Abstract
The molecular mechanisms that enable cyclooxygenase-2 (COX-2) and its mediator prostaglandin E2 (PGE2) to inhibit transforming growth factor-β (TGF-β) signaling during mammary tumorigenesis remain unknown. We show here that TGF-β selectively stimulated the expression of the PGE2 receptor EP2, which increased normal and malignant mammary epithelial cell (MEC) invasion, anchorage-independent growth, and resistance to TGF-β-induced cytostasis. Mechanistically, elevated EP2 expression in normal MECs inhibited the coupling of TGF-β to Smad2/3 activation and plasminogen activator inhibitor-1 (PAI1) expression, while EP2 deficiency in these same MECs augmented Smad2/3 activation and PAI expression stimulated by TGF-β. Along these lines, engineering malignant MECs to lack EP2 expression prevented their growth in soft agar, restored their cytostatic response to TGF-β, decreased their invasiveness in response to TGF-β, and potentiated their activation of Smad2/3 and expression of PAI stimulated by TGF-β. More important, we show that COX-2 or EP2 deficiency both significantly decreased the growth, angiogenesis, and pulmonary metastasis of mammary tumors produced in mice. Collectively, this investigation establishes EP2 as a potent mediator of the anti-TGF-β activities elicited by COX-2/PGE2 in normal and malignant MECs. Our findings also suggest that pharmacological targeting of EP2 receptors may provide new inroads to antagonize the oncogenic activities of TGF-β during mammary tumorigenesis.—Tian, M., Schiemann, W. P. PGE2 receptor EP2 mediates the antagonistic effect of COX-2 on TGF-β signaling during mammary tumorigenesis.
Keywords: angiogenesis, metastasis, prostaglandins, signal transduction
Transforming growth factor-β (TGF-β) plays an essential role in maintaining tissue homeostasis by inducing cell cycle arrest, differentiation, and apoptosis and by preserving genomic stability (1, 2). Interestingly, tumorigenesis typically elicits aberrations in the TGF-β pathway that engenders resistance to the cytostatic activities of TGF-β, thereby enhancing the development and progression of human malignances (2). Moreover, these genetic and epigenetic events conspire to convert TGF-β from a suppressor of tumor formation to a promoter of their growth, invasion, and metastasis (2). TGF-β initiates transmembrane signaling by binding to the TGF-β type I (TβR-I) and TβR-II receptors, the latter of which transphosphorylates and activates TβR-I, which then phosphorylates and stimulates Smad2/3. Activated Smad2/3 translocate into the nucleus with the co-Smad, Smad4, and regulate the expression of TGF-β-responsive genes in conjunction with additional transcription activators or repressors in a cell- and promoter-specific manner (1, 2). In addition to its stimulation of canonical Smad2/3 signaling, TGF-β also activates numerous noncanonical effectors (e.g., ERK1/2, p38MAPK, PI3K/AKT, RhoA, and NF-κB) that readily contribute to the complexity of TGF-β function, as well as promote its initiation of oncogenic signaling in developing neoplasms (2).
Dysregulated expression of the inducible cyclooxygenase COX-2 has been associated with breast cancer invasion, angiogenesis, metastasis, and inflammation (3,4,5,6,7). COX-2 is the key enzyme that converts arachidonic acid to several prostaglandins, including prostaglandin E2 (PGE2), which promotes cancer progression in part by increasing cell proliferation and angiogenesis and by inhibiting apoptosis (8,9,10,11). PGE2 exerts its biological functions by activating 4 receptors, namely EP1, 2, 3, and 4 (12), which are 7 transmembrane G protein-coupled receptors that activate distinct downstream effectors and second messengers. For instance, EP1 is a Gq-coupled receptor that increases intracellular calcium levels, while EP2 and EP4 are Gs-coupled receptors that increase cAMP levels via activation of adenylate cyclase. In stark contrast, EP3 is a Gi-coupled receptor that decreases cAMP levels.
The role of EP2 in mediating the biological functions of COX-2/PGE2, particularly cell proliferation, angiogenesis, and apoptosis, has been described in several reports (12,13,14,15,16,17,18,19,20,21). Homozygous deletion of the gene for EP2 decreases the number and size of intestinal polyps in APCΔ716 mice in part by reducing their expression of COX-2 and VEGF (13). Accordingly, EP2 regulates angiogenesis by inducing VEGF expression in pancreatic and prostate cancer cells and by stimulating endothelial cell motility and survival (14, 15, 17). Activation of EP2 by PGE2 also regulates Th17 cell differentiation and proinflammatory reactions via a cAMP-dependent pathway (22). In addition, EP2 plays critical roles in skin tumor development (19, 20), while elevated EP2 levels are associated with the poor prognosis and depth of tumor invasion (T-status) observed in esophageal squamous cell carcinomas (16). With respect to the mammary gland, EP2 is required for the ability of COX-2 to induce mammary hyperplasia, and EP2 overexpression in breast cancers mediates increased VEGF production in response to either PGE2 or an EP2 agonist via a cAMP/PKA-dependent pathway (23, 24).
We demonstrated recently that TGF-β induces COX-2 expression and subsequent PGE2 production in normal and malignant MECs, cellular reactions that contribute to the oncogenic activities of TGF-β in vitro (25). In addition, up-regulated COX-2 expression enhances TGF-β stimulation of epithelial-mesenchymal transition (EMT), invasion, and anchorage-independent growth in MECs, which transpires in part through the ability of COX-2 to inhibit Smad3 activation (25). At present, the effectors of the COX-2/PGE2 pathway responsible for mediating their anti-TGF-β activities remain unknown. Thus, we aimed to identify which EP receptor elicits anti-TGF-β signals in normal and malignant MECs and to determine how this PGE2 receptor affects the oncogenic activities of TGF-β during breast cancer progression.
MATERIALS AND METHODS
Reagents and materials
AH6809, GW627368X, butaprost, and PGE1-alcohol were purchased from Cayman (Ann Arbor, MI, USA), while the TβR-I inhibitor II was purchased from Calbiochem (San Diego, CA, USA). Lentiviral vectors (pLKO.1-puromycin) encoding for control [i.e., nonsilencing short hairpin RNA (shRNA)] or murine EP2 shRNA (catalog no. RMM4534) were purchased from Open Biosystems (Huntsville, AL, USA). The human EP2 cDNA (PER020TN00) was purchased from Missouri S&T cDNA Resource Center (Rolla, MO, USA). Normal murine NMuMG mammary gland and murine metastatic 4T1 breast cancer cells were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA), and were cultured as described previously (25). All additional supplies or reagents were routinely available.
Cell culture and transgene expression
NMuMG or 4T1 cells were transfected overnight (20 μg cDNA/10-cm plate) with either pcDNA3.1/neomycin or pcDNA3.1-EP2/neomycin and subsequently were subjected to neomycin selection (100 μg/ml for 4T1 cells and 300 μg/ml for NMuMG cells) for 2 wk. Whole-cell extracts from neomycin-resistant NMuMG or 4T1 cells were immunoblotted with anti-EP2 antibodies to monitor the extent of EP2 overexpression. The establishment of EP2-deficient NMuMG or 4T1 cells was accomplished by their overnight infection with control (i.e., scrambled shRNA) or EP2 shRNA lentiviral (pLKO.1-puro) supernatants produced by 293T cells that were transiently transfected with lentiviral packaging vectors (i.e., pMD2.G, pRRE and pRSV) as described previously (25). Cells expressing scrambled and EP2 shRNAs were isolated by puromycin selection (5 μg/ml) for 14 d. Afterward, NMuMG and 4T1 whole-cell extracts prepared from puromycin-resistant NMuMG and 4T1 cells were immunoblotted with antibodies against EP2 to monitor the extent of shRNA-mediated EP2 deficiency.
Cell biological assays
Analyzing the effect of EP2 expression on normal and malignant MEC in response to TGF-β was determined as follows: proliferation assays using 10,000 cells/well in 96-well plates, as described previously (26); cell invasion induced by 2% serum using 100,000 cells/well in a modified Boyden chamber coated with Matrigel matrices (BD Biosciences, San Jose, CA, USA), as described previously (27); anchorage-independent cell growth over a 10-d period, as described previously (27); and p-Smad-binding element (p-SBE)-, p-cAMP-responsive element (pCRE)-, and p-plasminogen activator inhibitor-1 (pPAI1)-luciferase reporter gene assays using 35,000 cells/well and performed in the absence or presence of either AH6809 (50 μM), GW627368X (20 μM), butaprost (10 μM), PGE1-alcohol (1 μM), or forskolin (30 μM), as described previously (27). In addition, indirect immunofluorescence was performed to monitor the subcellular localization of Smad2/3, as described previously (25, 28). Briefly, NMuMG (35,000 cells/well) or 4T1 (50,000 cells/well) were cultured overnight in 8-well plates, at which point they were washed extensively in PBS and incubated for 2 h in serum-free medium before their stimulation with TGF-β1 (5 ng/ml) for 30 min. Afterward, the cells were fixed in paraformaldehyde and processed for immunofluorescence with anti-Smad2/3 antibody (1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by biotinylated goat anti-rabbit antibody (5 μg/ml; Jackson ImmunoResearch, West Grove, PA, USA), and finally with Alexa-streptavidin (1.2 μg/ml; Molecular Probes, Portland, OR, USA). Following extensive washing with PBS, the coverslips were mounted on glass slides with Prolong mounting medium (Molecular Probes). Images were captured on a Nikon Diaphot microscope (Nikon, Tokyo, Japan) using identical exposure times that were determined initially by monitoring Smad2/3 signal intensity in TGF-β-stimulated parental MECs. Afterward, Smad2/3 immunofluorescent intensities were calculated using NIH ImageJ 1.40 g (National Institutes of Health, Bethesda, MD, USA) and were presented graphically as the percentage of Smad2/3-stained nuclei relative to TGF-β-treated parental MECs.
Semiquantitative real-time PCR analysis
Total RNA from NMuMG or 4T1 cells was purified using the RNeasy Plus Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Afterward, cDNA was synthesized by iScript reverse transcription (Bio-Rad, Hercules, CA, USA), which then were diluted 10-fold in H2O and used in semiquantitative real-time PCR reactions (25 μl) using the SYBR Green system (Bio-Rad) supplemented with 5 μl of diluted cDNA and 0.1 μM of oligonucleotide pairs listed below. PCR reactions were performed and analyzed on a Bio-Rad Mini-Opticon detection system, and differences in RNA concentration were controlled by normalizing individual gene signals to those of their corresponding β-actin reactions. The oligonucleotide primers used were as follows: EP1, forward 5′-GAAACACCAGAGGTGGGACT and reverse 5′-TTATGGAGAGCCCGAATCTT; EP2, forward 5′-CCTTGGGTCTTTGCCATACT and reverse 5′-TCCGACAACAGAGGACTGAG; EP3, forward 5′-TGGTCATCCTCGTGTACCTG and reverse 5′-AGGATAGCCCGAACACTGTC; and EP4, forward 5′-TACTCATCGCCACCTCTCTG and reverse 5′-GGGTTCACAGAAGCAATCCT. Primer pairs to amplify and monitor β-actin and PAI1 transcripts were described previously (27).
Western blotting analyses
NMuMG or 4T1 cells were incubated with TGF-β1 (5 ng/ml), TβR-I inhibitor II (100 ng/ml), or AH6809 (50 μM), and they subsequently were lysed in Buffer H/Triton X-100 (29) and solubilized on ice for 30 min. Afterward, the resulting clarified whole-cell extracts were resolved on 10% SDS-PAGE electrophoresis gels, transferred electrophoretically to nitrocellulose membranes, and blocked in 5% milk before incubation with the following primary antibodies (dilutions in parentheses): anti-COX-2 (1:500; Cayman); or anti-EP1, EP2, EP3, or EP4 (1:500; Cayman). The resulting immunocomplexes were visualized by enhanced chemiluminescence. Differences in protein loading were monitored by reprobing stripped membranes with anti-β-actin antibodies (1:1000; Sigma, St. Louis, MO, USA).
Tumor growth and metastasis studies
4T1 cells engineered to express either a scrambled shRNA, a COX-2 shRNA, or an EP2 shRNA were resuspended in sterile PBS and injected (12,000 cells/mouse) into the mammary fat pads of 6-wk-old female Balb/C mice (9 mice/condition; Jackson Laboratory, Bar Harbor, ME, USA). Mice were monitored daily for signs of disease and primary tumors were measured with digital calipers (Fisher Scientific, Pittsburgh, PA, USA) every other day beginning on d 10 postinoculation. Tumor volumes were calculated as 0.5 x2y, where x is tumor width and y is tumor length. Twenty-eight days postinoculation, the mice were killed and their primary tumors were excised, weighed, and processed for histopathological analysis in the Pathology Core at the University of Colorado Cancer Center. At the time of necropsy, the lungs were removed and weighed, and subsequently they were processed to assess their metastatic burden and histopathology. Finally, serial histological sections of the primary 4T1 tumors were stained with antibodies against phospho-Smad3 (1:50; Cell Signaling Technology, Danvers, MA, USA); Ki-67 (1:300; BD Biosciences); CD3 (1:1500; Sigma); COX-2 (1:100; Cayman); EP2 (1:100; Cayman); and CD31 (1:400; Dako, Glostrop, Denmark). Tumor sections also were stained with Masson’s trichrome reagent according to the manufacturer’s recommendations (Sigma) and with hematoxylin as described previously (30).
NMuMG cells (106) expressing either an empty vector (i.e., parental) or a COX-2 cDNA were injected into the mammary fat pads of athymic mice (6 mice/condition). Afterward, tumor growth was monitored over a span of 4 mo, at which point the mice were sacrificed and the tumors were removed, weighed, and sectioned for immunohistochemistry as described previously (27, 30). All animal studies were performed according to animal protocol procedures approved by the Institutional Animal Care and Use Committee of the University of Colorado.
Statistical analysis
Statistical values were defined using an unpaired Student’s t test, where a value of P < 0.05 was considered significant.
RESULTS
TGF-β induces EP2 expression in normal and malignant MECs
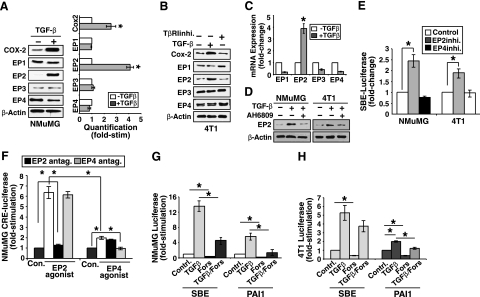
Up-regulated COX-2 expression is found frequently in many types of cancers, and its role in tumorigenesis has been supported by genetic, pharmacologic, and epidemiologic evidence (31, 32). Recently, we showed that TGF-β induces COX-2 expression and subsequent PGE2 production and that up-regulated COX-2 inhibits Smad3 activation during breast cancer progression (25). Unfortunately, the components and effectors lying downstream of COX-2/PGE2 that mediate their anti-TGF-β activities remain unknown. Given the association of PGE2 and its EP receptors in regulating tumorigenesis (33), we hypothesized that one or more of these PGE2 receptors would be targeted by TGF-β in normal NMuMG and metastatic 4T1 cells. Accordingly, we observed quiescent NMuMG (Fig. 1A) and 4T1 (Fig. 1B) cells to express all 4 EP receptors. Consistent with our previous study (25), TGF-β stimulation induced significant COX-2 expression in NMuMG (Fig. 1A) and 4T1 (Fig. 1B) cells. Interestingly, whereas the expression of EP1, EP3, and EP4 was unaffected by TGF-β, we did observe EP2 expression to be up-regulated significantly in NMuMG (Fig. 1A) and 4T1 (Fig. 1B) cells stimulated with TGF-β. We previously established autocrine TGF-β signaling as a major driver of COX-2 expression in 4T1 cells (25). As shown in Fig. 1B, treating 4T1 cells with TβR-I inhibitor II not only decreased COX-2 expression but also solely reduced that of EP2, which suggests that autocrine TGF-β signaling induces COX-2 and EP2 expression in metastatic MECs. Along these lines, only EP2 mRNA expression was dramatically induced by TGF-β (Fig. 1C). AH6809 is widely used as an EP2 receptor antagonist (21, 34, 35), and as such, we treated NMuMG and 4T1 cells with this antagonist to further determine the involvement of EP2 in the TGF-β signaling pathway. Figure 1D shows that administration of the EP2 receptor antagonist AH6809 prevented TGF-β from inducing EP2 expression in NMuMG and 4T1 cells, which suggests that EP2 receptor activation is necessary for maximal stimulation of EP2 expression by TGF-β.
Figure 1.
TGF-β induces EP2 expression in normal and malignant MECs. A) Quiescent NMuMG cells were stimulated with TGF-β1 (5 ng/ml) for 36 h. Detergent-solubilized whole-cell extracts were immunoblotted with antibodies against COX-2, EPs 1-4, and β-actin as indicated. Images represent 3 independent experiments. Immunoblot band densities were quantified using NIH ImageJ. Data are mean ± se pixel density of Cox2 and EPs 1-4 expression relative to control cells (n=3). B) Quiescent 4T1 cells were administered with TGF-β1 (5 ng/ml) or TβRI inhibitor (100 ng/ml; TβRIinhi) for 24 h as indicated. Detergent-solubilized whole-cell extracts were immunoblotted with antibodies against COX-2, EPs 1-4, and β-actin as indicated. Images represent 3 independent experiments. C) Quiescent 4T1 cells were treated with TGF-β1 (5 ng/ml) for 24 h as indicated. Total RNA was isolated and subjected to semiquantitative real-time PCR to monitor the expression of EPs 1-4. Data are mean ± se fold change in EP1-4 gene expression relative to control cells (n=3). D) Quiescent NMuMG or 4T1 cells were treated with TGF-β1 (5 ng/ml) with or without the EP2 antagonist AH6809 (50 μM) as indicated. Detergent-solubilized whole-cell extracts were immunoblotted with antibodies against EP2 or β-actin as shown. Images represent 3 independent experiments. E) Quiescent NMuMG or 4T1 cells were transiently transfected overnight with SBE-luciferase and β-gal cDNAs and subsequently were incubated with antagonists against EP2 (AH6809, 50 μM) or EP4 (GW627368X; 20 μM) for an additional 24 h. Afterward, luciferase and β-gal activities contained in detergent-solubilized whole-cell extracts were measured. Data are mean ± se luciferase activity relative to control cells (n=3). F) Quiescent NMuMG cells were transiently transfected overnight with CRE-luciferase and β-gal cDNAs, and subsequently were incubated with EP2 (butaprost; 10 μΜ) or EP4 (PGE1-alcohol; 1 μΜ) agonists in combination with EP2 (AH6809; 50 μM) or EP4 (GW627368X; 20 μM) antagonists for an additional 24 h as indicated. Afterward, luciferase and β-gal activities contained in detergent-solubilized whole-cell extracts were measured. Data are mean ± se luciferase activity relative to control cells (n=3). G, H) NMuMG (G) or 4T1 cells (H) were transiently transfected overnight with β-gal, together with either SBE- or PAI1-luciferase as indicated. Afterward, cells were stimulated with TGF-β1 (5 ng/ml), forskolin (30 μM), or both agents for 24 h as shown. Afterward, luciferase and β-gal activities contained in detergent-solubilized whole-cell extracts were determined. Data are mean ± se luciferase activity relative to control cells (n=3). *P < 0.05; Student’s t test.
Because EP2 and EP4 exhibit similar biological functions in part via their ability to increase cAMP levels in responsive cells (36), we next sought to determine the relative contribution of EP2 and EP4 in altering TGF-β signaling in normal and malignant MECs. In doing so, NMuMG and 4T1 cells were treated with inhibitors to either EP2 or EP4 to measure their ability to alter SBE-luciferase reporter gene expression, which serves as a measure of Smad2/3 transcription activity. As shown in Fig. 1E, administration of the EP2 inhibitor AH6809 significantly increased SBE-luciferase expression in both NMuMG and 4T1 cells, while no effect on SBE-luciferase expression was observed in normal or malignant MECs treated with the EP4 antagonist GW627368X. These findings suggest that autocrine PGE2 signaling suppresses Smad2/3 signaling in MECs and that this inhibitory reaction is likely mediated by EP2 receptors and their production of cAMP. To further test this supposition, we preformed CRE-luciferase assays as an indirect measure of cAMP production in NMuMG cells treated with either the EP2-selective agonist butaprost or the EP4-selective agonist PGE1-alcohol. Figure 1F shows that butaprost-mediated activation of EP2 elicited significantly more CRE-luciferase expression than did that induced EP4 receptors activated by PGE1-alcohol. Moreover, the ability of butaprost to induce CRE-luciferase expression was unaffected by inclusion of the EP4 antagonist GW627368X but was inhibited completely by addition of the EP2 antagonist AH6809 (Fig. 1F). Likewise, the modest induction of CRE-luciferase expression stimulated by PGE1-alcohol was unaffected by inclusion of the EP2 antagonist AH6809 but was abrogated by addition of the EP4 antagonist GW62368X (Fig. 1F). Thus, these findings implicate EP2 as the PGE2 receptor operant in suppressing TGF-β signaling. Along these lines, we found that mimicking EP2 activation by treating NMuMG and 4T1 cells with the adenylate cyclase activator forskolin blocked the coupling of TGF-β to SBE- and PAI-1-luciferase reporter gene expression in both cell lines (Fig. 1G, H). Taken together, our findings establish EP2 as a novel and selective TGF-β target that functions in mediating the anti-TGF-β activities of COX-2/PGE2 in normal and malignant MECs.
EP2 expression alters oncogenic TGF-β signaling in NMuMG cells
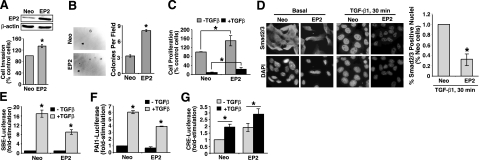
Previous studies (12,13,14, 19, 37) have shown that EP2 mediates the ability of PGE2 to regulate the proliferation, apoptosis, and angiogenesis of several tissues, especially the colon and mammary gland. Our findings above implicate EP2 as a novel mediator of TGF-β-induced COX-2 expression in MECs and, by extension, of their production of PGE2 (25); however, the extent to which EP2 expression affects the response of normal MECs to TGF-β remains unclear. To address this question, we established polyclonal NMuMG cell populations that stably expressed either empty vector or EP2 (Fig. 2A, top panel). Elevating EP2 expression significantly enhanced NMuMG cell invasion (Fig. 2A, bottom panel) and formation of colonies in soft agar (Fig. 2B). In addition, augmented EP2 expression moderately increased DNA synthesis in NMuMG cells, as well as partially reduced the extent to which TGF-β could inhibit DNA synthesis in these same MECs (Fig. 2C). Consistent with our previous study (25) that elucidated an antagonistic activity of COX-2 on Smad3 expression and function, we now show that EP2 overexpression also significantly reduced the ability of TGF-β to stimulate Smad2/3 nuclear translocation (Fig. 2D) and SBE-luciferase expression in NMuMG cells (Fig. 2E). PAI1 gene expression is tightly regulated by TGF-β (38), and as such, we examined the effects of EP2 expression on the activation of the PAI1 promoter by TGF-β. As shown in Fig. 2F, elevating EP2 expression in NMuMG cells decreased TGF-β stimulation of luciferase activity driven by the PAI1 promoter. Mechanistically, the anti-TGF-β activities mediated by EP2 overexpression likely transpire via increased levels of intracellular cAMP, as CRE-luciferase activity was elevated significantly in basal and TGF-β-stimulated EP2-expressing NMuMG cells relative to their control counterparts (Fig. 2G). Thus, EP2 activation appears to inhibit TGF-β signaling in part via a cAMP-dependent mechanism.
Figure 2.
EP2 expression alters oncogenic TGF-β signaling in NMuMG cells. A) Top panel: detergent-solubilized whole-cell extracts prepared from control (i.e., empty vector; Neo) or EP2-expressing NMuMG cells were immunoblotted with antibodies against EP2 and β-actin as indicated. Bottom panel: control (Neo) or EP2-expressing NMuMG cells were induced to invade through synthetic basement membrane by 2% serum for 48 h. Data are mean ± se invasion relative to control cells (n=4). B) Control (Neo) or EP2-expressing NMuMG cells were cultured on soft agar for 14 d (left panels), whereupon colony formation was quantified by light microscope (right panel). Data are mean ± se number of colonies per microscopic field (n=3). C) Control (Neo) or EP2-expressing NMuMG cells were stimulated with TGF-β1 (5 ng/ml) for 48 h. Cellular DNA was radiolabeled with [3H]thymidine and quantified by scintillation counting. Data are mean ± se [3H]thymidine incorporation relative to untreated control cells (n=4). D) Control (Neo) or EP2-expressing NMuMG cells were incubated in the absence or presence of TGF-β1 (5 ng/ml) for 30 min. Afterward, the cells were fixed in paraformaldehyde and processed for indirect immunofluorescence of Smad2/3. Images were captured using identical exposure times determined originally by Smad2/3 immunofluorescent signals observed in control cells stimulated with TGF-β1. Images are representative from a single experiment that was performed 2 times with identical results. Nuclear translocation was analyzed using NIH ImageJ program. Data are mean ± se pixel density relative to stimulated control cells (n=2). E–G) Control (Neo) or EP2-expressing NMuMG cells were transiently transfected overnight with cDNAs for SBE-luciferase and β-gal (E), for PAI1-luciferase and β-gal (F), or for CRE-luciferase and β-gal (G) as indicated. Afterward, transfectants were stimulated with TGF-β1 (5 ng/ml) for 24 h, at which point luciferase and β-gal activities were measured. Data are mean ± se luciferase activity relative to unstimulated control cells (n=3). *P < 0.05; Student’s t test.
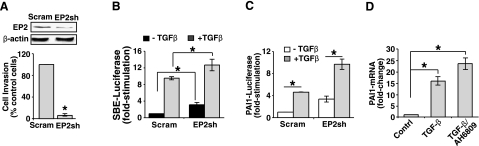
Because EP2 overexpression promoted tumorigenic cell behaviors (e.g., increased proliferation, invasion, and transformation; Fig. 2) and mediated anti-TGF-β activities (e.g., decreased Smad2/3 signaling; Figs. 1 and 2) in NMuMG cells, we reasoned that rendering NMuMG cells deficient in EP2 expression by lentiviral shRNA transduction would reverse these phenotypes in NMuMG cells. Accordingly, EP2 deficiency did indeed decrease the ability of NMuMG cells to invade through synthetic basement membranes (Fig. 3A) and to undergo growth arrest in response to TGF-β (decreased by ∼50%; data not shown). In addition, depleting EP2 expression in NMuMG cells moderately, but in a statistically significant manner, increased the coupling of TGF-β to SBE-luciferase expression (Fig. 3B), to PAI1-luciferase expression (Fig. 3C), and to PAI1 transcript production (Fig. 3D). Recently, a PGE2:EP2:COX-2 positive feedback loop was identified that functions in up-regulating COX-2 expression in response to PGE2-mediated activation of EP2 (10, 13, 39). We also found EP2 expression to be necessary for the induction of COX-2 by TGF-β in NMuMG cells (data not shown), suggesting that TGF-β plays a role in activating this PGE2:EP2:COX-2 positive feedback loop in MECs. Taken together, these findings demonstrate that EP2 mediates the inhibitory actions of COX-2/PGE2 on TGF-β in normal MECs.
Figure 3.
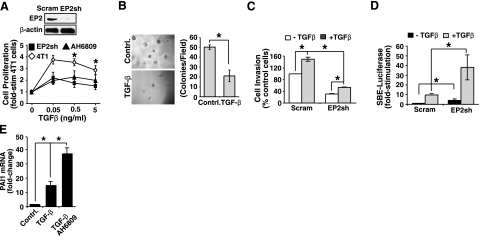
Altered EP2 expression affects oncogenic TGF-β signaling in malignant MECs. A) NMuMG cells were infected with lentivirus particles encoding for either a scrambled shRNA (Scram) or a murine EP2 shRNA (EP2sh). Extent of EP2 depletion was monitored by immunoblotting whole-cell extracts with antibodies against EP2 and β-actin as indicated (top panel). Control (Scram) or EP2-depleted NMuMG cells were induced to invade through synthetic basement membrane by 2% serum in the absence or presence of TGF-β1 (5 ng/ml) for 48 h as indicated. Data are mean ± se invasion relative to that induced by 2% serum in control cells (n=3). B, C) Control (Scram) or EP2-deficient NMuMG cells were transiently transfected overnight with cDNAs for SBE-luciferase and β-gal (B) or for PAI1-luciferase and β-gal (C) as indicated. Afterward, transfectants were stimulated with TGF-β1 (5 ng/ml) for 24 h, at which point luciferase and β-gal activities were measured. Data are mean ± se luciferase activity relative to unstimulated control cells (SBE, n=4; PAI1, n=3). D) NMuMG cells were treated with TGF-β1 (5 ng/ml) in the absence or presence of AH6809 (50 μM) for 36 h. Afterward, total RNA was isolated and subjected to semiquantitative real-time PCR to monitor PAI1 transcript expression. Data are mean ± se fold change in PAI1 mRNA expression relative to untreated control cells (n=3). *P < 0.05; Student’s t test.
Altered EP2 expression affects oncogenic TGF-β signaling in malignant MECs
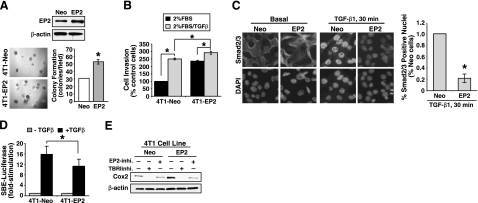
EP2 expression is essential for the ability of COX-2 to induce mammary gland hyperplasia in mice (23). Moreover, these hyperplastic reactions transpire in part via up-regulated amphiregulin expression stimulated by an EP2:cAMP signaling axis (23). Our findings thus far suggest that manipulations resulting in elevated EP2 expression will enhance the oncogenic activities of TGF-β in malignant MECs. As an initial test of this hypothesis, we established 4T1 cells that stably expressed either empty vector or EP2 (Fig. 4A, top panel). Consistent with our hypothesis, we observed elevated EP2 expression to significantly enhance soft agar colony formation by 4T1 cells (Fig. 4A, bottom panels), as well as to augment their invasion induced either by serum or by serum in combination with TGF-β (Fig. 4B). Analogous to our findings in NMuMG cells (Fig. 2D), we also found EP2 overexpression to antagonize the ability of TGF-β to stimulate Smad2/3 nuclear translocation (Fig. 4C) and SBE-luciferase activity (Fig. 4D) in 4T1 cells. Furthermore, elevating EP2 expression significantly up-regulated COX-2 expression in 4T1 cells, while treating these same cells with either the TβR-I inhibitor or the EP2 antagonist (AH6809) significantly reduced COX-2 expression, respectively (Fig. 4E). Collectively, these findings suggest that 4T1 cells are subjected to strong autocrine TGF-β signaling (25, 40,41,42) that induces COX-2 expression in part via the expression and activation of EP2 in malignant MECs.
Figure 4.
Altered EP2 expression affects oncogenic TGF-β signaling in malignant MECs. A) Top panel: detergent-solubilized whole-cell extracts prepared from control (i.e., empty vector; Neo) or EP2-expressing 4T1 cells were immunoblotted with antibodies against EP2 and β-actin as shown. Bottom panels: control (Neo) or EP2-expressing 4T1 cells were cultured in soft agar for 10 d (left), whereupon colony formation was quantified by light microscopy (right). Data are mean ± se number of colonies per microscopic field (n=3). B) Control (Neo) or EP2-expressing 4T1 cells were induced to invade through synthetic basement membrane by 2% serum in the absence or presence of TGF-β1 (5 ng/ml) for 48 h as indicated. Data are mean ± se invasion relative to that induced by 2% serum in control cells (n=3). C) Control (Neo) or EP2-expressing 4T1 cells were incubated in the absence or presence of TGF-β1 (5 ng/ml) for 30 min. Afterward, cells were fixed in paraformaldehyde and processed for indirect Smad2/3 immunofluorescence. Images were captured using identical exposure times determined originally by Smad2/3 immunofluorescent signals observed in control cells stimulated with TGF-β1. Images are representative from a single experiment that was performed 2 times with identical results. Nuclear translocation was analyzed using NIH ImageJ program. Data are mean ± se pixel density relative to stimulated control cells (n=2). D) Control (Neo) or EP2-expressing 4T1 cells were transiently transfected overnight with SBE-luciferase and β-gal cDNAs, and subsequently were stimulated with TGF-β1 (5 ng/ml) for 24 h. Afterward, luciferase and β-gal activities contained in detergent-solubilized whole-cell extracts were measured. Data are mean ± se luciferase activity relative to unstimulated control cells (n=3). E) Control (Neo) or EP2-expressing 4T1 cells were incubated in the absence or presence of either TβR-I inhibitor (100 ng/ml; TβRIinhi) or AH6809 (50 μM; EP2inhi) for 24 h as indicated. Afterward, whole-cell extracts were immunoblotted with antibodies against COX-2 and β-actin as shown. Images represent 3 independent experiments. *P < 0.05; Student’s t test.
A corollary of the above hypothesis states that manipulations resulting in diminished EP2 expression will suppress oncogenic TGF-β signaling in malignant MECs. To test this hypothesis, we engineered 4T1 cells to stably express either a scrambled (i.e., nontargeting shRNA) or an EP2 shRNA. Figure 5A shows that depleting 4T1 cells of EP2 expression significantly compromised the ability of TGF-β to induce DNA synthesis in these malignant MECs, as did the administration of the EP2 antagonist AH6809. In addition, EP2 deficiency significantly reduced the ability of TGF-β to stimulate the 4T1 cell colony formation (Fig. 5B) and invasion (Fig. 5C). More important, rendering 4T1 cells deficient in EP2 expression greatly enhanced their activation of Smad2/3 (Fig. 5D), while pharmacological inactivation of EP2 was sufficient to potentiate the coupling of TGF-β to PAI1 transcription in 4T1 cells (Fig. 5E). Taken together, our data demonstrate that EP2 signaling promotes the oncogenic activities of TGF-β by uncoupling this cytokine from its downstream effectors, particularly Smad2/3, during breast cancer progression. Thus, it stands to reason that the pharmacological targeting of EP2 signaling in developing and progressing mammary tumors may mitigate oncogenic TGF-β signaling, thereby restoring its tumor suppressing functions in cancers of the breast.
Figure 5.
EP2 deficiency abrogates the oncogenic TGF-β signaling in 4T1 cells. A) 4T1 cells were infected with lentivirus particles encoding for either a scrambled shRNA (Scram) or a murine EP2 shRNA (EP2sh). The extent of EP2 depletion was monitored by immunoblotting whole-cell extracts with antibodies against EP2 and β-actin as indicated (top panel). Control (Scram) or EP2-deficient 4T1 cells were stimulated with increasing concentrations of TGF-β1 (0–5 ng/ml) in the absence or presence of AH6809 (50 μM) for 48 h as indicated. Cellular DNA was radiolabeled with [3H]thymidine and quantified by scintillation counting. Data are mean ± se [3H]thymidine incorporation relative to untreated control cells (n=3). B) Control (Scram) or EP2-deficient 4T1 cells were cultured in soft agar with or without TGF-β1 (5 ng/ml) for 10 d (left panels), whereupon colony formation was quantified by light microscopy (right panel). Data are mean ±se number of colonies per microscopic field (n=3). C) Control (Scram) or EP2-deficient 4T1 cells were induced to invade through synthetic basement membrane by 2% serum in the absence or presence of TGF-β1 (5 ng/ml) for 48 h as indicated. Data are mean ± se invasion relative to that induced by 2% serum in control cells (n=4). D) Control (Scram) or EP2-deficient 4T1 cells were transiently transfected overnight with cDNAs for SBE-luciferase and β-gal, and subsequently were stimulated with TGF-β1 (5 ng/ml) for 24 h. Afterward, luciferase and β-gal activities contained in detergent-solubilized whole-cell extracts were measured. Data are mean ± se luciferase activity relative to unstimulated control cells (n=3). E) 4T1 cells were stimulated with TGF-β1 (5 ng/ml) in the absence or presence of AH6809 (50 μM) for 24 h as indicated. Afterward, total RNA was isolated and subjected to semiquantitative real-time PCR to monitor PAI1 transcript expression. Data are mean ± se fold change in PAI1 mRNA expression relative to unstimulated control cells (n=3). *P < 0.05; Student’s t test.
COX-2 deficiency reduces mammary tumor growth and pulmonary metastasis induced by TGF-β
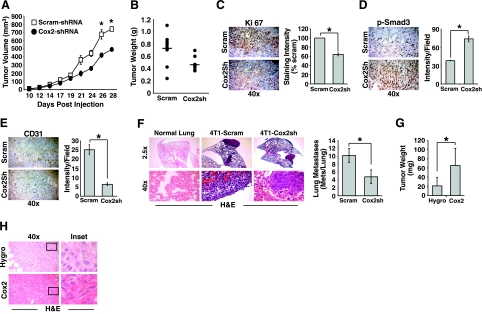
COX-2 expression contributes to oncogenic TGF-β signaling by uncoupling this cytokine from its activation of Smad2/3 in normal and malignant MECs in vitro (25). We therefore hypothesized that COX-2 deficiency would elevate Smad2/3 signaling in orthotopically established mammary tumors and, as such, would negatively affect the ability of TGF-β to induce mammary tumor progression in mice. In testing this hypothesis, we compared the tumorigenicity of parental (i.e., scrambled shRNA) and COX-2-deficient 4T1 cells following their orthotopic implantation into syngeneic Balb/C mice, which demonstrated that COX-2 deficiency significantly reduced the volume (Fig. 6A), weight (Fig. 6B), and proliferative indices (Fig. 6C) of 4T1 tumors produced in mice. The antitumor effects elicited by COX-2 deficiency reflected alterations within the TGF-β signaling system, as depleting COX-2 expression in 4T1 tumors significantly increased their phosphorylation of Smad3 as compared with their COX-2-expressing counterparts (Fig. 6D). Notably, these findings provide in vivo validation of our previously published in vitro studies (37, 43, 44) linking up-regulated COX-2 expression and PGE2 production to reduced Smad2/3 signaling in normal and malignant MECs (25). COX-2 and PGE2 both have been implicated in activating angiogenesis, including that occurring in breast cancers. As such, we also examined the angiogenic status in parental and COX-2-deficient 4T1 tumors and observed COX-2-deficient tumors to contain significantly fewer blood vessels (i.e., CD31 staining) as compared with their parental counterparts (Fig. 6E). Thus, COX-2 expression promotes vessel formation and subsequent progression of 4T1 tumors in mice. Along these lines, we observed COX-2 deficiency to significantly repress the pulmonary metastasis of 4T1 cells from the orthotopic site (Fig. 6F), which collectively suggests that COX-2 plays an important function in promoting the oncogenic activities of TGF-β in breast cancers.
Figure 6.
COX-2 deficiency reduces mammary tumor growth and pulmonary metastasis induced by TGF-β. A, B) Control (Scram) or COX-2-deficient 4T1 cells were injected into the mammary fat pads of Balb/C mice. At 10 d postinjection, tumor volumes were measured every second day until mice were sacrificed on d 28. Data are mean ± se tumor volume (A) or wet weight (B) (n=9). C–E) 4T1 tumor sections were stained with antibodies against Ki-67 (C), phospho-Smad3 (D), or CD31 (E) as indicated. Extent of immunoreactivity was quantified by measuring signal pixel density using NIH ImageJ. Data in graphs are mean ± se staining intensity relative to control 4T1 tumor sections. F) Lung sections were prepared from normal or 4T1 tumor-bearing mice and stained with hematoxylin and eosin (H&E; left panel). Pulmonary metastatic lesions were counted manually. Data are mean ± se metastatic lesions observed per microscopic field. G, H) Control (Hygro) or COX-2-expressing NMuMG cells were injected into the mammary fat pads of athymic mice (106 cells/injection; n=6). At 4 mo postinjection, resulting tumors were removed, weighed (G), and processed for H&E staining (H). Data are mean ± se wet weight (Hygro, n=2; Cox-2, n=5). *P < 0.05; Student’s t test.
Given the important contributions of COX-2 expression to the malignant conversion of normal MECs (5) and to the suppression of Smad2/3 signaling stimulated by TGF-β (Fig. 6D; ref. 25), we hypothesized that COX-2 overexpression in normal MECs would be sufficient to drive their transformation and elicit tumor formation in immunocompromised mice. As such, we inoculated parental (i.e., empty vector) or COX-2-expressing NMuMG cells into the mammary fat pads of nude mice, and we subsequently monitored tumor formation and growth. Interestingly, 33% (2 of 6) of the mice injected with parental NMuMG cells developed small, but detectable, well-differentiated tumors at 4 mo (Fig. 6G, H). In stark contrast, 83% (5 of 6) of the mice injected with COX-2-expressing NMuMG cells developed tumors at 4 mo. Notably, COX-2 expression not only enhanced the extent of NMuMG cell transformation but also promoted the development of larger tumors (Fig. 6G) that were poorly differentiated and clearly contained regions of sarcomatoid progression (Fig. 6H). Thus, these findings provide in vivo validation of our previously published in vitro studies linking up-regulated COX-2 expression to EMT in normal and malignant MECs (25). Collectively, our results implicate COX-2 as an important mediator of mammary tumor development and progression, doing so in part through its ability to negatively affect the coupling of TGF-β to its canonical effectors, Smad2/3.
EP2 deficiency inhibits mammary tumor growth and pulmonary metastasis induced by TGF-β
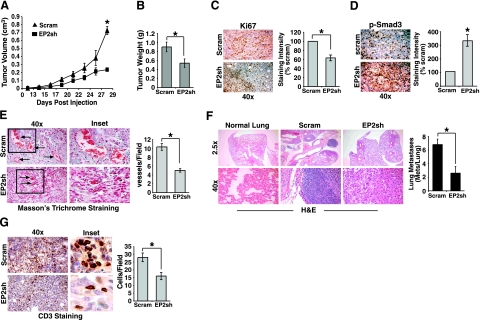
Homozygous deletion of EP2 in APCΔ716 mice decreases their formation of intestinal polyps, an effect that is reminiscent of COX-2 disruption and points to involvement of EP2 in mediating the actions of COX-2/PGE2 (13). In light of our findings that identified EP2 as a mediator of TGF-β-regulated COX-2/PGE2 activity in vitro (Figs. 12345), we reasoned that EP2 deficiency should mimic the defects in 4T1 tumor development elicited by COX-2 deficiency (Fig. 6). In testing this supposition, we engrafted parental (i.e., scrambled shRNA) or EP2-deficient 4T1 cells onto the mammary fat pads of Balb/C mice to monitor differences in tumor growth and metastasis. Similar to the effects of COX-2 deficiency, EP2 deficiency also significantly reduced the volume (Fig. 7A), weight (Fig. 7B), and proliferative indices (Fig. 7C) of 4T1 tumors, and it also significantly enhanced their phosphorylation of Smad3 (Fig. 7D). Thus, EP2 deficiency does indeed recapitulate the tumorigenic defects elicited by COX-2 deficiency in 4T1 cells, suggesting that EP2 functions in mediating the anti-TGF-β activities of COX-2/PGE2. Recently, EP2 and its induction of cAMP production were shown to mediate the ability of PGE2 to stimulate VEGF expression and angiogenesis (15, 17). Figure 7E shows that the functional parallels between COX-2 and EP2 deficiency also extend to angiogenic defects, as 4T1 tumors lacking EP2 expression contained fewer and formed smaller blood vessels as compared with their parental counterparts. In addition, depleting EP2 expression in 4T1 cells also significantly impaired their ability to undergo pulmonary metastasis (Fig. 7F), which together with the necessity of EP2 expression for maximal angiogenesis, suggests that EP2 plays an essential role in promoting mammary tumor development and progression. Finally, EP2 signaling has also been linked to cancer-associated immunodeficiency (45) and to the regulation of tumor-infiltrating lymphocytes (12). As such, we examined whether EP2 deficiency altered lymphocyte infiltration into developing 4T1 tumors. Figure 7G shows that 4T1 tumors lacking EP2 expression contained significantly less CD3-positive lymphocytes as compared with their EP2-expressing counterparts. Taken together, these findings demonstrate the crucial roles played by EP2 during mammary tumor growth, angiogenesis, and metastasis. In addition, our findings suggest that chemotherapeutic targeting of EP2 may provide novel inroads to alleviate the oncogenic activities of TGF-β in breast cancer.
Figure 7.
EP2 deficiency inhibits mammary tumor growth and pulmonary metastasis induced by TGF-β. A, B) Control (Scram) or EP2-deficient 4T1 cells were injected into the mammary fat pads of Balb/C mice. At 10 d postinjection, tumor volumes were measured every second day until mice were sacrificed on d 28. Data are mean ± se tumor volume (A) or wet weight (B) (n=9). C, D) 4T1 tumor sections were stained with antibodies against Ki-67 (C) or phospho-Smad3 (D) as indicated. Extent of immunoreactivity was quantified by measuring signal pixel density using NIH ImageJ. Data in graphs are mean ± se staining intensity relative to control 4T1 tumor sections. E) 4T1 tumor sections were stained with Masson’s trichrome reagent, which stains collagen fibers blue and erythrocytes red (left panel). Data are mean ± se number of vessels formed per microscopic field. F) Lung sections were prepared from normal or 4T1 tumor-bearing mice and stained with H&E (left panel). Pulmonary metastatic lesions were counted manually. Data are mean ± se metastatic lesions observed per microscopic field. G) 4T1 tumor sections were stained with antibodies against CD3 (left panel). Data in graph are mean ± se number of CD3 positive cells per microscopic field. *P < 0.05; Student’s t test.
DISCUSSION
The molecular mechanisms underlying the oncogenic switch of TGF-β function from that of a tumor suppressor that inhibits the uncontrolled proliferation of MECs to that of a tumor promoter that stimulates their invasion and metastasis remain to be thoroughly elucidated (2). Indeed, the dynamics and interplay between canonical and noncanonical TGF-β signaling inputs underlie the complexity of this intricate signaling system in regulating the pathophysiological functions of TGF-β, which manifest in response to genetic disruptions and signaling imbalances that ultimately contribute to the dichotomous behaviors exhibited by TGF-β during breast cancer progression (2). Our previous work (25) established up-regulated COX-2 expression and its production of PGE2 as being important in vitro mediators of oncogenic TGF-β signaling in part by uncoupling TGF-β from activating Smad3 and by promoting TGF-β stimulation of EMT. We show here for the first time that the expression of EP2 is selectively targeted by TGF-β in normal and malignant MECs and, more important, that EP2 mediates the anti-TGF-β activities of COX-2 and PGE2, including their ability to suppress Smad2/3 signaling both in vitro (Figs. 12345) and in vivo (Fig. 7). The net result of these EP2 activities manifest in part through the enhanced proliferation and morphological transformation of normal MECs (Fig. 2), suggesting an important function for EP2 signaling in promoting the early stages of mammary tumorigenesis. Along these lines, EP2 activity is essential in promoting cell proliferation in a murine model of skin cancer, wherein the levels of EP2 expression correlate positively with the ability of carcinogens or PGE2 to induce tumor growth (19, 20). EP2 expression is also necessary for the ability of COX-2 to promote mammary gland hyperplasia (23), and in fact, we show that EP2 expression and activity were necessary in mediating COX-2 expression in metastatic 4T1 cells (Fig. 4). Thus, in addition to its ability to promote the early stages of mammary tumorigenesis, EP2 signaling also figures prominently in late-stage breast cancers where it induces their invasion, angiogenesis, and metastasis (Figs. 4, 5, and 7). Another striking finding of this study was the complete pathological redundancy existing between COX-2 and EP2 in promoting mammary tumor development and progression, including their ability to enhance oncogenic TGF-β signaling (Figs. 6 and 7). Indeed, the antitumor activities resulting from EP2 deficiency were indistinguishable from those elicited by COX-2 deficiency, presumably due to the necessity of both molecules to establish a PGE2:EP2:COX-2 positive feedback loop in malignant cells (10, 13, 39). Thus, our findings provide the first preclinical evidence that chemotherapeutic targeting of EP2 may engender antitumor and anti-TGF-β activities reminiscent of those elicited by COX-2 antagonism, and as such, the use of EP2 antagonists may circumvent the cardiovascular and renal health risks associated with the administration of COX-2 inhibitors (3,4,5).
A number of recent studies (12,13,14,15,16,17,18,19,20,21) have established important roles for EP2 during tumor development and progression. For instance, EP2 deficiency has been associated with a reduction in the growth, angiogenesis, inflammation, and survival of skin tumors in mice, while EP2 overexpression significantly enhanced these same tumorigenic behaviors (19, 20). In addition, EP2 expression has also been linked to the angiogenesis activation and polyp formation in the colon (13) and to angiogenesis in cancers of the breast (24). Accordingly, we showed that COX-2 and EP2 deficiency both significantly impair the growth and pulmonary metastasis of 4T1 tumors in mice in part by reducing tumor angiogenesis. Another particularly interesting finding of this study was the sufficiency of COX-2 overexpression to transform NMuMG cells following their engraftment into the mammary fat pads of athymic mice. Indeed, not only did COX-2 expression increase the incidence of tumor formation by NMuMG cells (i.e., 83% of COX-2 expressers vs. 33% of parentals), but this cellular condition also produced larger and more poorly differentiated tumors that contained distinct regions of sarcomatoid progression. Thus, COX-2 expression promotes EMT in cultured MECs in response to TGF-β (25) and, more important, in developing and progressing mammary tumors produced in mice (Fig. 6). Given the parallels between COX-2 and EP2 in promoting mammary tumorigenesis, future studies need to assess the relative contribution of EP2 expression and TGF-β function in mediating the transforming activity of COX-2. Despite these knowledge gaps, our findings do suggest that EP2 antagonism will suppress mammary tumorigenesis by uncoupling COX-2/PGE2 from stimulating breast cancer growth, angiogenesis, and immunosuppression; and diminishing the oncogenic activities of TGF-β, particularly its ability to induce EMT and metastasis.
Finally, our findings indicate that the anti-TGF-β activities mediated by COX-2/PGE2 in normal and malignant MECs require EP2 receptors and their production of intracellular cAMP, the activities of which were recapitulated by forskolin administration (Fig. 1). An especially intriguing finding of our study was the selective antagonism of TGF-β signaling specifically by EP2 receptors and not their related EP4 receptors, despite the fact that both receptor subtypes are expressed in normal and malignant MECs (Fig. 1) and couple to the production of cAMP (36). The inability of EP4 receptors to inhibit TGF-β signaling could be explained by the fundamental differences between EP2 and EP4 receptor structure and relative efficiency to couple to cAMP production (12, 46). Indeed, when EP2 and EP4 receptors are expressed at a comparable receptor number, PGE2-mediated activation of EP2 receptors results in the production of ∼7-fold more cAMP than that induced by EP4 receptors (12), findings that are remarkably similar to the differences in CRE-luciferase expression we measured in NMuMG cells (Fig. 1F). Alternatively, the specificity of EP2 vs. EP4 receptors to inhibit TGF-β signaling may also reflect differences in their ability to internalize in response to PGE2 stimulation (47, 48), as well as in their preferential coupling to cAMP · PKA (EP2 receptors) vs. PI3K · AKT · ERK1/2 (EP4 receptors; ref. 49). Along these lines, it has been suggested that the growth, angiogenesis, and inflammation observed in developing skin tumors is largely accounted for by the actions of EP2/cAMP/PKA (20). Moreover, the transcription factor cAMP-responsive element binding protein (CREB) plays an important role in governing cell proliferation, angiogenesis, and apoptosis in part by regulating the expression of Bcl-2, IAP, VEGF, and the cyclins D1 and A (50,51,52,53). Similarly, EP2 expression mediates the ability of COX-2/PGE2 to induce the activation of PKA/CREB necessary in promoting mouse skin proliferation (54). We also found TGF-β to be capable of activating CREB in a EP2-dependent manner (Fig. 2), and as such, future studies are warranted to determine the downstream effectors and genes targeted by the COX-2/PGE2/EP2/cAMP signaling axis and to elucidate how these events negatively affect TGF-β function in developing and progressing mammary tumors.
In summary, our findings have established EP2 as a novel mediator of the anti-TGF-β activities stimulated by COX-2/PGE2 in normal and malignant MECs. Moreover, the similarities between COX-2 and EP2 in initiating the oncogenic activities of TGF-β both in vitro and in vivo suggest a crucial and essential role for EP2 during breast cancer progression. Thus, chemotherapeutic targeting of EP2 may provide new inroads to alleviate the tumor promoting properties of TGF-β in late-stage breast cancers and to circumvent the severe cardiovascular and renal side effects associated with the administration of COX-2 antagonists to cancer patients (3,4,5).
Acknowledgments
The authors thank Dr. Paul Jedlicka for pathology help and assistance. The authors also thank the members of the W.P.S. laboratory for critical comments and reading of the manuscript. W.P.S. was supported by grants from the National Institutes of Health (CA-114039 and CA-129359).
References
- Siegel P M, Massague J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- Tian M, Schiemann W P. The TGF-β paradox in human cancer: an update. Future Oncol. 2009;5:259–271. doi: 10.2217/14796694.5.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazhar D, Ang R, Waxman J. COX inhibitors and breast cancer. Br J Cancer. 2006;94:346–350. doi: 10.1038/sj.bjc.6602942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Ranger G, Salhab M, Mokbel K. The role of cyclooxygenase-2 in breast cancer: review. Breast Cancer Res Treat. 2008;109:189–198. doi: 10.1007/s10549-007-9641-5. [DOI] [PubMed] [Google Scholar]
- Howe L R. Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res. 2007;9:210. doi: 10.1186/bcr1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn A J, Gupta G P, Siegel P M, Bos P D, Shu W, Giri D D, Viale A, Olshen A B, Gerald W L, Massague J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Berry J A, Shoher A, Ayers G D, Wei C, Lucci A. COX-2 involvement in breast cancer metastasis to bone. Oncogene. 2007;26:3789–3796. doi: 10.1038/sj.onc.1210154. [DOI] [PubMed] [Google Scholar]
- Sheng H, Shao J, Washington M K, DuBois R N. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276:18075–18081. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- Hoper M M, Voelkel N F, Bates T O, Allard J D, Horan M, Shepherd D, Tuder R M. Prostaglandins induce vascular endothelial growth factor in a human monocytic cell line and rat lungs via cAMP. Am J Respir Cell Mol Biol. 1997;17:748–756. doi: 10.1165/ajrcmb.17.6.2888. [DOI] [PubMed] [Google Scholar]
- Lu X, Xie W, Reed D, Bradshaw W S, Simmons D L. Nonsteroidal antiinflammatory drugs cause apoptosis and induce cyclooxygenases in chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1995;92:7961–7965. doi: 10.1073/pnas.92.17.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara H, Kizaka-Kondoh S, Insel P A, Eckmann L. Inhibition of apoptosis in normal and transformed intestinal epithelial cells by cAMP through induction of inhibitor of apoptosis protein (IAP)-2. Proc Natl Acad Sci U S A. 2003;100:8921–8926. doi: 10.1073/pnas.1533221100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan J W. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74:143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, Oshima M, Taketo M M. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(delta 716) knockout mice. Nat Med. 2001;7:1048–1051. doi: 10.1038/nm0901-1048. [DOI] [PubMed] [Google Scholar]
- Eibl G, Bruemmer D, Okada Y, Duffy J P, Law R E, Reber H A, Hines O J. PGE(2) is generated by specific COX-2 activity and increases VEGF production in COX-2-expressing human pancreatic cancer cells. Biochem Biophys Res Commun. 2003;306:887–897. doi: 10.1016/s0006-291x(03)01079-9. [DOI] [PubMed] [Google Scholar]
- Kamiyama M, Pozzi A, Yang L, DeBusk L M, Breyer R M, Lin P C. EP2, a receptor for PGE2, regulates tumor angiogenesis through direct effects on endothelial cell motility and survival. Oncogene. 2006;25:7019–7028. doi: 10.1038/sj.onc.1209694. [DOI] [PubMed] [Google Scholar]
- Kuo K T, Wang H W, Chou T Y, Hsu W H, Hsu H S, Lin C H, Wang L S. Prognostic role of PGE2 receptor EP2 in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16:352–360. doi: 10.1245/s10434-008-0242-2. [DOI] [PubMed] [Google Scholar]
- Wang X, Klein R D. Prostaglandin E2 induces vascular endothelial growth factor secretion in prostate cancer cells through EP2 receptor-mediated cAMP pathway. Mol Carcinog. 2007;46:912–923. doi: 10.1002/mc.20320. [DOI] [PubMed] [Google Scholar]
- Chun K S, Akunda J K, Langenbach R. Cyclooxygenase-2 inhibits UVB-induced apoptosis in mouse skin by activating the prostaglandin E2 receptors, EP2 and EP4. Cancer Res. 2007;67:2015–2021. doi: 10.1158/0008-5472.CAN-06-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Y M, He G, Fischer S M. Lack of expression of the EP2 but not EP3 receptor for prostaglandin E2 results in suppression of skin tumor development. Cancer Res. 2005;65:9304–9311. doi: 10.1158/0008-5472.CAN-05-1015. [DOI] [PubMed] [Google Scholar]
- Sung Y M, He G, Hwang D H, Fischer S M. Overexpression of the prostaglandin E2 receptor EP2 results in enhanced skin tumor development. Oncogene. 2006;25:5507–5516. doi: 10.1038/sj.onc.1209538. [DOI] [PubMed] [Google Scholar]
- Banu S K, Lee J, Speights V O, Jr, Starzinski-Powitz A, Arosh J A. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 induces apoptosis of human endometriotic cells through suppression of ERK1/2, AKT, NF-κB, and β-catenin pathways and activation of intrinsic apoptotic mechanisms. Mol Endocrinol. 2009;23:1291–1305. doi: 10.1210/me.2009-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniface K, Bak-Jensen K S, Li Y, Blumenschein W M, McGeachy M J, McClanahan T K, McKenzie B S, Kastelein R A, Cua D J, de Waal Malefyt R. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S H, Ai Y, Breyer R M, Lane T F, Hla T. The prostaglandin E2 receptor EP2 is required for cyclooxygenase 2-mediated mammary hyperplasia. Cancer Res. 2005;65:4496–4499. doi: 10.1158/0008-5472.CAN-05-0129. [DOI] [PubMed] [Google Scholar]
- Chang S H, Liu C H, Wu M T, Hla T. Regulation of vascular endothelial cell growth factor expression in mouse mammary tumor cells by the EP2 subtype of the prostaglandin E2 receptor. Prostaglandins Other Lipid Mediat. 2005;76:48–58. doi: 10.1016/j.prostaglandins.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Neil J R, Johnson K M, Nemenoff R A, Schiemann W P. Cox-2 inactivates Smad signaling and enhances EMT stimulated by TGF-β through a PGE2-dependent mechanisms. Carcinogenesis. 2008;29:2227–2235. doi: 10.1093/carcin/bgn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliher A J, Schiemann W P. β3 integrin and Src facilitate TGF-β mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2006;8:R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Schiemann W P. Preclinical efficacy of cystatin C to target the oncogenic activity of TGF-β in breast cancer. Translat Oncol. 2009;2:174–183. doi: 10.1593/tlo.09145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann B J, Neil J R, Schiemann W P. SPARC inhibits epithelial cell proliferation in part through stimulation of the TGF-β-signaling system. Mol Biol Cell. 2003;14:3977–3988. doi: 10.1091/mbc.E03-01-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann W P, Pfeifer W M, Levi E, Kadin M E, Lodish H F. A deletion in the gene for TGF-β type I receptor abolishes growth regulation by TGF-β in a cutaneous T-cell lymphoma. Blood. 1999;94:2854–2861. [PubMed] [Google Scholar]
- Galliher-Beckley A J, Schiemann W P. Grb2 binding to Tyr284 in TβR-II is essential for mammary tumor growth and metastasis stimulated by TGF-β. Carcinogenesis. 2008;29:244–251. doi: 10.1093/carcin/bgm245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A J, Subbaramaiah K. Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell. 2003;4:431–436. doi: 10.1016/s1535-6108(03)00310-6. [DOI] [PubMed] [Google Scholar]
- Cao Y, Prescott S M. Many actions of cyclooxygenase-2 in cellular dynamics and in cancer. J Cell Physiol. 2002;190:279–286. doi: 10.1002/jcp.10068. [DOI] [PubMed] [Google Scholar]
- Greenhough A, Smartt H J, Moore A E, Roberts H R, Williams A C, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- Lee B P, Juvet S C, Zhang L. Prostaglandin E2 signaling through E prostanoid receptor 2 impairs proliferative response of double negative regulatory T cells. Int Immunopharmacol. 2009;9:534–539. doi: 10.1016/j.intimp.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Hudis C, Chang S H, Hla T, Dannenberg A J. EP2 and EP4 receptors regulate aromatase expression in human adipocytes and breast cancer cells. Evidence of a BRCA1 and p300 exchange. J Biol Chem. 2008;283:3433–3444. doi: 10.1074/jbc.M705409200. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- Chang S H, Liu C H, Conway R, Han D K, Nithipatikom K, Trifan O C, Lane T F, Hla T. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci U S A. 2004;101:591–596. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins-Port C E, Higgins P J. Regulation of extracellular matrix remodeling following TGF-β1/epidermal growth factor-stimulated epithelial-mesenchymal transition in human premalignant keratinocytes. Cells Tissues Organs. 2007;185:116–122. doi: 10.1159/000101312. [DOI] [PubMed] [Google Scholar]
- Bradbury D A, Newton R, Zhu Y M, El-Haroun H, Corbett L, Knox A J. Cyclooxygenase-2 induction by bradykinin in human pulmonary artery smooth muscle cells is mediated by the cyclic AMP response element through a novel autocrine loop involving endogenous prostaglandin E2, E-prostanoid 2 (EP2), and EP4 receptors. J Biol Chem. 2003;278:49954–49964. doi: 10.1074/jbc.M307964200. [DOI] [PubMed] [Google Scholar]
- Nam J S, Terabe M, Mamura M, Kang M J, Chae H, Stuelten C, Kohn E, Tang B, Sabzevari H, Anver M R, Lawrence S, Danielpour D, Lonning S, Berzofsky J A, Wakefield L M. An anti-TGF-β antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res. 2008;68:3835–3843. doi: 10.1158/0008-5472.CAN-08-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil J R, Schiemann W P. Altered TAB1:IκB kinase interaction promotes TGF-β-mediated NF-κB activation during breast cancer progression. Cancer Res. 2008;68:1462–1470. doi: 10.1158/0008-5472.CAN-07-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil J R, Tian M, Schiemann W P. X-linked inhibitor of apoptosis protein (xIAP) and its E3 ligase activity promotes TGF-β-mediated NF-κB activation during breast cancer progression. J Biol Chem. 2009;284:21209–21217. doi: 10.1074/jbc.M109.018374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Cao W, Wen R, Steinberg R H, LaVail M M. Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Muller cells. Invest Ophthalmol Vis Sci. 1998;39:581–591. [PubMed] [Google Scholar]
- Williams C S, Tsujii M, Reese J, Dey S K, DuBois R N. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest. 2000;105:1589–1594. doi: 10.1172/JCI9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Yamagata N, Yadav R, Brandon S, Courtney R L, Morrow J D, Shyr Y, Boothby M, Joyce S, Carbone D P, Breyer R M. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest. 2003;111:727–735. doi: 10.1172/JCI16492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chell S, Kaidi A, Williams A C, Paraskeva C. Mediators of PGE2 synthesis and signalling downstream of COX-2 represent potential targets for the prevention/treatment of colorectal cancer. Biochim Biophys Acta. 2006;1766:104–119. doi: 10.1016/j.bbcan.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Desai S, April H, Nwaneshiudu C, Ashby B. Comparison of agonist-induced internalization of the human EP2 and EP4 prostaglandin receptors: role of the carboxyl terminus in EP4 receptor sequestration. Mol Pharmacol. 2000;58:1279–1286. doi: 10.1124/mol.58.6.1279. [DOI] [PubMed] [Google Scholar]
- Nishigaki N, Negishi M, Ichikawa A. Two Gs-coupled prostaglandin E receptor subtypes, EP2 and EP4, differ in desensitization and sensitivity to the metabolic inactivation of the agonist. Mol Pharmacol. 1996;50:1031–1037. [PubMed] [Google Scholar]
- Fujino H, Xu W, Regan J W. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. J Biol Chem. 2003;278:12151–12156. doi: 10.1074/jbc.M212665200. [DOI] [PubMed] [Google Scholar]
- Desdouets C, Matesic G, Molina C A, Foulkes N S, Sassone-Corsi P, Brechot C, Sobczak-Thepot J. Cell cycle regulation of cyclin A gene expression by the cyclic AMP-responsive transcription factors CREB and CREM. Mol Cell Biol. 1995;15:3301–3309. doi: 10.1128/mcb.15.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Evers B M, Sheng H. Prostaglandin E2 synergistically enhances receptor tyrosine kinase-dependent signaling system in colon cancer cells. J Biol Chem. 2004;279:14287–14293. doi: 10.1074/jbc.M313276200. [DOI] [PubMed] [Google Scholar]
- Shaywitz A J, Greenberg M E. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Shao J, Lee S B, Guo H, Evers B M, Sheng H. Prostaglandin E2 stimulates the growth of colon cancer cells via induction of amphiregulin. Cancer Res. 2003;63:5218–5223. [PubMed] [Google Scholar]
- Ansari K M, Sung Y M, He G, Fischer S M. Prostaglandin receptor EP2 is responsible for cyclooxygenase-2 induction by prostaglandin E2 in mouse skin. Carcinogenesis. 2007;28:2063–2068. doi: 10.1093/carcin/bgm011. [DOI] [PubMed] [Google Scholar]