Abstract
Heparanase is a mammalian endo-β-d-glucuronidase that can cleave heparan sulfate side chains, an activity strongly implicated in tumor cell dissemination. The current study aimed to identify and characterize heparanase splice variants. LEADS, Compugen’s alternative splicing modeling platform (Compugen, Tel Aviv, Israel), was used to search for splice variants in silico; tumor-derived cell lines (i.e., CAG myeloma) and tumor biopsies were utilized to validate T5 expression in vivo; signaling (i.e., Src phosphorylation) was evaluated following T5 gene silencing or overexpression and correlated with cell proliferation, colony formation, and tumor xenograft development. A novel spliced form of human heparanase, termed T5, was identified. In this splice variant, 144 bp of intron 5 are joined with exon 4, which results in a truncated, enzymatically inactive protein. T5 overexpression resulted in increased cell proliferation and larger colonies in soft agar, mediated by Src activation. Furthermore, T5 overexpression markedly enhanced tumor xenograft development. T5 expression is up-regulated in 75% of human renal cell carcinoma biopsies examined, which suggests that this splice variant is clinically relevant. Controls included cells overexpressing wild-type heparanase or an empty plasmid and normal-looking tissue adjacent the carcinoma lesion. T5 is a novel functional splice variant of human heparanase endowed with protumorigenic characteristics.—Barash, U., Cohen-Kaplan, V., Arvatz, G., Gingis-Velitski, S., Levy-Adam, F., Nativ, O., Shemesh, R., Ayalon-Sofer, M., Ilan, N., Vlodavsky, I. A novel human heparanase splice variant, T5, endowed with protumorigenic characteristics.
Keywords: cell proliferation, colony formation, xenograft, Src, phosphorylation
Heparanase is an endo-β-d-glucuronidase that can cleave heparan sulfate (HS) side chains at a limited number of sites. The consequence of this activity combines structural alteration of the extracellular matrix (ECM) underlying epithelial and endothelial cells, which makes it more susceptible to cellular invasion, and liberation of a multitude of biological mediators sequestered in the ECM or tethered to HS on the cell membrane. Heparanase activity has long been associated with metastatic potential of tumor-derived cells (1, 2), a notion that has been established by using specific anti-heparanase siRNA and ribozyme methodologies (3). Although heparanase in cellular invasion and tumor metastasis plays a decisive role (4,5,6,7), its function in primary tumor progression remains largely unknown but likely involves tumor angiogenesis. Several in vitro and in vivo model systems, including wound healing (8, 9), tumor xenografts (10), Matrigel plug assay (8) and tube-like structure formation (11), have confirmed the angiogenic potency of heparanase. Moreover, microvessel density was significantly reduced in tumor xenografts developed by T-lymphoma cells transfected with anti-heparanase ribozyme (3), and clinical findings concluded with a proangiogenic feature of heparanase (7, 12). These observations, the anticancerous effect of heparanase gene silencing (3) and of heparanase-inhibiting molecules (4, 13), as well as the unexpected identification of a single functional heparanase (6, 14), suggest that the enzyme is a valid target for anticancer drug development and a promising tumor marker (4, 13, 15,16,17).
Apart from the well-studied enzymatic feature of the enzyme, findings indicated that heparanase exerts biological functions apparently independent of its enzymatic activity. Inactive heparanase was noted to enhance adhesion and migration of normal and tumor-derived cells (10, 11, 18, 19) and to promote the phosphorylation of signaling molecules such as Akt (10, 11, 20, 21), which likely support cell survival (22). Similarly, enzymatically inactive heparanase was noted to enhance the phosphorylation of p38 and Src, associated with induced tissue factor (TF) and VEGF expression (23, 24). Heparanase also augmented the phosphorylation of EGFR in a Src-dependent manner (25). EGFR activation by heparanase was associated with enhanced cell proliferation and colony formation in soft agar (25). Furthermore, head and neck carcinoma showed a correlation between heparanase and EGFR phosphorylation levels (25), with the heparanase-Src-EGFR axis playing an important route in tumor progression (26). A splice variant of human heparanase was described (27), yet its function remains obscure. Here, we describe a novel splice form of human heparanase termed T5, in which 144 bp of intron 5 are joined with exon 4, which results in a truncated 169-aa protein. We provide evidence that T5 augments Src phosphorylation to levels comparable with the full-length heparanase. Moreover, T5 overexpression was associated with increased cell proliferation, larger colonies in soft agar, and markedly enhanced tumor xenograft development. T5 is overexpressed in the majority (75%) of human renal cell carcinoma biopsies examined, which suggests that this splice variant is clinically relevant.
MATERIALS AND METHODS
Discovery of heparanase splice variants
The discovery of T4 and T5 heparanase splice variants was carried out using LEADS, Compugen’s alternative splicing modeling platform (Compugen, Tel Aviv, Israel) (28,29,30,31). Briefly, human ESTs and cDNAs were obtained from the U.S. National Center for Biotechnology Information GenBank (www.ncbi.nlm.nih.gov/Genebank) and aligned to the human genome build (www.ncbi.nlm.nih.gov/genome/guide/human) using the LEADS clustering and assembly algorithms. The platform cleans the expressed sequences from vectors and immunoglobulins, masks them for repeats and low-complexity regions, and aligns the expressed sequences to the genome while modeling alternative splicing. The discovery of the skip 10 splice variant was carried out using a “non-EST-based method for exon-skipping prediction” (30). In principle, this method relies on a gene’s exon structure/size and the human/mouse homology of the exon and its surrounding sequences.
T5 Cloning and gene constructs
3′-Rapid amplification of cDNA ends (RACE) analysis (Ambion, Austin, TX, USA) was performed using the forward heparanase primers 5′-GAGAATTCAGGTGAGCCCAAGATGCTGCTG-3′, 5′-GGAATTCATGCTGCTGCGCTCG-3′, according to the manufacturer’s instructions. The PCR product was purified by Wizard SV Gel PCR Clean-Up System (Promega, Madison, WI, USA), and sequenced. The T5 cDNA was subcloned into eukaryotic expression plasmids pcDNA3, pSecTag2A, or pTK208 lentivirus vector, essentially as described (32).
Antibodies and reagents
Anti-Myc-tag (sc-40), anti-Src (sc-18), and anti-calnexin (sc-11397) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-phospho-Src (Tyr416) antibody was purchased from Cell Signaling Technologies (Beverly, MA, USA). Anti-heparanase 1453 and 733 have previously been characterized (33). Anti-mouse platelet endothelial cell adhesion molecule (PECAM)-1 (CD31) polyclonal antibody was kindly provided by Dr. Joseph A. Madri (Yale University, New Haven, CT, USA) (23). Concanavalin A-Sepharose and bromodeoxyuridine (BrdU) were purchased from GE Healthcare (Little Chalfont, UK) and anti-BrdU monoclonal antibody-HRP conjugated was purchased from Roche (Mannheim, Germany). Fluorescein wheat germ agglutinin was purchased from Vector Laboratories (Burlingame, CA, USA). Src inhibitors PP1, PP2, and Src inhibitor I (Merck Biosciences, GmbH, Germany) were dissolved in DMSO as stock solution. DMSO was added to the cell culture as a control.
Cells and cell culture
HEK 293, human choriocarcinoma JAR, U87-MG glioma, Colo205 and HT29 colon carcinoma, PC-3 prostate carcinoma, MDA-MB-231 breast carcinoma, and PANC-1 pancreatic carcinoma cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). FaDu pharynx carcinoma cells were kindly provided by Dr. Eben L. Rosenthal (University of Alabama at Birmingham, Birmingham, AL, USA) (34) and CAG myeloma cells were kindly provided by Dr. Ben-Zion Katz (Tel Aviv Sourasky Medical Center, Tel Aviv, Israel) (35). Cells were grown in Dulbecco’s modified Eagle’s medium (Biological Industries, Beit Haemek, Israel) supplemented with 10% fetal calf serum (FCS) and antibiotics. Human umbilical vein endothelial cells (HUVECs) were isolated and grown essentially as described previously (11).
Gene silencing and PCR analysis
Anti-heparanase and anti-T5 siRNA oligonucleotides (siGenome On-Target plus SMART pool duplex) were purchased from Dharmacon (Thermo Fisher Scientific Inc, Waltham, MA, USA), and transfection was carried out with DharmaFECT reagent (Dharmacon), according to the manufacturer’s instructions. PCR was preformed with the following primer sets: heparanase, F 5′-ACAGTTCTAATGCTCAGTTGCTC-3′ and R 5′-TTGCCTCATCACCACTTCTATT-3′; T5, F 5′-TTGGCCAGAGGCTTGTCTCC-3′ and R 5′-CCCATTTGGGAAATGCTTCAG-3′; GAPDH, F 5′-CCAGCCGAGCCACATCGCTC-3′ and R 5′-ATGAGCCCCAGCCTTCTCCAT-3′.
Immunocytochemistry
Immunofluorescent staining was performed essentially as described previously (34).
Cell lysates, activity assay, and protein blotting
Preparation of cell lysates, protein blotting, and measurement of heparanase enzymatic activity were carried out as described previously (24, 25, 34).
Cell proliferation
Cell proliferation was analyzed by BrdU incorporation using cell proliferation labeling reagent (1:1000, GE Healthcare), as described previously (10). At least 1000 cells were counted for each cell type.
Colony formation in soft agar
DMEM (3 ml) containing 0.5% low-melt agarose (Bio-Rad, Hercules, CA, USA) and 10% FCS was poured into 60-mm Petri dishes. The layer was covered with cell suspension (0.5×104 cells) in 1.5 ml DMEM containing 0.3% low-melt agarose and 10% FCS, followed by addition of 2 ml DMEM containing 10% FCS. Medium was exchanged every 3 d. Colonies were visualized and counted under a microscope after 2–5 wk, as described previously (25).
Tumorigenicity and immunohistochemistry
Cells from exponential cultures of control-, heparanase-, and T5-infected CAG myeloma cells were detached with trypsin/EDTA, washed with PBS, and brought to a concentration of 1 × 107 cells/ml. Cell suspension (1×106/0.1 ml) was inoculated subcutaneously at the right flank of 5-wk-old female athymic nude mice (n=7). Xenograft size was determined 2×/wk by externally measuring tumors in 2 dimensions using a caliper. At the end of the experiment, mice were sacrificed, and xenografts were removed, weighed, and fixed in formalin. Paraffin-embedded 5-μm sections were subjected to immunostaining with anti-PECAM-1 (CD31) or anti-smooth muscle actin (1A4; Sigma) antibodies, using the Envision kit (Dako, Glostrup, Denmark) according to the manufacturer’s instructions, as described previously (25, 34). The Animal Care Committee of the Technion (Haifa, Israel), approved all animal experiments.
Statistics
Data are presented as means ± se. Statistical significance was analyzed by 2-tailed Student’s t test. Values of P < 0.05 were considered significant. All experiments were repeated ≥3 times with similar results.
RESULTS
Discovery, cloning, and expression of heparanase splice variant T5
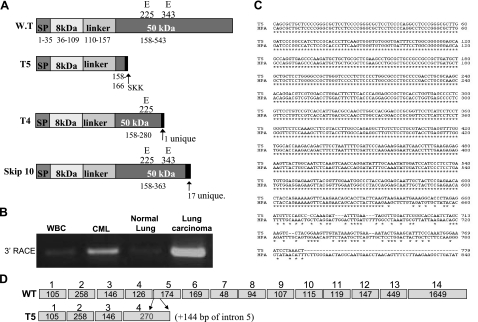
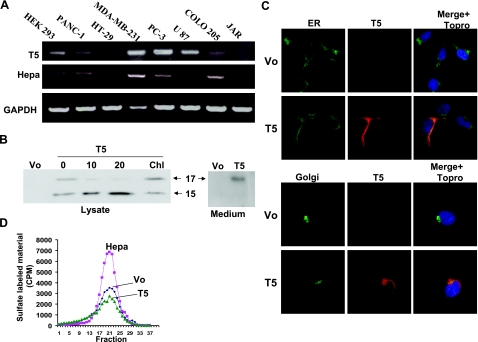
We have computationally discovered three different splice forms of heparanase (T4, T5, and skip 10; Fig. 1A) using Compugen’s alternative splicing modeling platform LEADS (T4 and T5), and a non-EST-based method for exon-skipping prediction (skip 10). 3′-RACE analysis yielded a product of 687 bp that was highly enriched in RNA extracted from lung carcinoma tissue and chronic myeloid leukemia (CML) cells compared with controls [adjacent normal lung tissue and white blood cells (WBCs) collected from healthy donors, respectively; Fig. 1B]. Sequencing of the 3′-RACE product matched the expected T5 sequence (Fig. 1C), indicating expression of the T5 splice variant. The expression of T4 and skip 10 splice variants could not be detected under these experimental settings. T5 expression was subsequently confirmed by RT-PCR analysis of total RNA extracted from several cancer-derived cell lines applying T5-specific primers (Fig. 2A, top panel). T5 expression is detected, along with heparanase, to various levels in essentially all cell lines examined. JAR cells reported to be negative for heparanase (36) (Fig. 2A, middle panel) were also negative for T5 expression (Fig. 2A, top panel). To verify that T5 encodes for a protein, the T5 sequence was cloned into a mammalian expression vector, and lysate samples prepared from stably transfected 293 cells were subjected to immunoblotting. Anti-heparanase antibody clearly detected an ∼15-kDa protein band, a molecular mass slightly higher than that anticipated from the deduced amino acid sequence (14,207; Fig. 2B, 0), whereas no reactivity was detected in control cells transfected with an empty plasmid (Fig. 2B, Vo). In addition, an ∼17-kDa protein band was also seen (Fig. 2B, 0). We considered two possibilities for the appearance of two T5 protein bands: different glycosylation pattern or proteolytic cleavage of the high-molecular-mass protein. We examined the first possibility by incubating T5-overexpressing cells with increasing concentrations of tunicamycin, an inhibitor of protein N-glycosylation. Indeed, tunicamycin treatment markedly reduced the amount of the ∼17-kDa band, while levels of the ∼15-kDa protein were increased (Fig. 2B, 10, 20). In contrast, chloroquine, reported to inhibit proteolytic processing of heparanase within lysosomes (33), did not significantly affect the ratio between the two T5 forms (Fig. 2, Chl). These results indicate that T5 is being expressed as an ∼15-kDa protein and a more glycosylated 17-kDa form and that T5 is not subjected to processing within lysosomes. As would be expected from the presence of a signal peptide, T5 is secreted and found in the cell conditioned medium (Fig. 2B, right). This finding is in agreement with its localization to the ER and Golgi apparatus (Fig. 2C), as demonstrated previously for heparanase (34). Subjecting lysates of stably transfected cells to heparanase activity assay indicated that T5 lacks heparanase enzymatic activity (Fig. 2D, T5 vs. Hepa) as would be expected from a heparanase variant comprising only a small portion of the 50-kDa subunit (Fig. 1A, T5). Moreover, T5 expression did not seem to impinge significantly on the activity of endogenous heparanase (Fig. 2D, T5 vs. Vo), which suggests that T5 does not function as a modulator of heparanase enzymatic activity.
Figure 1.
Expression of T5 splice variant. A) Schematic structure of heparanase splice variants emerging from Compugen’s LEADS search. B) 3′-RACE analysis of RNA extracted from white blood cells collected from healthy donor (WBC), patient with chronic myeloid leukemia (CML), lung carcinoma, and adjacent normal lung tissue. C) Sequence alignment indicating that the 3′-RACE product corresponds to the predicted T5 splice variant sequence. D) Schematic presentation of heparanase (WT) and T5 exon composition.
Figure 2.
Cloning and expression of T5. A) RT-PCR analysis. Total RNA was extracted from the indicated cell line and subjected to RT-PCR analysis applying T5 (top panel), heparanase (middle panel), and GAPDH (bottom panel) specific primers, specified in the Materials and Methods section. B) T5 transfection. T5 was cloned into mammalian expression vector (pcDNA3), and transfected HEK 293 cells were left untreated (0) or incubated with tunicamycin (10, 20 μg/ml) or chloroquine (Chl; 100 μM). Control cells were transfected with an empty vector (Vo). Cell lysates (left panel) and cell conditioned medium (right panel) were blotted with anti-heparanase 1453 antibody. C) Cellular localization. Stably transfected HEK 293 cells were triple stained for Myc-tag (T5; red), the ER marker calnexin (ER; top panels, green), and merged with cell nuclei labeled with TO-PRO (top panels, blue). Cells were similarly stained with anti-Myc-tag (T5; red), the Golgi marker wheat germ agglutinin-FITC (Golgi; bottom panels, green) and merged with cell nuclei labeled with TO-PRO (bottom panels, blue). Shown is one image in which the two colors are of equal intensities, representative of many images, all exhibiting similar localization patterns. D) Enzymatic activity. HEK 293 cells transfected with heparanase (Hepa), T5, or control empty vector (Vo) were subjected to 3 freeze-thaw cycles and applied onto culture dishes coated with 35S-labeled ECM. Release of sulfate-labeled material eluted in fractions 15–30 was evaluated as measure of heparanase activity, as described in Materials and Methods.
T5 activates Src and facilitates cell proliferation
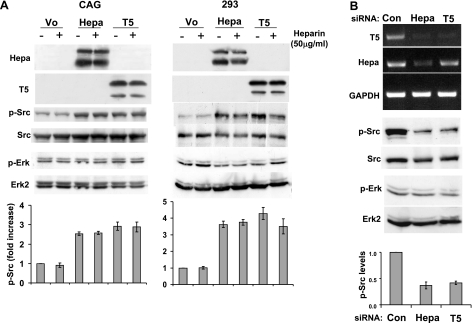
Since T5 does not appear to modulate heparanase enzymatic activity (Fig. 2D), we sought to determine an alternative function of the splice variant and examined whether T5 facilitates the phosphorylation of Src, shown previously to be induced by heparanase (24,25,26). Src phosphorylation was increased nearly 3-fold in CAG myeloma cells infected with heparanase (Fig. 3A, left; third and bottom panels, Hepa), in agreement with a similar effect noted in diverse cell systems (24, 25). Notably, CAG cells infected with T5 exhibited comparable induction of Src phosphorylation (Fig. 3A, left; third panel, T5), as determined by densitometry analysis (Fig. 3A, left; bottom panel). Even higher enhancement of Src phosphorylation (∼4-fold) was found in 293 cells overexpressing heparanase or T5 proteins (Fig. 3A, right panels). Src phosphorylation enhanced by heparanase or T5 was not affected by heparin (Fig. 3A, +), which suggests that the observed stimulation is not mediated by cell surface HS (26, 37). In contrast, Erk phosphorylation was not affected by heparanase or T5 overexpression (Fig. 3A, fifth and sixth panels), suggesting a specific trait.
Figure 3.
T5 augments Src phosphorylation. A) Overexpression. CAG myeloma (left) and 293 (right) cells infected with heparanase (Hepa), T5, or control empty vector (Vo) were grown in the absence (–) or presence (+) of heparin (50 μg/ml) under serum-free conditions. Total cell lysates were subjected to immunoblotting, applying anti-heparanase 1453 (top and second panels), anti-phospho-Src (p-Src, third panels), anti-Src (fourth panels) anti-phospho-Erk (p-Erk, fifth panels), and anti-Erk2 antibodies (sixth panels). Bottom panels: Src phosphorylation index was calculated by densitometry analysis of phosphorylated Src levels divided by total Src values. Data are presented as average ± se fold increase of Src phosphorylation vs. control Vo cells (set to arbitrary value of 1) of 5 independent experiments. B) Gene silencing. Parental 293 cells were transfected with anti-heparanase, anti-T5, or control siRNAs. Total RNA was extracted, and RT-PCR analysis was performed, applying T5 (top panel), heparanase (Hepa; second panel), and GAPDH (third panel) specific primers. Corresponding cell lysates were subjected to immunoblotting, applying phospho-Src (p-Src; fourth panel), Src (fifth panel), phospho-Erk (p-Erk; sixth panel) and Erk2 (seventh panel) antibodies. Bottom panel: Src phosphorylation index, calculated as in A. Note decreased Src phosphorylation levels in response to T5 down-regulation.
We next utilized the opposite approach and designed siRNA specific for the inhibition of endogenous heparanase or T5 expression by 293 cells (Fig. 3B). Anti-T5 siRNA significantly and specifically decreased T5 expression while heparanase expression was not inhibited (Fig. 3B, top and second panels, T5). In contrast, anti-heparanase siRNA inhibited both heparanase and T5 expression (Fig. 3B, top and second panels). Heparanase gene silencing was associated with reduced Src phosphorylation (Fig. 3B, fourth panel, Hepa), which is in agreement with the notion that endogenous heparanase is engaged in Src regulation (25). Notably, not only heparanase but also T5 silencing resulted in reduced Src phosphorylation (Fig. 3B, fourth panel, T5). Densitometry analysis revealed >2-fold decrease in Src phosphorylation levels following heparanase and T5 silencing (Fig. 3B, bottom panel). In contrast, the phosphorylation levels of Erk were not affected (Fig. 3C, sixth panel), which suggests a specific effect.
We have reported previously that interaction of heparanase with cell membrane HS results in clustering of syndecans (37). Syndecan clustering by heparanase or by a peptide (termed KKDC) corresponding to the heparin-binding domain of heparanase, stimulated the adhesion and spreading of various cell types, involving PKC, Src, and Rac1 activity (37). The heparin-binding sequence Lys158-Asn162 (38) is retained in the T5 splice variant and is followed by the addition of three unique amino acids, SKK, thought to comprise additional binding sites for heparin (Fig. 1A). Src activation by T5, noted above, may therefore result from clustering and activation of cell membrane HSPG. Our findings argue, however, against this possibility. First, inclusion of heparin did not inhibit Src activation by T5 (Fig. 3A, +). In fact, T5 failed to bind heparin (Supplemental Fig. 1A, left; bottom panel), while interaction with the lectin concanavalin A was retained (Supplemental Fig. 1A, bottom panel, ConA), in agreement with T5 being glycosylated (Fig. 2B). Similarly, T5 levels in the cell culture medium were not affected by the addition of heparin, whereas heparanase levels are markedly increased (Supplemental Fig. 1A, right). Second, a peptide harboring the heparin binding domain, including the unique SKK residues (CKKFKNSTYSSKK), failed to interact with heparin (Supplemental Fig. 1B, CKKK) or to inhibit heparanase enzymatic activity (Supplemental Fig. 1C), both accomplished by the KKDC peptide (Supplemental Fig. 1B, C). We, therefore, concluded that Src activation by T5 is HS-independent but rather likely involves interaction with other cell surface molecules.
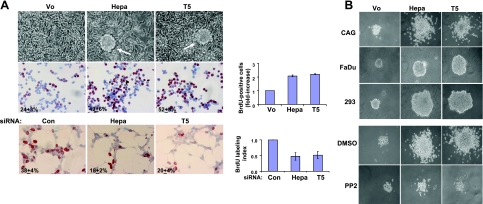
The intimate involvement of Src in different aspects of tumor progression, including cell proliferation (39), led us to examine this aspect in heparanase- and T5-overexpressing cells (Fig. 4). Control CAG cells mainly grow while adhering to the culture dish as a monolayer (Fig. 4A, top panel, Vo) (35). In contrast, heparanase- and T5-infected CAG cells were noted to form large foci (Fig. 4A, top panel; Hepa, T5; arrows), indicating their overgrowth. Increased cell proliferation was revealed further by BrdU incorporation (Fig. 4A, middle panel). Thus, while 24 ± 3% of control CAG cells incorporated BrdU (Fig. 4A, middle panel, Vo), heparanase- and T5-infected cells exhibited 49 ± 6% and 52 ± 5% BrdU incorporation, respectively, an increase that is statistically highly significant (P=0.0002 and 0.00001 for heparanase vs. Vo and T5 vs. Vo, respectively), reflecting >2-fold increase in cell proliferation (Fig. 4A, middle panel, right). Similar results also were observed in heparanase- and T5-overexpressing 293 cells (data not shown). Likewise, heparanase- and T5-silencing was associated with a comparable ∼50% decrease in BrdU incorporation (Fig. 4A, bottom panel).
Figure 4.
T5 augments cell proliferation. A) BrdU incorporation. Top panel: morphology of control (Vo)-, heparanase (Hepa)-, and T5-infected CAG myeloma cell cultures. Middle panel: direct evaluation of DNA synthesis is demonstrated by BrdU incorporation. Subconfluent cultures of Vo-, Hepa-, and T5-infected CAG cells were grown in serum-free medium for 20 h, followed by incubation with BrdU (1:1000) for 2 h. Cells were then fixed and immunostained with anti-BrdU monoclonal antibody. Positively stained, red-brown nuclei were counted vs. blue, hematoxilin counterstained nuclei. At least 1000 cells were counted for each cell type; percentage of positively stained cells is noted in each panel. Fold increase in BrdU incorporation is shown graphically at right. Bottom panel: gene silencing. HEK 293 cells were transfected with anti-GFP (si-GFP), anti-heparanase (si-Hepa), or anti-T5 siRNA oligonucleotides, and BrdU incorporation was evaluated as above, except that cells were kept in serum-containing medium. Note 2-fold decrease in BrdU incorporation following heparanase or T5 gene silencing (right panel). B) Colony formation in soft agar. Vo-, Hepa-, and T5-infected CAG (top panel), Fadu (second panel) and 293 (third panel) cells (5×103 cells/dish) were mixed with soft agar and cultured for 3–5 wk. CAG cells were similarly grown in the absence (DMSO; fourth panels) or presence of Src inhibitor (PP2, 0.4 nM; bottom panels). Photomicrographs of colonies are representative (×100 view).
The effect of heparanase and T5 on cell proliferation was evaluated further by monitoring cell growth and colony formation in soft agar (Fig. 4B and Supplemental Fig. 2). Colony number (Supplemental Fig. 2), size, and cell density were increased markedly following heparanase- or T5-overexpression in CAG myeloma (Fig. 4B, top panel), FaDu pharynx carcinoma (Fig. 4B, second panel) and 293 cells (Fig. 4B, third panel). Enhanced colony formation on heparanase or T5-overexpression appeared to be mediated by Src activation, because colonies formed in the presence of the Src inhibitor PP2 (Fig. 4B, fourth and bottom panels) as well as two additional Src inhibitors (PP1, Src inhibitor I; data not shown) were similar in shape and size to control colonies. Collectively, these findings strongly imply that heparanase and the T5 splice variant enhance cell proliferation and anchorage-independent cell growth by activating Src.
T5 enhances myeloma xenograft development
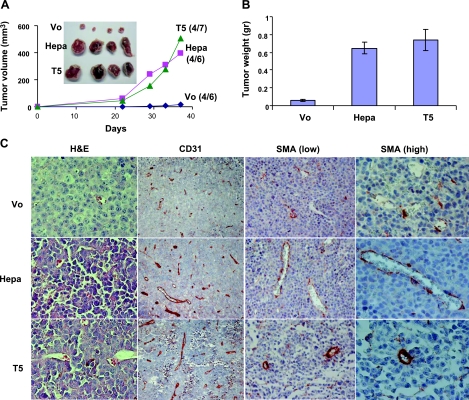
Having demonstrated a role for T5 in cell proliferation and colony formation, we next examined whether T5 enhances tumor development. Until d 21, xenografts produced by control (Vo)-, heparanase-, and T5-infected CAG myeloma cells exhibited similar growth rates on subcutaneous inoculation into athymic nude mice (Fig. 5A). Thereafter, however, the development of tumor xenografts produced by heparanase- and T5-infected cells was enhanced markedly compared with xenografts generated by control cells (Fig. 5A). On termination of the experiment on d 37, noticeable differences in tumor xenograft development were observed (Fig. 5A, inset). Thus, average weight of control xenografts was 58 ± 11 mg compared with 648 ± 69 and 738 ± 114 mg for heparanase and T5 xenografts, respectively, differences that are statistically highly significant (P=0.00007 and 0.0005 for heparanase vs. Vo and T5 vs. Vo, respectively). We next evaluated microvessel density in the resulting CAG xenografts by immunohistochemical analysis of the endothelial cell marker, CD31. A 2-fold increase in vessel density was found following heparanase overexpression (Fig. 5C; CD31, Hepa), in agreement with previous studies utilizing this cell system (40), and a similar elevation of vessel density was observed on T5 overexpression (Fig. 5C; CD31, T5). Not only vessel density but also vessel maturation was affected by heparanase and T5 overexpression. Staining for smooth muscle actin, which labels vessel-associated pericytes, showed a significantly increased number of pericytes associated with blood vessels in xenografts produced by heparanase- and T5-infected CAG cells (Fig. 5C, SMA low), in agreement with previous findings (9). Closer examination revealed that while the pericyte coverage appeared discontinuous in xenografts produced by heparanase-overexpressing CAG cells (Fig. 5C; SMA high, Hepa), a dense pericyte layer surrounded even small capillaries following T5 overexpression (Fig. 5C, SMA high, T5). The proangiogenic feature of T5 was further concluded from endothelial cell organization (tube formation) on reconstituted basement membrane (Matrigel) that was maintained for a relatively long period of time vs. control (Vo) cells (Supplemental Fig. 3A). These results suggest that enhanced xenograft progression is due, in part, to augmented tumor angiogenesis and blood vessel maturation.
Figure 5.
T5 enhances tumor xenograft development. A, B) Control (Vo)-, heparanase-, and T5-infected CAG myeloma cells were injected subcutaneously (1×106/ 0.1 ml) and tumor volume was calculated (A). At the end of the experiment on d 37, tumors were resected, photographed (A, inset) and weighed (B). C) Immunohistochemical analysis. Paraffin-embedded 5-μm sections were stained with hematoxilin and eosin (H&E; left column), anti-CD31 (left center column), and anti-smooth muscle actin antibodies (SMA; right center and right columns) antibodies. Note increased blood vessel density and maturation (recruitment of SMA-positive cells) in xenografts produced by heparanase- and T5-infected cells.
T5 is abundantly expressed in renal cell carcinoma
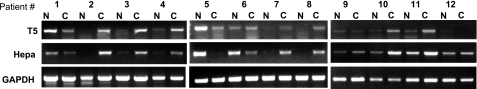
To investigate the clinical significance of T5, we examined its expression in renal cell carcinoma biopsies by applying RT-PCR analysis (Fig. 6). T5 expression was observed readily in the carcinoma (Fig. 6, C) samples and appeared elevated in 75% of the cases available for us (8/12) compared to the adjacent, normal-looking tissue (Fig. 6, N). Moreover, renal cell carcinomas appeared to contain similar levels of heparanase and T5, suggesting that both wild-type and spliced heparanase T5 contribute to the progression of this and likely other carcinomas.
Figure 6.
Clinical relevance of T5. Total RNA was extracted from biopsies of renal cell carcinoma (C) and adjacent normal looking tissue (N) and subjected to RT-PCR analysis applying T5 (top panel), heparanase (middle panel), and GAPDH primers (bottom panel).
DISCUSSION
Almost all protein-coding genes contain introns that are removed in the nucleus by RNA splicing and are often alternatively spliced. Alternative splicing increases the coding capacity of the genome, generating multiple proteins from a single gene. The resulting protein isoforms frequently exhibit different biological properties that may play an essential role in tumorigenesis (41,42,43). T5 appears to be such a protumorigenic splice variant. Splice variant of human heparanase has been recognized previously, yet no biological function was demonstrated (27). Clearly, the T5 splice variant is endowed with protumorigenic properties, enhancing cell proliferation, anchorage independent growth, and tumor xenograft development (Figs. 4 and 5). These features were observed in several tumor-derived cell lines overexpressing T5, or following T5 gene silencing (Figs. 3 and 4), suggesting that its function is relevant to multiple tumor types. The clinical relevance of T5 critically emerged from analysis of renal cell carcinoma biopsies, where T5 and heparanase expression appeared to be induced in 75% of the cases (Fig. 6).
Compelling evidence indicates that heparanase expression is elevated in the vast majority of primary hematological and solid malignancies (7, 12, 17), yet the role that heparanase plays in primary tumor progression is incompletely understood. In several instances, heparanase induction was associated with tumors larger in size, attributed, in part, to the proangiogenic function of heparanase (8, 9, 12). The discovery of the T5 splice variant provides another explanation for the protumorigenic and proangiogenic properties of heparanase. This probably is because T5 is expressed along with heparanase in tumor-derived cells (Fig. 2A) and in clinical specimens (Fig. 6) to comparable levels and appears to promote cell proliferation (Fig. 4A), colony formation (Fig. 4B), xenograft progression (Fig. 5A), and tumor angiogenesis (Fig. 5C). Thus, while inhibitors directed against the enzymatic activity of heparanase are currently evaluated in clinical trials (44,45,46,47), T5 as well as the heparanase C domain responsible for enzymatic activity-independent functions of heparanase (26, 34) are not expected to be affected by these inhibitors. It appears, therefore, that a well-defined enzymatic activity thought to be relatively easy to target, turned, at least in certain tumor systems, into a complex matter as more knowledge accumulates and the biology of the protein is revealed.
Heparanase and T5 share several biochemical characteristics. The signal peptide of heparanase is retained in T5, enabling its delivery to the ER and Golgi apparatus (Fig. 2C), glycosylation (evident by sensitivity to tunicamycin and binding to Con A; Fig. 2B and Supplemental Fig. 1A), and secretion (Fig. 2B). Protein N-glycosylation likely occurs on a single asparagine residue (Asn162) preserved in T5 and shown to be required for protein secretion (48). Not surprisingly, T5 is devoid of heparanase enzymatic activity, nor did it compete with the endogenous heparanase activity (Fig. 2D). This is largely because T5 is incapable of heparin binding (Supplemental Fig. 1). Although the basic heparin binding domain of heparanase (Lys158-Asn162) is retained in T5 and is followed by additional positively charged amino acids (KK) thought to create an even more potent heparin binding domain, T5 failed to interact with heparin (Supplemental Fig. 1A). Similarly, a peptide corresponding to this sequence including the unique amino acids introduced in T5 (CKKK; Supplemental Fig. 1B) failed to interact with heparin and to inhibit heparanase enzymatic activity, as opposed to the KKDC peptide, which efficiently accomplished both tasks (Supplemental Fig. 1B, C) (38). Thus, the mere presence of Cardin-Weintraub consensus sequence (49) appears insufficient to ensure high-affinity binding to heparin and neighboring amino acids dictate the functional outcome (38). Therefore, Src activation by T5 cannot be explained by interaction with HS and/or clustering of syndecans. The rational behind this function is likely structural. According to this notion, the alternative splicing generated a truncated protein that acquired a novel conformation not normally present in the heparanase molecule (50). This may also be the case with Spalax heparanase splice variant 7, noted to enhance U87 xenograft development (51), while Spalax splice variant 36, which did not advance tumor progression, assume an inactive conformation. It should be noted that Spalax splice variants (splice 7 and splice 36) appear unique to this mammal, and equivalent splice variants could not be identified in humans, which leaves T5 the only functional human heparanase splice variant reported so far. Both heparanase and T5 still await detailed structural analysis.
Interestingly, heparanase and T5 appear to be localized in endocytic vesicles (Supplemental Fig. 3B). While heparanase is subjected to cellular uptake together with syndecans and accumulates in the lysosomal compartment (52, 53), where it undergoes proteolytic processing by cathepsin L (54,55,56), T5 does not interact with HS. Its delivery to endocytic vesicles is, therefore, likely mediated by interaction with other cell surface molecules responsible for its signaling and protumorigenic properties.
Taken together, we describe here, for the first time, a functional splice variant of heparanase, discovered through Compugen’s LEADS platform. The T5 splice variant possesses protumorigenic properties, facilitating cell proliferation, colony formation, and tumor xenograft development. Furthermore, T5 is expressed in the majority of renal cell carcinoma biopsies examined, strongly supporting the clinical relevance of this protein variant. T5 appears, therefore, as a valid target for the development of anticancer drugs, introducing another level of complexity to the heparanase field, and the attempts to neutralize its growing repertoire of biological activities. Prospective analysis of a large cohort of tumor biopsies is required to appreciate adequately the outcome of patients expressing high vs. low levels of T5. In addition, staining of tumor biopsies with monoclonal antibody specific for T5, but not heparanase will enable, possibly, to distinguish between T5-positive and -negative cases and reveal its association with patient outcome.
Supplementary Material
Acknowledgments
This work was supported by grants from the Israel Science Foundation (549/06); the National Cancer Institute, U.S. National Institutes of Health (RO1-CA106456); the DKFZ-MOST cooperation program in cancer research; the Technion Fine Postdoctoral Fellowship; and the Israel Cancer Research Fund (ICRF) Postdoctoral Fellowship. I.V. is a Research Professor of the ICRF.
References
- Nakajima M, Irimura T, DiFerrante D, DiFerrante N, Nicolson G L. Heparan sulfate degradation: relation to tumor invasion and metastatic properties of mouse B 16 melanoma sublines. Science. 1983;220:611–613. doi: 10.1126/science.6220468. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I, Fuks Z, Bar-Ner M, Ariav Y, Schirrmacher V. Lymphoma cells mediated degradation of sulfated proteoglycans in the subendothelial extracellular matrix: relation to tumor cell metastasis. Cancer Res. 1983;43:2704–2711. [PubMed] [Google Scholar]
- Edovitsky E, Elkin M, Zcharia E, Peretz T, Vlodavsky I. Heparanase gene silencing, tumor invasiveness, angiogenesis, and metastasis. J Natl Cancer Inst. 2004;96:1219–1230. doi: 10.1093/jnci/djh230. [DOI] [PubMed] [Google Scholar]
- Casu B, Vlodavsky I, Sanderson R D. Non-anticoagulant heparins and inhibition of cancer. Pathophysiol Haemost Thromb. 2008;36:195–203. doi: 10.1159/000175157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey L A, Brunn G J, Platt J L. Heparanase, a potential regulator of cell-matrix interactions. Trends Biochem Sci. 2000;25:349–351. doi: 10.1016/s0968-0004(00)01619-4. [DOI] [PubMed] [Google Scholar]
- Parish C R, Freeman C, Hulett M D. Heparanase: a key enzyme involved in cell invasion. Biochim Biophys Acta. 2001;1471:M99–108. doi: 10.1016/s0304-419x(01)00017-8. [DOI] [PubMed] [Google Scholar]
- Vreys V, David G. Mammalian heparanase: what is the message? J Cell Mol Med. 2007;11:427–452. doi: 10.1111/j.1582-4934.2007.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkin M, Ilan N, Ishai-Michaeli R, Friedmann Y, Papo O, Pecker I, Vlodavsky I. Heparanase as mediator of angiogenesis: mode of action. FASEB J. 2001;15:1661–1663. doi: 10.1096/fj.00-0895fje. [DOI] [PubMed] [Google Scholar]
- Zcharia E, Zilka R, Yaar A, Yacoby-Zeevi O, Zetser A, Metzger S, Sarid R, Naggi A, Casu B, Ilan N, Vlodavsky I, Abramovitch R. Heparanase accelerates wound angiogenesis and wound healing in mouse and rat models. FASEB J. 2005;19:211–221. doi: 10.1096/fj.04-1970com. [DOI] [PubMed] [Google Scholar]
- Zetser A, Bashenko Y, Miao H-Q, Vlodavsky I, Ilan N. Heparanase affects adhesive and tumorigenic potential of human glioma cells. Cancer Res. 2003;63:7733–7741. [PubMed] [Google Scholar]
- Gingis-Velitski S, Zetser A, Flugelman M Y, Vlodavsky I, Ilan N. Heparanase induces endothelial cell migration via protein kinase B/Akt activation. J Biol Chem. 2004;279:23536–23541. doi: 10.1074/jbc.M400554200. [DOI] [PubMed] [Google Scholar]
- Ilan N, Elkin M, Vlodavsky I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol. 2006;38:2018–2039. doi: 10.1016/j.biocel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Ferro V, Hammond E, Fairweather J K. The development of inhibitors of heparanase, a key enzyme involved in tumour metastasis, angiogenesis and inflammation. Mini Rev Med Chem. 2004;4:693–702. doi: 10.2174/1389557043403729. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I, Friedmann Y. Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. J Clin Invest. 2001;108:341–347. doi: 10.1172/JCI13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie E A. Heparanase: a target for drug discovery in cancer and inflammation. Br J Pharmacol. 2007;151:1–14. doi: 10.1038/sj.bjp.0707182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H Q, Liu H, Navarro E, Kussie P, Zhu Z. Development of heparanase inhibitors for anti-cancer therapy. Curr Med Chem. 2006;13:2101–2111. doi: 10.2174/092986706777935230. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I, Ilan N, Naggi A, Casu B. Heparanase: structure, biological functions, and inhibition by heparin-derived mimetics of heparan sulfate. Curr Pharm Des. 2007;13:2057–2073. doi: 10.2174/138161207781039742. [DOI] [PubMed] [Google Scholar]
- Goldshmidt O, Zcharia E, Cohen M, Aingorn H, Cohen I, Nadav L, Katz B-Z, Geiger B, Vlodavsky I. Heparanase mediates cell adhesion independent of its enzymatic activity. FASEB J. 2003;17:1015–1025. doi: 10.1096/fj.02-0773com. [DOI] [PubMed] [Google Scholar]
- Sotnikov I, Hershkoviz R, Grabovsky V, Ilan N, Cahalon L, Vlodavsky I, Alon R, Lider O. Enzymatically quiescent heparanase augments T cell interactions with VCAM-1 and extracellular matrix components under versatile dynamic contexts. J Immunol. 2004;172:5185–5193. doi: 10.4049/jimmunol.172.9.5185. [DOI] [PubMed] [Google Scholar]
- Ben-Zaken O, Gingis-Velitski S, Vlodavsky I, Ilan N. Heparanase induces Akt phosphorylation via a lipid raft receptor. Biochem Biophys Res Commun. 2007;361:829–834. doi: 10.1016/j.bbrc.2007.06.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doviner V, Maly B, Kaplan V, Gingis-Velitski S, Ilan N, Vlodavsky I, Sherman Y. Spatial and temporal heparanase expression in colon mucosa throughout the adenoma-carcinoma sequence. Mod Pathol. 2006;19:878–888. doi: 10.1038/modpathol.3800603. [DOI] [PubMed] [Google Scholar]
- Cohen I, Pappo O, Elkin M, San T, Bar-Shavit R, Hazan R, Peretz T, Vlodavsky I, Abramovitch R. Heparanase promotes growth, angiogenesis and survival of primary breast tumors. Int J Cancer. 2006;118:1609–1617. doi: 10.1002/ijc.21552. [DOI] [PubMed] [Google Scholar]
- Nadir Y, Brenner B, Zetser A, Ilan N, Shafat I, Zcharia E, Goldshmidt O, Vlodavsky I. Heparanase induces tissue factor expression in vascular endothelial and cancer cells. J Thromb Haemost. 2006;4:2443–2451. doi: 10.1111/j.1538-7836.2006.02212.x. [DOI] [PubMed] [Google Scholar]
- Zetser A, Bashenko Y, Edovitsky E, Levy-Adam F, Vlodavsky I, Ilan N. Heparanase induces vascular endothelial growth factor expression: correlation with p38 phosphorylation levels and Src activation. Cancer Res. 2006;66:1455–1463. doi: 10.1158/0008-5472.CAN-05-1811. [DOI] [PubMed] [Google Scholar]
- Cohen-Kaplan V, Doweck I, Naroditsky I, Vlodavsky I, Ilan N. Heparanase augments epidermal growth factor receptor phosphorylation: correlation with head and neck tumor progression. Cancer Res. 2008;68:10077–10085. doi: 10.1158/0008-5472.CAN-08-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fux L, Ilan N, Sanderson R, Vlodavsky I. Heparanase: busy at the cell surface. Trends Biochem Sci. 2009;34:511–519. doi: 10.1016/j.tibs.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser N J, Avivi A, Shushy M, Vlodavsky I, Nevo E. Cloning, expression, and characterization of an alternatively spliced variant of human heparanase. Biochem Biophys Res Commun. 2007;354:33–38. doi: 10.1016/j.bbrc.2006.12.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorek R, Ast G, Graur D. Alu-containing exons are alternatively spliced. Genome Res. 2002;12:1060–1067. doi: 10.1101/gr.229302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorek R, Safer H M. A novel algorithm for computational identification of contaminated EST libraries. Nucleic Acids Res. 2003;31:1067–1074. doi: 10.1093/nar/gkg170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorek R, Shemesh R, Cohen Y, Basechess O, Ast G, Shamir R. A non-EST-based method for exon-skipping prediction. Genome Res. 2004;14:1617–1623. doi: 10.1101/gr.2572604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Zhu W Y, Wasserman A, Grebinskiy V, Olson A, Mintz L. Computational analysis of alternative splicing using EST tissue information. Genomics. 2002;80:326–330. doi: 10.1006/geno.2002.6841. [DOI] [PubMed] [Google Scholar]
- Ben-Zaken O, Shafat I, Gingis-Velitski S, Bangio H, Kelson I K, Alergand T, Amor Y, Maya R B, Vlodavsky I, Ilan N. Low and high affinity receptors mediate cellular uptake of heparanase. Int J Biochem Cell Biol. 2008;40:530–542. doi: 10.1016/j.biocel.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetser A, Levy-Adam F, Kaplan V, Gingis-Velitski S, Bashenko Y, Schubert S, Flugelman M Y, Vlodavsky I, Ilan N. Processing and activation of latent heparanase occurs in lysosomes. J Cell Sci. 2004;117:2249–2258. doi: 10.1242/jcs.01068. [DOI] [PubMed] [Google Scholar]
- Fux L, Feibish N, Cohen-Kaplan V, Gingis-Velitski S, Feld S, Geffen C, Vlodavsky I, Ilan N. Structure-function approach identifies a COOH-terminal domain that mediates heparanase signaling. Cancer Res. 2009;69:1758–1767. doi: 10.1158/0008-5472.CAN-08-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadav L, Katz B Z, Baron S, Cohen N, Naparstek E, Geiger B. The generation and regulation of functional diversity of malignant plasma cells. Cancer Res. 2006;66:8608–8616. doi: 10.1158/0008-5472.CAN-06-1301. [DOI] [PubMed] [Google Scholar]
- Shteper P J, Zcharia E, Ashhab Y, Peretz T, Vlodavsky I, Ben-Yehuda D. Role of promoter methylation in regulation of the mammalian heparanase gene. Oncogene. 2003;22:7737–7749. doi: 10.1038/sj.onc.1207056. [DOI] [PubMed] [Google Scholar]
- Levy-Adam F, Feld S, Suss-Toby E, Vlodavsky I, Ilan N. Heparanase facilitates cell adhesion and spreading by clustering of cell surface heparan sulfate proteoglycans. PLoS ONE. 2008;3:e2319. doi: 10.1371/journal.pone.0002319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Adam F, Abboud-Jarrous G, Guerrini M, Beccati D, Vlodavsky I, Ilan N. Identification and characterization of heparin/heparan sulfate binding domains of the endoglycosidase heparanase. J Biol Chem. 2005;280:20457–20466. doi: 10.1074/jbc.M414546200. [DOI] [PubMed] [Google Scholar]
- Thomas S M, Brugge J S. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Kelly T, Miao H-Q, Yang Y, Navarro E, Kussie P, Huang Y, MacLeod V, Casciano J, Joseph L, Zhan F, Zangari M, Barlogie B, Shaughnessy J, Sanderson R D. High heparanase activity in multiple myeloma is associated with elevated microvessel density. Cancer Res. 2003;63:8749–8756. [PubMed] [Google Scholar]
- Cooper T A, Wan L, Dreyfuss G. RNA and disease. Cell. 2009;136:777–793. doi: 10.1016/j.cell.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazi J, Bakkour N, Stamm S. Alternative splicing and disease. Biochim Biophys Acta. 2009;1792:14–26. doi: 10.1016/j.bbadis.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alphen R J, Wiemer E A, Burger H, Eskens F A. The spliceosome as target for anticancer treatment. Br J Cancer. 2009;100:228–232. doi: 10.1038/sj.bjc.6604801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather J K, Hammond E, Johnstone K D, Ferro V. Synthesis and heparanase inhibitory activity of sulfated mannooligosaccharides related to the antiangiogenic agent PI-88. Bioorg Med Chem. 2008;16:699–709. doi: 10.1016/j.bmc.2007.10.044. [DOI] [PubMed] [Google Scholar]
- Ferro V, Dredge K, Liu L, Hammond E, Bytheway I, Li C, Johnstone K, Karoli T, Davis K, Copeman E, Gautam A. PI-88 and novel heparan sulfate mimetics inhibit angiogenesis. Semin Throm Hemost. 2007;33:557–568. doi: 10.1055/s-2007-982088. [DOI] [PubMed] [Google Scholar]
- Lewis K D, Robinson W A, Millward M J, Powell A, Price T J, Thomson D B, Walpole E T, Haydon A M, Creese B R, Roberts K L, Zalcberg J R, Gonzalez R. A phase II study of the heparanase inhibitor PI-88 in patients with advanced melanoma. Invest New Drugs. 2008;26:89–94. doi: 10.1007/s10637-007-9080-5. [DOI] [PubMed] [Google Scholar]
- Basche M, Gustafson D L, Holden S N, O'Bryant C L, Gore L, Witta S, Schultz M K, Morrow M, Levin A, Creese B R, Kangas M, Roberts K, Nguyen T, Davis K, Addison R S, Moore J C, Eckhardt S G. A phase I biological and pharmacologic study of the heparanase inhibitor PI-88 in patients with advanced solid tumors. Clin Cancer Res. 2006;12:5471–5480. doi: 10.1158/1078-0432.CCR-05-2423. [DOI] [PubMed] [Google Scholar]
- Simizu S, Ishida K, Wierzba M K, Osada H. Secretion of heparanase protein is regulated by glycosylation in human tumor cell lines. J Biol Chem. 2004;279:2697–2703. doi: 10.1074/jbc.M300541200. [DOI] [PubMed] [Google Scholar]
- Cardin A D, Weintraub H J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- Birzele F, Csaba G, Zimmer R. Alternative splicing and protein structure evolution. Nucleic Acids Res. 2008;36:550–558. doi: 10.1093/nar/gkm1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser N J, Avivi A, Shafat I, Edovitsky E, Zcharia E, Ilan N, Vlodavsky I, Nevo E. Alternatively spliced Spalax heparanase inhibits extracellular matrix degradation, tumor growth, and metastasis. Proc Natl Acad Sci U S A. 2009;106:2253–2258. doi: 10.1073/pnas.0812846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingis-Velitski S, Zetser A, Kaplan V, Ben-Zaken O, Cohen E, Levy-Adam F, Bashenko Y, Flugelman M Y, Vlodavsky I, Ilan N. Heparanase uptake is mediated by cell membrane heparan sulfate proteoglycans. J Biol Chem. 2004;279:44084–44092. doi: 10.1074/jbc.M402131200. [DOI] [PubMed] [Google Scholar]
- Goldshmidt O, Nadav L, Aingorn H, Irit C, Feinstein N, Ilan N, Zamir E, Geiger B, Vlodavsky I, Katz B Z. Human heparanase is localized within lysosomes in a stable form. Exp Cell Res. 2002;281:50–62. doi: 10.1006/excr.2002.5651. [DOI] [PubMed] [Google Scholar]
- Abboud-Jarrous G, Atzmon R, Peretz T, Palermo C, Gadea B B, Joyce J A, Vlodavsky I. Cathepsin L is responsible for processing and activation of proheparanase through multiple cleavages of a linker segment. J Biol Chem. 2008;283:18167–18176. doi: 10.1074/jbc.M801327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abboud-Jarrous G, Rangini-Guetta Z, Aingorn H, Atzmon R, Elgavish S, Peretz T, Vlodavsky I. Site-directed mutagenesis, proteolytic cleavage, and activation of human proheparanase. J Biol Chem. 2005;280:13568–13575. doi: 10.1074/jbc.M413370200. [DOI] [PubMed] [Google Scholar]
- Cohen E, Atzmon R, Vlodavsky I, Ilan N. Heparanase processing by lysosomal/endosomal protein preparation. FEBS Lett. 2005;579:2334–2338. doi: 10.1016/j.febslet.2005.03.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.