary antibody as negative controls (Fig. 5B). Neither time of treatment (P5 vs. P22) nor age of testing (PI-2 vs. PI-30) had any effect on the observed lack of immune response. Results from this assay are in keeping with our previous findings and suggest that rds+/− eyes do not exhibit an acute macrophage response or an acute increase in expression of TNF-α or IL-6 after nanoparticle injection.
Figure 4.
MOP-NMP nanoparticle injection does not elicit an acute cytokine response. qRT-PCR for TNF-α and IL-6 was completed at PI-2 and PI-30 on cDNA prepared from whole eyes for animals injected at P5 or P22. No significant differences were observed between TNF-α (A) or IL-6 (B) levels in either injected sample groups (MOP-NMP nanoparticle, naked DNA, or saline treatment) or uninjected animals. Significant cytokine expression was detected in identically prepared positive control samples from the B. cereus endophthalmitis model.
Figure 5.
MOP-NMP nanoparticle injection does not elicit an acute macrophage response. A) Immunolabeling with the macrophage/microglia marker F4/80 was performed at PI-2 and PI-30 in eyes injected with nanoparticles or naked DNA at P5 or P22. No labeling was detected. B) Positive control image is from an experimental endophthalmitis model to confirm antibody recognition (arrows indicate infiltrating macrophages). Negative controls include saline-injected and uninjected sections, and sections from nanoparticle-injected eyes on which normal rat IgG was used instead of the F4/80 primary antibody. GCL, ganglion cell layer. Scale bar = 20 μm.
MOP-NMP nanoparticles lead to increased expression of OS proteins
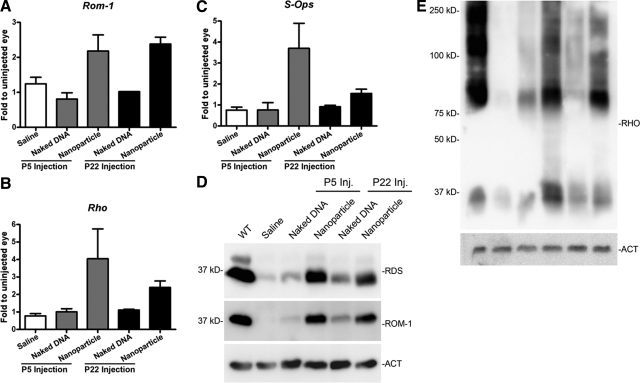
Since expression of many photoreceptor OS specific genes is reduced in the rds+/− model due to its abnormal OS structure, one sign of nanoparticle-mediated rescue would be increased expression of these proteins. At PI-30, qRT-PCR and Western blotting from P5 and P22 nanoparticle-injected samples and controls were performed (Fig. 6). Consistent with the qRT-PCR data presented in Fig. 1, Western blots probed with anti-RDS-CT antibody indicate that nanoparticle treatment leads to increased RDS protein level (Fig. 6D). The RDS binding partner ROM-1 is expressed at lower levels than normal in the rds+/− eye. After nanoparticle injection, however, qRT-PCR and Western blot results suggest that expression of ROM-1 is increased on both the message (Fig. 6A) and protein (Fig. 6D) levels. Rom-1 message was increased by ∼2-fold, relative to uninjected control eyes, consistent with the observed increase in Rds message. Another protein of particular interest to us is rhodopsin, the rod photopigment. Normally, levels of rhodopsin are decreased in the rds+/− retina, commensurate with the reduced amount of OS membrane mass; however, in nanoparticle-treated eyes, there were increases in the expression of rhodopsin message and protein (Fig. 6B, E). Finally, since MOP-NMP nanoparticles also drive gene expression in cones, we examined the message levels of short-wavelength cone opsin (S-opsin) and found that it too was increased after nanoparticle treatment at P5, although no obvious changes were detected after P22 (Fig. 6C) or in S-opsin protein levels at either time point (not shown). There were no significant differences in ROM-1, rhodopsin, or S-opsin message or protein levels when animals injected at P5 were compared with those injected at P22.
Figure 6.
Transferred NMP leads to increased expression of photoreceptor-specific proteins in the rds+/− retina. A–C) Message levels of photoreceptor proteins at PI-30 were analyzed by qRT-PCR for both P5 and P22 injected mice. MOP-NMP nanoparticle injections (but not naked DNA or saline) lead to increases in Rom-1 (A), rhodopsin (B), and cone S-opsin (C) message after P5 and P22 injection. n = 3 animals/group. D, E) Protein levels at PI-30 after nanoparticle, naked DNA, or saline P5 and P22 injections were examined. Representative SDS-PAGE/Western blots from individual retinas are shown (n=5–6/group). MOP-NMP nanoparticle injections at P5 and P22 increase RDS and ROM-1 (20 μg/lane; D), and rhodopsin (RHO; 10 μg/lane; E). Actin is shown as a loading control.
Improvement in retinal function after MOP-NMP nanoparticle treatment
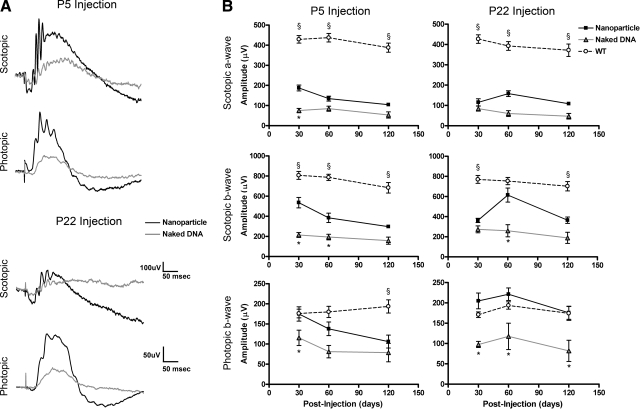
To assess retinal function after P5 and P22 injection, full-field scotopic and photopic ERGs were measured at PI-30, -60, and -120 in nanoparticle-treated and control animals. Consistent with previous studies (12, 36) on the rds+/−, uninjected and control-injected eyes (naked DNA and saline) exhibited reduced scotopic ERG amplitudes at PI-30 compared with WT. Based on a 1-way ANOVA, average scotopic a-wave amplitudes in nanoparticle-injected eyes were improved with significance after P5 treatment (at PI-30, 2.5-fold improvement compared with naked DNA injected controls, P<0.001). Scotopic a-wave values also improved after P22 injection but without significance (Fig. 7 and Table 1). Scotopic b-wave amplitudes were also increased with significance after both P5 (at PI-30) and P22 injection (at PI-60). The increase in scotopic b-wave amplitudes was not accounted for by concurrent improvements in a-wave amplitude, suggesting that both rod function and overall retinal health (as measured by second-order neuron signaling) were improved after nanoparticle treatment. Based on 2-way ANOVA, age was not an interacting variable in any case. Despite improvements, average scotopic levels did not reach those seen in age-matched untreated WT animals. In addition, we observed some variation in the extent of rescue; some animals exhibited better rescue than others. Average scotopic ERG levels in nanoparticle-treated eyes remained above those observed in naked DNA-treated eyes (baseline levels) to PI-120 but not with statistical significance at this latest time point (Table 1).
Figure 7.
Expression of transferred NMP leads to partial functional rescue of the rds+/− phenotype. A) Representative scotopic and photopic traces from naked DNA-injected (gray trace) and nanoparticle-injected eyes (black trace) at PI-30. B) Scotopic a-wave (top) and b-wave (middle) amplitudes from eyes injected with MOP-NMP nanoparticles are elevated compared with naked DNA injected eyes after P5 and P22 injection but do not reach levels seen in age-matched WT animals. Photopic b-wave (bottom) amplitudes from eyes injected with MOP-NMP nanoparticles are elevated compared with naked DNA-injected eyes after P5 and P22 injection and meet or exceed levels seen in age-matched WT animals. Amplitudes are means ± se (N values in Table 1). *P < 0.05, §P < 0.05 vs. nanoparticle treatment.
In contrast to the modest effects on the scotopic (rod-based) function, average photopic (cone-based) values in nanoparticle-injected eyes not only improved compared with baseline (naked DNA-injected eyes), but they approached or exceeded WT values. As shown in Fig. 7 and Table 1, photopic b-wave amplitudes in nanoparticle-injected eyes were increased with significance compared with those treated with naked DNA at all time points after P22 injection and at PI-30 after P5 injection and were not significantly different from photopic b-wave amplitudes recorded in age-matched WT animals except for P5 injected eyes at PI-120.
To determine whether visual behavior correlated with retinal signaling, photopic optomotor testing was undertaken on a small subset of treated and untreated mice. Results from 2 nanoparticle-treated mice (right eye injected, left eye uninjected), 5 uninjected mice, and 4 WT mice are shown in Supplemental Fig. 5. The 2 MOP-NMP-injected eyes have visual acuities (left panel) and contrast sensitivities (right panel) substantially higher than the untreated control animals and similar to those observed in the WT animals. Interestingly, acuity and contrast sensitivity values for uninjected contralateral eyes [MOP-NMP(1)-L and (2)-L] are lower than for age-matched uninjected animals (uninjected rds+/−). This is most likely an adaptive response arising from the improved vision in the treated eye, i.e., these mice use the treated eye almost exclusively. Note that the improved acuity and contrast sensitivity observed in these behavioral tests track cone-related visual functions, which correspond nicely to the significantly improved cone ERG function observed in Fig. 7.
Structural improvement after NMP nanoparticle delivery
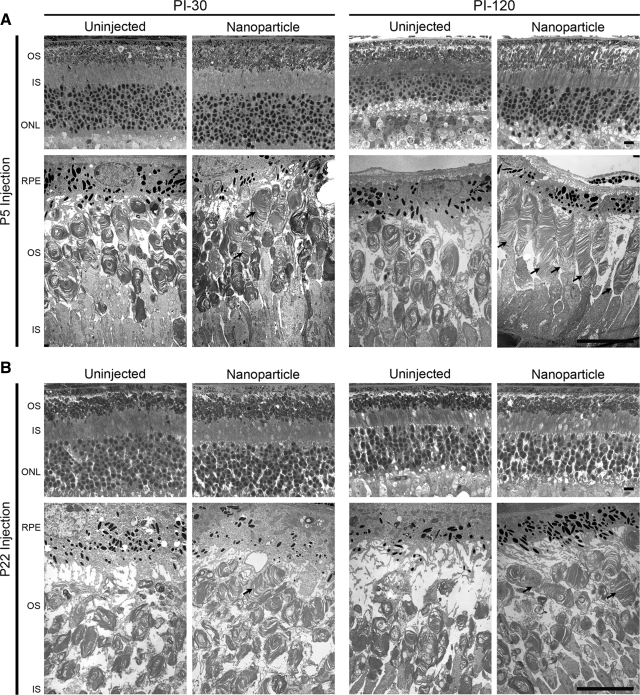
Finally, we examined the retina by histology and EM to determine whether nanoparticles resulted in an improvement in retinal structure. Eyes were collected at PI-30 and PI-120 after treatment at P5 or P22. Representative images from the temporal region are shown in Fig. 8. After P5 injection (at both PI-30 and PI-120), the thickness of the OS layer and the ONL appeared to be enhanced in nanoparticle-injected eyes compared with uninjected controls, although this change was not observed after P22 injection. To determine whether these improvements occurred throughout the retina or only proximal to the site of injection, a series of morphometric studies was undertaken on a small subset of eyes (see Supplemental Methods). As shown in Supplemental Fig. 5, at PI-30, improvement in the thickness of the ONL (number of vertical rows of nuclei) was quite variable; shown are 2 representative eyes, 1 demonstrating improvement and 1 without. Eyes demonstrating improvement did so on the injected side of the eye, suggesting possible attenuation of photoreceptor degeneration in that region, but the results were variable.
Figure 8.
Transferred NMP leads to structural rescue of the rds+/− phenotype. Light micrographs (top rows) and electron micrographs (bottom rows, n=3–5/group) from the temporal side of rds+/− eyes were examined. After P5 (A) or P22 (B) injection, moderate ultrastructural rescue is detected in the OSs of nanoparticle-injected eyes (arrows) at PI-30; significant ultrastructural improvement in OSs of nanoparticle-injected eyes is apparent by PI-120. OS discs are properly aligned and flattened, and improved OSs do not exhibit the swirl-like structures typical of the rds+/−. IS, inner segment layer; RPE, retinal pigment epithelium. Scale bars = 10 μm.
We observed substantial ultrastructural improvement in rod photoreceptor OSs after P5 and modest improvement after P22 injection. Rod OSs in the rds+/− retinas are typically short and rounded and, characteristically, contain poorly aligned discs and an abundance of membranous whorl-like formations, in addition to considerable areas of cystic space in the OS layer. The improvement in nanoparticle-injected eyes was first noticeable at PI-30 (Fig. 8, left panels), as evidenced by the increased amount of membrane mass in the OS layer. At PI-30 after P5 injection, there also were some OSs that exhibited signs of improved structure (Fig. 8A, arrows, left 2 panels), but very few of these were seen at PI-30 after P22 injection (Fig. 8B, arrows, left 2 panels). Ultrastructural improvement was much more pronounced at PI-120 after P5 injection; we observed many rod OSs with neatly aligned and properly formed discs (Fig. 8, right 2 panels; cf. Supplemental Fig. 6). There was considerable variability in the degree of structural rescue, consistent with the variability observed in the ERG data (see above): some OSs appeared to be almost comparable to WT in length and contained tightly stacked discs (Supplemental Fig. 6, top right), while others showed more modest improvement. Based on examination of images captured across the retina, ∼30–50% of the whole retina showed ultrastructural improvement after P5 injection; however, structural rescue of rods was slight after P22 injection (Fig. 8B; Supplemental Fig. 6, bottom right).
DISCUSSION
Taken together, the evidence presented in the present work clearly demonstrates that CK30PEG10K nanoparticles containing the WT Rds (NMP) gene under the control of the MOP promoter provide a therapeutically effective gene delivery system for rescuing the disease phenotype in a mouse RP model (rds+/−). Nanoparticles produced elevated (2-fold higher than uninjected control eyes) and stable levels (up to PI-120) of transgene expression in rod and cone photoreceptors after treatment at either P5 or P22 without any signs of acute inflammatory response. Eyes treated at both P5 and P22 exhibited improvements in the levels of photoreceptor-specific gene transcripts, such as rhodopsin, S-opsin, and ROM-1, as well as improved retinal function and visual behavior. Notably, improvement in cone function was quite pronounced; levels in treated eyes matched or exceeded those in age-matched WT controls. Ultrastructural rescue was observed after P5 injection at both PI-30 and PI-120 in the form of improved OS structure, length, disc alignment, and membrane density. ONL thickness was improved in some mice dosed with nanoparticles at P5. Despite some modest functional improvements, little structural rescue was observed after P22 injection.
Although our previous studies demonstrated that nanoparticle injection is not associated with reductions in retinal function in the WT eye, and preliminary results from our comprehensive immune study have shown that nanoparticles do not induce an acute immune response in the WT eye (25), we were concerned that the diseased rds+/− retina might be more prone to developing toxic responses. This issue is of particular importance given that any eyes needing treatment are, by definition, diseased. It was therefore reassuring to detect no overt signs of acute inflammatory response to the nanoparticles (as measured by macrophage immunoreactivity and TNF-α/IL-6 expression). These findings complement those of nanoparticle dosing in the lung, which also showed minimal inflammatory response (23).
As expected for the photoreceptor-specific Rds gene as well as the MOP promoter, the product of the transgene was localized almost exclusively to the OSs or nascent OSs of photoreceptor cells, not in other organelles. This expression pattern is similar to what was previously shown in the retinas of transgenic Xenopus in which a full-length Rds cDNA fused with GFP was driven by a Xenopus opsin promoter (43) and similar to our previous results using NMP nanoparticles (under the control of a different promoter) in the rds+/− retina (19). Interestingly, we see nanoparticle expression throughout the retina, i.e., not limited to the area of injection, but do not see expression in every photoreceptor cell. Although we predicted that the MOP promoter would be rod specific (29, 44), our results showed that it was capable of driving gene expression in both rods and cones. The rare finding of NMP expression in RPE cells may be due to ectopic transgene expression and/or engulfment of rod OSs expressing NMP. There is a precedent for MOP-directed expression in cones: when a 500-bp fragment of the MOP promoter was used for AAV-mediated gene delivery, expression was also observed in both rods and cones (26). Our primary goal in choosing this promoter was to rescue rod photoreceptors, since they are the most affected by Rds haploinsufficiency. Despite choosing this promoter, however, the majority of the improvement we observed was in cone photoreceptors, with rods showing a more modest rescue effect.
This result is consistent with our previous study (19) demonstrating that treatment of neonatal rds+/− with IRBP-NMP nanoparticles (expressing in cones and rods) provided more substantial structural and functional rescue to cones than rods. Despite the similarly small magnitude of rod functional rescue with either the MOP-NMP or IRBP-NMP nanoparticles, ultrastructural improvement in rod OSs was much more pronounced with the MOP-NMP nanoparticles, many of which looked nearly identical to WT OSs. In terms of gene expression levels (and persistence thereof), localization, and biochemical improvement, the 2 nanoparticles (MOP-NMP and IRBP-NMP) performed similarly. Given our positive results regarding improvements in photopic (cone) retinal function, either the MOP-NMP or IRBP-NMP nanoparticles would be suitable choices for future studies.
We hypothesize that the enhanced cone improvement (vs. rod) arises from a combination of 2 factors. First, given the complexity of photoreceptor degeneration and our observation that structural rescue is more pronounced after P5 injection than P22, it is likely that it is much easier to prevent degeneration of photoreceptors than to rescue those that have already begun to degenerate. Since cones retain normal structure/function longer than rods in the rds+/− retina, it stands to reason that they would be easier to protect. Second, we have previously shown that RDS may play different roles in rods vs. cones (33, 45) and that the sensitivity of the 2 cell types to RDS deficiency or mutations are quite different. One of the most obvious examples of this phenomenon is that rods need more RDS to function normally than do cones (12, 33; hence, the earlier degeneration of rods in the rds+/− mouse), suggesting that a less exogenous gene would need to be introduced to promote improvements in cone function. These factors combined provide ample evidence to suggest that full cone rescue is quite feasible after nanoparticle-mediated treatment.
The P5 and P22 treatment ages were selected for this study based on the fact that at P5 the murine retina is still undergoing mitosis, cellular differentiation and maturation; in contrast, by P22 the rds+/− retina is almost fully developed and postmitotic and also has begun to degenerate. There have been several studies (27, 28, 38, 46, 47) reporting large differences in transfection or transduction efficiency depending on the age of treatment. In those reports, transgene expression levels were usually higher after treatment of young mice compared with treatment of adults. In this study, we did not observe a difference in efficiency of vector delivery or expression based on age of treatment (Figs. 1 and 2). Nanoparticles drove sustained, elevated levels of gene expression regardless of treatment age, consistent with the previously reported ability of CK30PEG10K nanoparticles to transfect both mitotic and postmitotic cells (ref. 22; for reviews, see refs. 17, 21). However, in common with other viral studies, we report that structural and functional rescue of the diseased retina after nanoparticle treatment is more pronounced after P5 injection than after P22. In our case, this difference is likely a result of the ongoing degeneration rather than the efficiency in transduction. After treatment at both P5 and P22, gene expression in nanoparticle-injected eyes stabilized at levels ∼2-fold higher than in uninjected rds+/− controls (Fig. 1). This suggests that the transfection is similar after P5 and P22 treatment but that after neonatal delivery the cells are being corrected before the onset of death, while P22 delivery occurs after the onset of the death signal. The gene expression levels are similar to what would be seen in WT animals, and although we have previously demonstrated that overexpression of Rds is not toxic to the photoreceptor (12), overexpression of other genes (e.g., rhodopsin; ref. 35) can be toxic; hence, it is important to be able to titrate expression of exogenous genes to match native expression levels.
Although we used a rod-dominant RP model for this study (rds+/−), many RDS mutations cause cone-dominant macular degenerations (48). These disease mutations are often gain-of-function mutations, but our results suggest that nanoparticle-based gene supplementation with RDS could provide excellent phenotype improvement when coupled with concurrent knockdown therapy to eliminate the gain-of-function allele. As part of our groundwork for this gene therapy study, we previously showed that gene supplementation with WT Rds (NMP) via transgenesis could be used to provide full rod and cone rescue to the rds+/− and the C214S/rds+/− RP models (7, 12). Interestingly, we also demonstrated that gene supplementation could provide substantial rod and cone rescue in a cone-dominant macular degeneration model (36). The R172W mutation causes macular degeneration in patients and early onset severe cone degeneration (with subsequent rod degeneration) in mice. Despite the presence of the gain-of-function R172W mutant allele, transgenic animals coexpressing NMP exhibited improved cone (and rod) structure and function. Although cone rescue lasted only 3 mo, these results coupled with those presented here suggest that gene replacement therapy for dominant mutations might be of some use even without knockdown therapy.
In summary, we have demonstrated that treatment of an RP mouse model with nanoparticles containing WT Rds driven by the MOP promoter, either at an early neonatal (P5) or juvenile (P22) stage, can partially rescue the rds+/− disease phenotype (although less effectively at P22 than at P5). Subretinal delivery of NMP nanoparticles did not demonstrate evidence of cytokine induction or macrophage activation in the rds+/− retina. These data, coupled with our previous work on ocular use of compacted DNA nanoparticles (20), suggest that this technology has great promise as a therapeutic option for treatment of human hereditary ocular diseases, as well as possibly other genetic diseases.
Supplementary Material
Acknowledgments
The authors thank Barbara Nagel for excellent EM and Lauren Brush for visual behavior studies. The authors also thank Rasha Makkia and Ehsan Nour-Mohammadi for excellent technical assistance. Drs. Robert Barlow and Yumiko Umino provided assistance with the visual behavior testing, and Dr. Michelle Callegan generously shared positive control specimens. This work was supported by National Institutes of Health grants EY-010609 and EY-018656 (to M.I.N.) and EY-007361 (to S.J.F.); the Foundation Fighting Blindness, Baltimore, MD, USA (to M.I.N.); Oklahoma Center for the Advancement of Science and Technology (OCAST), Oklahoma City, OK, USA (to M.I.N.); a vision COBRE (to M.I.N.); and an unrestricted grant from Research to Prevent Blindness (to S.J.F.). M.I.N. and S.J.F. are recipients of the Research to Prevent Blindness James S. Adams Scholar Award and Senior Scientific Investigator Award, respectively. M.J.C. is an employee of Copernicus Therapeutics and owns stock in the company.
References
- Weleber R. Phenotypic variation in patients with mutation in the peripherin/RDS gene. [Online] Digit J Opthalmol. 1999;5(2) [Google Scholar]
- Connell G, Bascom R, Molday L, Reid D, McInnes R R, Molday R S. Photoreceptor peripherin is the normal product of the gene responsible for retinal degeneration in the rds mouse. Proc Natl Acad Sci U S A. 1991;88:723–726. doi: 10.1073/pnas.88.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis G H, Sutcliffe J G, Bok D. The retinal degeneration slow (rds) gene product is a photoreceptor disc membrane-associated glycoprotein. Neuron. 1991;6:61–70. doi: 10.1016/0896-6273(91)90122-g. [DOI] [PubMed] [Google Scholar]
- Keen T J, Inglehearn C F. Mutations and polymorphisms in the human peripherin-RDS gene and their involvement in inherited retinal degeneration. Hum Mutat. 1996;8:297–303. doi: 10.1002/(SICI)1098-1004(1996)8:4<297::AID-HUMU1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Kohl S, Giddings I, Besch D, Apfelstedt-Sylla E, Zrenner E, Wissinger B. The role of the peripherin/RDS gene in retinal dystrophies. Acta Anat. 1998;162:75–84. doi: 10.1159/000046471. [DOI] [PubMed] [Google Scholar]
- Cheng T, Peachey N S, Li S, Goto Y, Cao Y, Naash M I. The effect of peripherin/rds haploinsufficiency on rod and cone photoreceptors. J Neurosci. 1997;17:8118–8128. doi: 10.1523/JNEUROSCI.17-21-08118.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour M, Fliesler S J, Naash M I. Genetic supplementation of RDS alleviates a loss-of-function phenotype in C214S model of retinitis pigmentosa. Adv Exp Med Biol. 2008;613:129–138. doi: 10.1007/978-0-387-74904-4_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalloniatis M, Fletcher E L. Retinitis pigmentosa: understanding the clinical presentation, mechanisms and treatment options. Clin Exp Optom. 2004;87:65–80. doi: 10.1111/j.1444-0938.2004.tb03152.x. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C, Sung C H, Nathans J, Adler R. Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa. Proc Natl Acad Sci U S A. 1994;91:974–978. doi: 10.1073/pnas.91.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G Q, Hao Y, Wong F. Apoptosis: final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. Neuron. 1993;11:595–605. doi: 10.1016/0896-6273(93)90072-y. [DOI] [PubMed] [Google Scholar]
- Chang G Q, Gaitan A, Hao Y, Wong F. Correlation of DNA fragmentation and chromatin condensation in apoptotic nuclei of the Ser 6 mouse retina. Microsc Res Tech. 1997;36:123–129. doi: 10.1002/(SICI)1097-0029(19970115)36:2<123::AID-JEMT5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Nour M, Ding X Q, Stricker H, Fliesler S J, Naash M I. Modulating expression of peripherin/rds in transgenic mice: critical levels and the effect of overexpression. Invest Ophthalmol Vis Sci. 2004;45:2514–2521. doi: 10.1167/iovs.04-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Cheng M, Yang H, Peachey N S, Naash M I. Age-related changes in the mouse outer retina. Optom Vis Sci. 2001;78:425–430. doi: 10.1097/00006324-200106000-00015. [DOI] [PubMed] [Google Scholar]
- Kedzierski W, Nusinowitz S, Birch D, Clarke G, McInnes R R, Bok D, Travis G H. Deficiency of rds/peripherin causes photoreceptor death in mouse models of digenic and dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 2001;98:7718–7723. doi: 10.1073/pnas.141124198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R K, Jansen H G, Sanyal S. Development and degeneration of retina in rds mutant mice: photoreceptor abnormalities in the heterozygotes. Exp Eye Res. 1985;41:701–720. doi: 10.1016/0014-4835(85)90179-4. [DOI] [PubMed] [Google Scholar]
- Ma J, Norton J C, Allen A C, Burns J B, Hasel K W, Burns J L, Sutcliffe J G, Travis G H. Retinal degeneration slow (rds) in mouse results from simple insertion of a t haplotype-specific element into protein-coding exon II. Genomics. 1995;28:212–219. doi: 10.1006/geno.1995.1133. [DOI] [PubMed] [Google Scholar]
- Cai X, Conley S, Naash M. Nanoparticle applications in ocular gene therapy. Vis Res. 2008;48:319–324. doi: 10.1016/j.visres.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagali P S, Balya D, Awatramani G B, Munch T A, Kim D S, Busskamp V, Cepko C L, Roska B. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci. 2008;11:667–675. doi: 10.1038/nn.2117. [DOI] [PubMed] [Google Scholar]
- Cai X, Nash Z, Conley S M, Fliesler S J, Cooper M J, Naash M I. A partial structural and functional rescue of a retinitis pigmentosa model with compacted DNA nanoparticles. PLoS ONE. 2009;4:e5290. doi: 10.1371/journal.pone.0005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjo R, Skaggs J, Quiambao A B, Cooper M J, Naash M I. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS ONE. 2006;1:e38. doi: 10.1371/journal.pone.0000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P B, Cooper M J. Vectors for airway gene delivery. AAPS J. 2007;9:E11–17. doi: 10.1208/aapsj0901002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Li D, Pasumarthy M K, Kowalczyk T H, Gedeon C R, Hyatt S L, Payne J M, Miller T J, Brunovskis P, Fink T L, Muhammad O, Moen R C, Hanson R W, Cooper M J. Nanoparticles of compacted DNA transfect postmitotic cells. J Biol Chem. 2003;278:32578–32586. doi: 10.1074/jbc.M305776200. [DOI] [PubMed] [Google Scholar]
- Ziady A G, Gedeon C R, Muhammad O, Stillwell V, Oette S M, Fink T L, Quan W, Kowalczyk T H, Hyatt S L, Payne J, Peischl A, Seng J E, Moen R C, Cooper M J, Davis P B. Minimal toxicity of stabilized compacted DNA nanoparticles in the murine lung. Mol Ther. 2003;8:948–956. doi: 10.1016/j.ymthe.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Fink T L, Klepcyk P J, Oette S M, Gedeon C R, Hyatt S L, Kowalczyk T H, Moen R C, Cooper M J. Plasmid size up to 20 kbp does not limit effective in vivo lung gene transfer using compacted DNA nanoparticles. Gene Ther. 2006;13:1048–1051. doi: 10.1038/sj.gt.3302761. [DOI] [PubMed] [Google Scholar]
- Ding X Q, Quiambao A B, Fitzgerald J B, Cooper M J, Conley S M, Naash M I. Ocular delivery of compacted DNA nanoparticles does not elicit toxicity in the mouse retina. PLoS ONE. 2009;4:e7410. doi: 10.1371/journal.pone.0007410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glushakova L G, Timmers A M, Issa T M, Cortez N G, Pang J, Teusner J T, Hauswirth W W. Does recombinant adeno-associated virus-vectored proximal region of mouse rhodopsin promoter support only rod-type specific expression in vivo? Mol Vis. 2006;12:298–309. [PubMed] [Google Scholar]
- Ali R R, Sarra G M, Stephens C, Alwis M D, Bainbridge J W, Munro P M, Fauser S, Reichel M B, Kinnon C, Hunt D M, Bhattacharya S S, Thrasher A J. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat Genet. 2000;25:306–310. doi: 10.1038/77068. [DOI] [PubMed] [Google Scholar]
- Sarra G M, Stephens C, de Alwis M, Bainbridge J W, Smith A J, Thrasher A J, Ali R R. Gene replacement therapy in the retinal degeneration slow (rds) mouse: the effect on retinal degeneration following partial transduction of the retina. Hum Mol Genet. 2001;10:2353–2361. doi: 10.1093/hmg/10.21.2353. [DOI] [PubMed] [Google Scholar]
- Schlichtenbrede F C, da Cruz L, Stephens C, Smith A J, Georgiadis A, Thrasher A J, Bainbridge J W, Seeliger M W, Ali R R. Long-term evaluation of retinal function in Prph2Rd2/Rd2 mice following AAV-mediated gene replacement therapy. J Gene Med. 2003;5:757–764. doi: 10.1002/jgm.401. [DOI] [PubMed] [Google Scholar]
- Quiambao A B, Peachey N S, Mangini N J, Rohlich P, Hollyfield J G, al-Ubaidi M R. A 221-bp fragment of the mouse opsin promoter directs expression specifically to the rod photoreceptors of transgenic mice. Vis Neurosci. 1997;14:617–625. doi: 10.1017/s095252380001258x. [DOI] [PubMed] [Google Scholar]
- Connell G J, Molday R S. Molecular cloning, primary structure, and orientation of the vertebrate photoreceptor cell protein peripherin in the rod outer segment disk membrane. Biochemistry. 1990;29:4691–4698. doi: 10.1021/bi00471a025. [DOI] [PubMed] [Google Scholar]
- Nour M, Quiambao A B, Peterson W M, Al-Ubaidi M R, Naash M I. P2Y(2) receptor agonist INS37217 enhances functional recovery after detachment caused by subretinal injection in normal and rds mice. Invest Ophthalmol Vis Sci. 2003;44:4505–4514. doi: 10.1167/iovs.03-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjo R, Skaggs J S, Nagel B A, Quiambao A B, Nash Z A, Fliesler S J, Naash M I. Retention of function without normal disc morphogenesis occurs in cone but not rod photoreceptors. J Cell Biol. 2006;173:59–68. doi: 10.1083/jcb.200509036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X Q, Nour M, Ritter L M, Goldberg A F, Fliesler S J, Naash M I. The R172W mutation in peripherin/rds causes a cone-rod dystrophy in transgenic mice. Hum Mol Genet. 2004;13:2075–2087. doi: 10.1093/hmg/ddh211. [DOI] [PubMed] [Google Scholar]
- Tan E, Wang Q, Quiambao A B, Xu X, Qtaishat N M, Peachey N S, Lem J, Fliesler S J, Pepperberg D R, Naash M I, Al-Ubaidi M R. The relationship between opsin overexpression and photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2001;42:589–600. [PubMed] [Google Scholar]
- Conley S, Nour M, Fliesler S J, Naash M I. Late-onset cone photoreceptor degeneration induced by R172W mutation in Rds and partial rescue by gene supplementation. Invest Ophthalmol Vis Sci. 2007;48:5397–5407. doi: 10.1167/iovs.07-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Nash Z A, Conley S M, Fliesler S J, Cooper M J, Naash M. A partial structural and functional rescue of a retinitis pigmentosa model with compacted DNA nanoparticles. PLoS ONE. 2009;4:e5290. doi: 10.1371/journal.pone.0005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C L, Austin C P, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci U S A. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich S J, Polverini P J, Shepard H M, Wiseman D M, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987;329:630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- Davies M H, Eubanks J P, Powers M R. Microglia and macrophages are increased in response to ischemia-induced retinopathy in the mouse retina. Mol Vis. 2006;12:467–477. [PubMed] [Google Scholar]
- Espinosa-Heidmann D G, Suner I J, Hernandez E P, Monroy D, Csaky K G, Cousins S W. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–3592. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- Lee E S, Burnside B, Flannery J G. Characterization of peripherin/rds and rom-1 transport in rod photoreceptors of transgenic and knockout animals. Invest Ophthalmol Vis Sci. 2006;47:2150–2160. doi: 10.1167/iovs.05-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Y Z, Zheng L, Zheng W, Ash J D, Agbaga M P, Zhu M, Anderson R E. Mouse opsin promoter-directed Cre recombinase expression in transgenic mice. Mol Vis. 2006;12:389–398. [PubMed] [Google Scholar]
- Farjo R, Fliesler S J, Naash M I. Effect of Rds abundance on cone outer segment morphogenesis, photoreceptor gene expression, and outer limiting membrane integrity. J Comp Neurol. 2007;504:619–630. doi: 10.1002/cne.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge J W, Stephens C, Parsley K, Demaison C, Halfyard A, Thrasher A J, Ali R R. In vivo gene transfer to the mouse eye using an HIV-based lentiviral vector; efficient long-term transduction of corneal endothelium and retinal pigment epithelium. Gene Ther. 2001;8:1665–1668. doi: 10.1038/sj.gt.3301574. [DOI] [PubMed] [Google Scholar]
- Pang J, Cheng M, Haire S E, Barker E, Planelles V, Blanks J C. Efficiency of lentiviral transduction during development in normal and rd mice. Mol Vis. 2006;12:756–767. [PubMed] [Google Scholar]
- Farjo R, Naash M I. The role of Rds in outer segment morphogenesis and human retinal disease. Ophthalmic Genet. 2006;27:117–122. doi: 10.1080/13816810600976806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.