Abstract
This paper describes a method for the purification of monoclonal antibodies (rat anti-2,4-dinitrophenyl IgG: IgGDNP; and mouse anti-digoxin IgG: IgGDgn) from ascites fluid. This procedure (for IgGDNP) has three steps: i) precipitation of proteins heavier than immunoglobulins with ammonium sulfate; ii) formation of cyclic complexes of IgGDNP by causing it to bind to synthetic multivalent haptens containing multiple DNP groups; iii) selective precipitation of these dimers, trimers, and higher oligomers of the target antibody, followed by regeneration of the free antibody. This procedure separates the targeted antibody from a mixture of antibodies, as well as from other proteins and globulins in a biological fluid. This method is applicable to antibodies with a wide range of monovalent binding constants (0.1 μM to 0.1 nM). The multivalent ligands we used (derivatives of DNP and digoxin) isolated IgGDNP and IgGDgn from ascites fluid in yields of > 80% and with > 95% purity. This technique has two advantages over conventional chromatographic methods for purifying antibodies: i) it is selective for antibodies with two active Fab binding sites (both sites are required to form the cyclic complexes) over antibodies with one or zero active Fab binding sites; ii) it does not require chromatographic separation. It has the disadvantage that the structure of the hapten must be compatible with the synthesis of bi and/or trivalent analogs.
Introduction
This paper describes a non-chromatographic procedure for purifying monoclonal IgG antibodies (mAbs) from a biological fluid. This procedure is based on selective precipitation of cyclic complexes of the targeted antibody and multivalent haptens with ammonium sulfate (AMS) from a biological fluid (e.g., acites fluid or a cell lysate). Because the cyclic oligomers of [IgG]n (n = 2, 3) have molecular weights that are two or three times that of the monomeric IgG (150 kDa), the complexes precipitate at lower concentrations of AMS than does monomeric IgG. The key step in this procedure is the precipitation that separates the oligomeric [IgG]n complexes from other monomeric antibodies that do not form complexes (including IgGs that are not bivalently active), and from other proteins in the ascites fluid. We used two commercial IgGs (rat anti-2,4-dinitrophenyl, IgGDNP and mouse anti-digoxin, IgGDgn) as model systems in developing this method. To the best of our knowledge, this procedure is the first for purifying monoclonal IgGs that selects for bivalently active antibodies. We have successfully precipitated complexes of IgGDNP using bi- and trivalent DNP (2,4-dinitrophenyl) haptens (Kdaffinity ∼ 0.8 nM), and bi- and trivalent 4-NP (4-nitrophenyl, Kdaffinity ∼ 0.5 μM), and complexes of IgGDgn using bivalent digoxin (Kdaffinity ∼ 0.1 nM). We believe, based on these results, it should be possible to apply this technique to antibody-ligand systems that have monovalent dissociation constants ranging from micro- to nanomolar (provided that the bi- and/or trivalent analogues of these haptens are synthetically accessible).
Monoclonal antibodies are important in biomedical research, and antibody-based therapies have become increasingly important in the last decade:1-7 55% of the drugs that are under development currently are mAbs. 8 The pharmaceutical industry is considering a target for production of mAbs of 10 tons per year. 9 Bioreactors with a capacity from 15,000 to 25,000 L are becoming more common, and an expression rate of 5 g of antibody/L is standard. 9 Purification of mAbs—both at process scales, and for research—remains an expense and inconvenience. The current processes for purifying mAb use multiple steps that may have detrimental effects on the specific activity of isolated mAbs. Methods for purification that are faster, less expensive, more convenient at research scales, and result in greater yields (of specifically active product) would be useful. 10-12
Immunoglobulin (e.g., IgD, IgE and IgG antibodies) consists of two antigen recognition sites and is bivalent (Figure 1a). The bivalency of IgG increases its avidity for antigens displayed on cell surfaces, and also allows it to form high-molecular-weight complexes with soluble, multivalent antigens and allergens. 13
Figure 1.
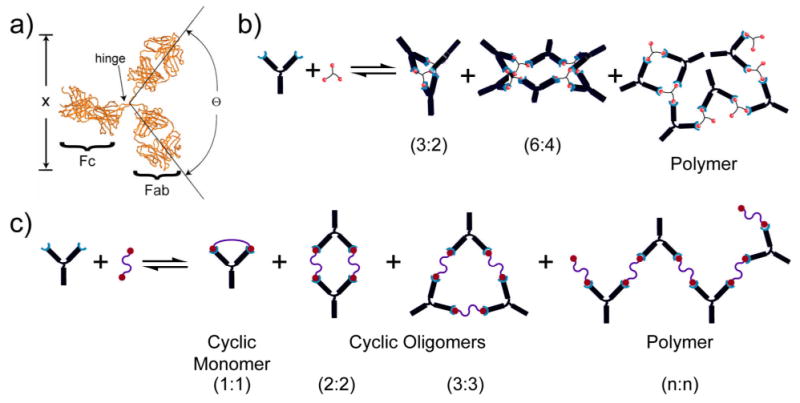
a) Crystal structure of an antibody with the dimensions labeled. The flexibility of the hinge region gives rise to a range of values for Θ and x (distance between binding sites). The complexes of antibodies (IgG) that can be formed by incubation with b) trivalent hapten include: bicyclic antibody trimer, tricyclic antibody hexamer, and branched polymer; c) bivalent hapten include: cyclic dimer, cyclic trimer, and linear polymer.
During purification, several processes may yield monoclonal antibodies with only one active Fab binding site; examples include: i) protein unfolding, misfolding, and aggregation; ii) covalent modification (e.g., oxidation); iii) enzymatic proteolysis, and iv) the “scrambling” of light chains. 12 The biological significance of bivalently active (as opposed to monovalently active) immunoglobulins is presently unclear, because there has been no consistent route for preparing either. 14-16
Procedures for purifying antibodies must remove a number of contaminants that are associated with their expression, such as host cell proteins, DNA, endotoxins, and cell culture media additives. In addition, antibody-derived impurities, such as high-molecular-weight aggregates and proteolytic fragments of immunoglobulin, can also contaminate the desired product. Current procedures for purifying therapeutic antibodies typically rely on protein A (proA) chromatography (proA binds to the Fc domain of IgGs), where the elution of the antibody is achieved by decreasing the pH to 2-3. 9 Although proA affinity chromatography can yield products with 95% purity after a single chromatographic step 17, it introduces a number of additional challenges and new routes for contamination: hydrolysis and proteolysis can contaminate the isolated product with cleaved proA and its truncated derivatives, and leached proA can adhere to the eluting product. 18-20 Furthermore, the acidic pH of the mobile phase can cause the mAb to unfold and lose activity, and/or aggregate non-specifically and precipitate.
Because chromatographic procedures are labor-intensive, expensive, and operationally demanding at large scales, there is substantial effort directed toward developing new methods of purification of mAbs. The Cohn fractionation process, which currently provides yields of 80 tons per year for IgIV prufication, is a potential candidate for the ultimate goal for producing 10 tons per year of mAbs. The Cohn fractionation process employs selective precipitation steps through control of pH, temperature, concentration of ethanol, and ionic strength, but does not employ any chromatographic steps;21, 22 additional unit operations include microfiltration, ultrafiltration, and centrifugation. Nevertheless, the Cohn process is not yet a viable method for the production of mAbs, and neither the Cohn process nor the chromatographic techniques under development explicitly select for bivalently active IgG.
The technique we introduce here is based on the formation of discrete, cyclic complexes of antibodies, and therefore can avoid many of these disadvantages when the appropriate oligovalent ligand can be prepared. This procedure is an affinity-based method, with the strengths and weaknesses of such methods. In particular, it is specific for a hapten, but requires that that hapten be accessible and amenable to synthetic manipulation. Importantly, this procedure differs from conventional techniques in that the formation of the complexes requires both Fab sites of an antibody to have binding activity.
Early in the development of molecular immunology, Pecht, Baird, Posner, and others described the formation of discrete, cyclic dimers and trimers resulting from the interaction of IgE's and IgG's with bivalent haptens (Figure 1b). 23, 24 Based on analytical modeling of the assembly of antibody complexes, Dembo and Goldstein predicted that the concentration of bivalent hapten (Ctotal) at which maximum conversion (CTmax) took place would depend on the monovalent dissociation constant (Kdaffinity) and the total concentration of antibody ([IgG]total) according to equation 1. 25 Hence, in order to achieve maximum conversion to cyclic complexes, the dissociation constant (Kdaffinity) should be lower than the concentration of antibody, and the ratio of the bivalent ligand to antibody should be 1.
| (1) |
Results
Summary of Purification
Using this purification procedure, we isolated pure, bivalently active anti-2,4-DNP and anti-digoxin from rat and mouse ascites fluids; ascites fluid and the supernatant from hybridoma bioreactors are the two most common biological sources for monoclonal antibodies for both small and large scales. 10, 26 Ascites fluid contains 1-10 mg/mL of globulins, and 10-30 mg/mL of other serum proteins including albumin (MW ∼ 66 kD) and transferrin (MW ∼ 80 kD).
The procedure consists of three steps: i) Addition of ammonium sulfate (AMS) to a final concentration of 35% of the saturated concentration precipitated of all proteins and complexes heavier than an IgG (150 kDa). ii) After removing the precipitate, addition of bi- or trivalent hapten formed cyclic, higher molecular-weight complexes (described in Figure 1) of the IgG of interest (here, anti-2,4-DNP or anti-digoxin) (Figure 2).13, 27-29 These complexes—with molecular weights of 300, 450 or 600 kDa—precipitated from the 35% AMS solution immediately. iii) Centrifugation separated the precipitated complexes from the supernatant, which retained IgG molecules incapable of forming complexes with the multivalent haptens (i.e., those with different specificity, or with one or no active Fab sites), as well as other proteins with MW ≤ 150 kDa. We finally solubilized and dissociated the complexes by incubation with a large excess of monovalent hapten, and removed the haptens by dialysis.
Figure 2.
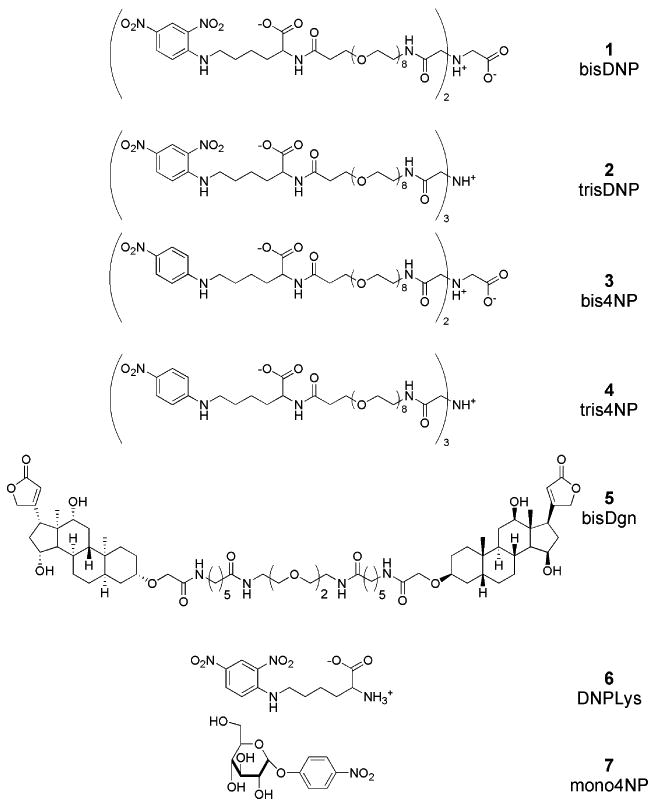
Structures of the bi- and trivalent DNP and 4-NP haptens (1, 2, 3, and 4), bivalent digoxin hapten (5), monovalent 2,4-dinitrophenyl lysine (6), and 4-nitrophenylglucose (7).
Anti-2,4-DNP rat monoclonal IgG1κ (from clone LO-DNP-2) and anti-digoxin mouse monoclonal IgG1 (from clone DI-22) antibodies are appropriate for proof-of-principle demonstrations for five reasons: i) The purified antibodies and the ascites fluids are both commercially available. ii) Both antibodies have high affinity (KdDNP ∼ 0.8 nM and KdDgn ∼ 0.1 nM) for their monovalent haptens (a requirement to observe and isolate the complexes by SE-HPLC). iii) IgGDNP has a substantially lower affinity (Kd = ∼ 0.5 μM) for monovalent 4-NP than for 2,4-DNP; we used this low-affinity interaction to demonstrate the range of values of Kd for which this procedure is applicable. (iv) The syntheses of bi- and trivalent haptens are straightforward. v) The lifetime of the IgG-DNP complex is sufficiently long to allow the use of chromatography to separate the aggregates relevant to this work for analysis and to understand the mechanisms underlying the process.
Analytical Methods
We determined the efficiency of the purification with size-exclusion chromatography (SE-HPLC).30, 31 This analytical technique can resolve antibody complexes of different molecular weights, provided that these complexes do not dissociate over the time required to carry out a separation (∼ 20 min). 13 Using this technique, we separated the complexes formed by mixing commercially available IgGDNP with bi- and trivalent derivatives of DNP, as well as the complexes that formed by mixing commercially available IgGDgn with bivalent digoxin. (Figure 3). The procedure developed with these compounds also works with ligands that bind less tightly than DNP and dissociate more rapidly (as we show using 4-nitrophenyl hapten, Kdaffinity ∼ 0.5 μM), but the ability to resolve aggregates was very important in understanding the mechanism of the purification. SE-HPLC was an effective tool for measuring the amount of IgGDNP at each step of the purification procedure (except in the starting ascites fluid), as well as determining the mole-fraction of each complex.
Figure 3.
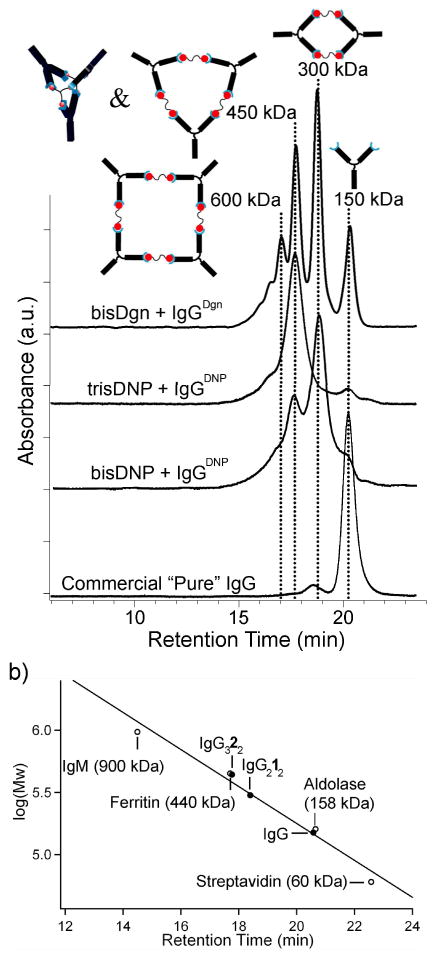
(a) SE-HPLC chromatograms of IgGDgn and IgGDNP complexes formed upon binding to multivalent ligands. Each antibody-ligand complex is labeled by the chromatogram. The schematic structures show the aggregates expected to be formed from IgG; other proteins with the same MW also seem to be present in small quantities. The products in a mixture of bis-DNP ligand 1 and IgGDNP showed the cyclic antibody dimer (IgG)212 (MW = 300 kDa and trimer (IgG)313 (MW = 450 kDa). The product of a mixture of tris-DNP ligand 2 to IgGDNP, as expected from previous work, was a bicyclic trimer complex. Mixing bis-Dgn ligand 5 with IgGDgn yielded multiple peaks that corresponded to cyclic dimer, trimer and tetramer as well as monomeric antibody. 13 (b) Calibration of the size-exclusion column using proteins with known molecular masses.
By contrast, although this procedure was applicable to the purification of IgG using the much more weakly binding 4-NP as a hapten, SE-HPLC could not resolve complexes formed by bi- and trivalent 4-NP ligands (the chromatograms showed only a monomeric IgG peak) as a result of the kinetic instability of these complexes. All IgGDNP complexes dissociated rapidly (<1 min) in the presence of excess monovalent hapten (6).
The presence of many other proteins precluded the use of SE-HPLC for determining the amount of IgGDNP in the ascites, as many proteins eluted together with the antibody. Therefore, we used an Enzyme-Linked Immunosorbent Assay (ELISA) to quantify the concentration of IgGDNP in the ascites fluid. We calculated the percentage of IgGDNP separated after each step of the protocol by comparing the intensity of fluorescence from the sample to the intensity of fluorescence from the original ascites fluid. We did not carry out the ELISA procedure for IgGDgn. The chromatograms in Figure 4 are of IgGDgn-containing ascites with and without the bivalent Dgn ligand, and after purification. The concentration of IgGDgn was the same in all three injections and the peak intensities demonstrate the relative concentration of IgGDgn to the rest of the proteins in the ascites fluid.
Figure 4.
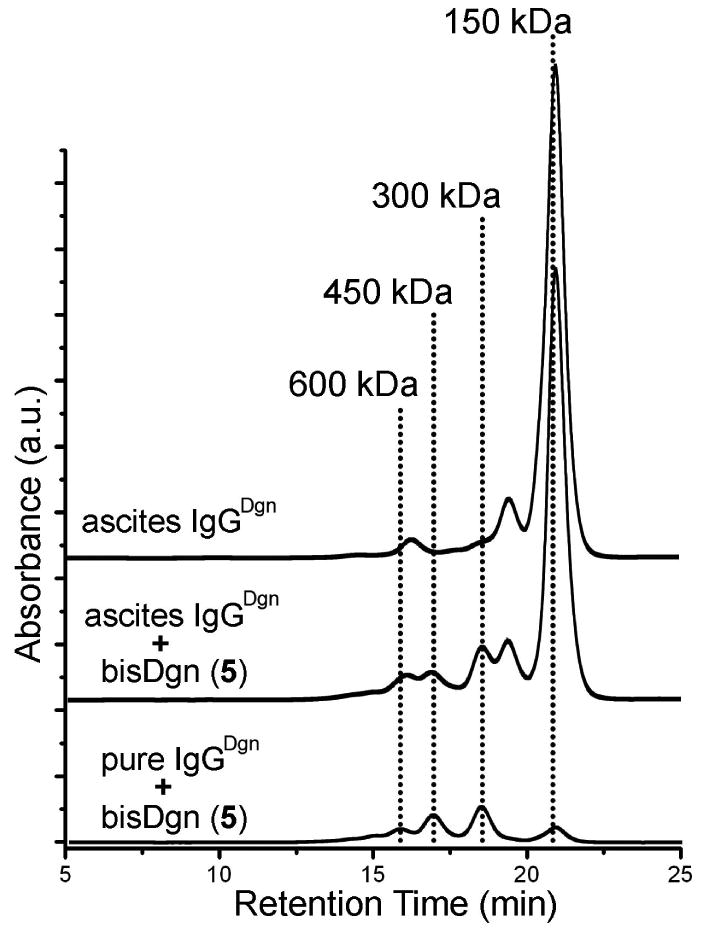
Size exclusion chromatograms of anti-digoxin ascites fluid. Top SE-HPLC trace labeled “ascites IgGDgn” is untreated ascites fluid. Anti-digoxin ascites fluid after incubation with bis-Dgn (5) (2 μM) is labeled “ascites IgGDgn”” + bis-Dgn (5)”. The bottom trace is purified IgGDgn mixed with bis-Dgn (this sample was prepared to match the original IgGDgn concentration in the ascites). The peaks at 300, 450, and 600 kDa correspond to cyclic dimer, trimer, and tetramer (Figure 3). The differences in the absorbance illustrate that the concentration of the IgG in the ascites was low relative to the other proteins.
Step 1. Removal of High Molecular Weight Impurities
The first step consisted of filtering the ascites fluid (0.5 mL) through glass wool to remove the majority of the liposaccharides. We rinsed the glass wool with an additional 0.5 mL of phosphate-buffered saline (PBS: pH 7.4, 10 mM phosphate, 150 mM NaCl) for a final volume of 1 mL (diluting the ascites fluid two-fold). Addition of saturated AMS solution (540 μL; to a final concentration of 1.4 M (35%)) to the filtered ascites fluid, followed by centrifugation, separated the proteins having molecular weights > 150 kDa (Figure 5). The proteins that precipitated have retention times similar to that of the cyclic complexes that form upon addition of multivalent ligands. Using an ELISA, we determined that this initial precipitation step resulted in the loss of only ∼ 4% of the IgGDNP.
Figure 5.
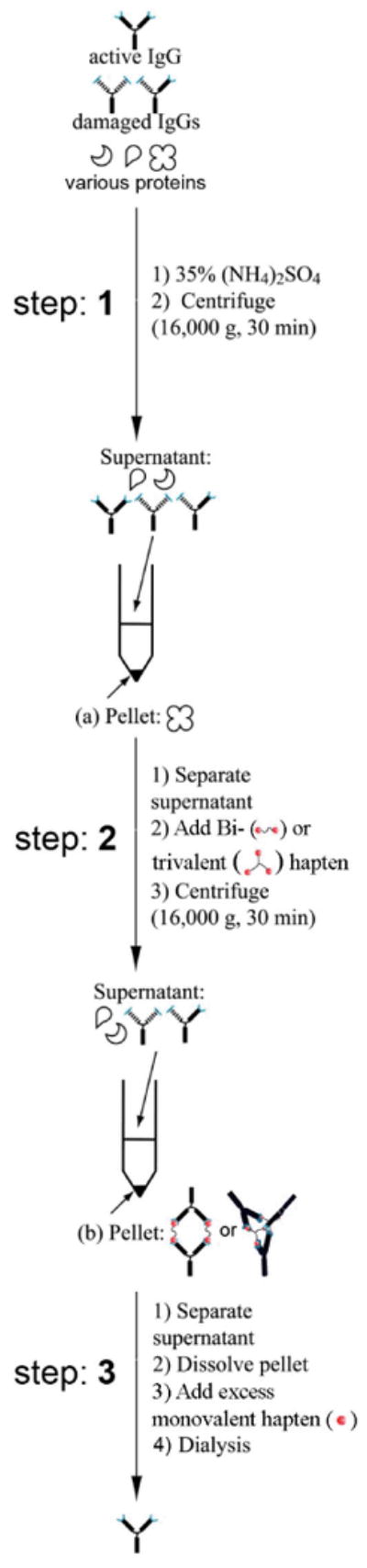
A schematic representation of the three steps (1 – 3) used to purify bivalently active monoclonal IgGDNP from ascites fluid using AMS precipitation. The starting material was ascites fluid, which contained a mixture of: IgGDNP with two active Fab binding sites that recognize 2,4-DNP (active IgG), improperly folded or denatured IgGDNP (damaged IgG), and IgG fragments (heavy or light chain), and other proteins present in ascites with a range of molecular weights. (1) A low concentration of AMS (35%) precipitated high molecular weight (≥ 300 kDa) proteins. These proteins (as a precipitate) were separated by centrifugation as a pellet (a). (We carried the supernatant, which contained all IgG and low molecular weight serum proteins, to the next step.) (2) The addition of bi or trivalent hapten molecules to the supernatant formed complexes of IgGDNP (represented here as cyclic dimer, and bicyclic trimer Figure 1b), which immediately precipitated from the solution. This precipitate (b) was isolated by centrifugation. The supernatant, which now contained any damaged IgGDNP, immunoglobulins against other antigens, and other serum proteins, was discarded. (3) The pellet (b) was dissolved in PBS, and the IgGDNP complexes were dissociated by the addition of excess monovalent 4-NP 7 (∼ 1 mM). Dialysis of this solution against phosphate buffered saline (pH 7.4) removed both the monovalent and multivalent haptens, and gave the final product as monomeric, bivalently active, IgGDNP.
Step 2. Isolation of Bivalently Active IgGDNP as Complexes
The supernatant from Step 1 contained active IgG, inactive IgG, and serum proteins with molecular weights equal to or lower than that of IgG. Adding multivalent haptens 1-4 (to a final concentration of 5 μM) to the supernatant induced the aggregation of bivalently active IgG; these complexes immediately formed a precipitate. To ensure maximum recovery, we incubated the sample overnight at 4 °C. We then centrifuged (16,000 g, 30 min) these samples, isolated the pellets, and redissolved them in PBS for analysis by SE-HPLC (Supplementary Figure S.1.).
SE-HPLC chromatograms of the pellets isolated using bis- and tris-DNP haptens 1 and 2 had peaks that corresponded to the IgG monomer as well as the cyclic antibody complexes (dimer and trimer). This distribution of antibody complexes indicated that an excess of multivalent ligand precipitated with the complexes. This excess ligand probably precipitated with the antibody/ligand complexes as a result of non-specific binding, and did not interfere with the rest of the purification.
Analysis of pellets from AMS precipitation with bi- and trivalent 4-NP haptens 3 and 4 with SE-HPLC showed a single peak at a retention time that corresponded to monomeric IgGDNP; neither chromatogram showed peaks corresponding to higher aggregates. This observation indicated that, although the complexes that formed upon the interaction of antibody with these haptens were sufficiently stable to facilitate precipitation with AMS, the complexes were not sufficiently stable kinetically to survive a 20-minute SE-HPLC separation. The absence of peak broadening indicates that the rate constant for dissociation must be > 2.5×103 sec-1.
Accordingly, ELISA determined that the amounts of purified IgGDNP after step 2 of the procedure using each ligand was: 16 ± 2% from bis-DNP, 11 ± 4% from tris-DNP, 78 ± 12% from bis-4NP, and 82 ± 20% from tris-4NP. The yields of purified IgG with the multivalent DNP haptens appear to be much lower than the yields with the multivalent 4-NP haptens; this order is opposite of what we expected. In addition, the yield calculated by ELISA differed significantly from that calculated by integrating the peak area of the antibody in the SE-HPLC chromatogram of the re-dissolved pellets; the SE-HPLC indicated the actual yield obtained by precipitation with 1 was closer to 66% (the peak area was ∼80% of the peak area of the pellet generated using tris-4-NP). We hypothesized that the reason for the low apparent yield of these samples by ELISA was that the aggregates precipitated with DNP-containing ligands remained stable when the pellets re-dissolved in PBS, and were not accessible (either kinetically or thermodynamically, we have not determined which) to the DNP on the surface of the ELISA plate.
To test this hypothesis, we allowed commercial, affinity purified IgGDNP to react with tris-DNP (ligand 2) and form the bicyclic complex (IgG322), and ran an ELISA assay with this sample. As predicted, the concentration of IgGDNP in this sample according to ELISA was only ∼6% of its actual concentration. Therefore, the IgGDNP purified using the multivalent DNP haptens required purification step 3: dissociation of the complexes by the addition of monovalent DNP (ligand 6), and removal of the monovalent and multivalent ligands from the antibody by dialysis.
Step 3. Dissociation of the Cyclic Complexes with Monovalent DNP
We solubilized the pellets from step 2 in PBS buffer, and added excess (∼1 mM) DNP-Lysine (6); this monovalent ligand completely dissociated the cyclic IgG complexes (as confirmed by SE-HPLC). We dialyzed (10 kDa MWCO membrane, 4 °C) the sample against monovalent 4-NP 7 (in order to prevent the reformation of IgG complexes) and eliminated all the multivalent ligands from the dialysis chamber. Upon the completion of dialysis, the chamber contained monovalent ligand 7 together with IgGDNP. A second dialysis step against PBS at 4 °C removed the low molecular weight monovalent ligand 7 (as monitored by UV absorbance, λ = 360 nm). The final product was ∼ 80% of the total amount of the IgGDNP in the starting ascites fluid (as estimated by ELISA) and yielded a clean single peak corresponding to 150 kDa on the SE-HPLC (Figure 6a).
Figure 6.
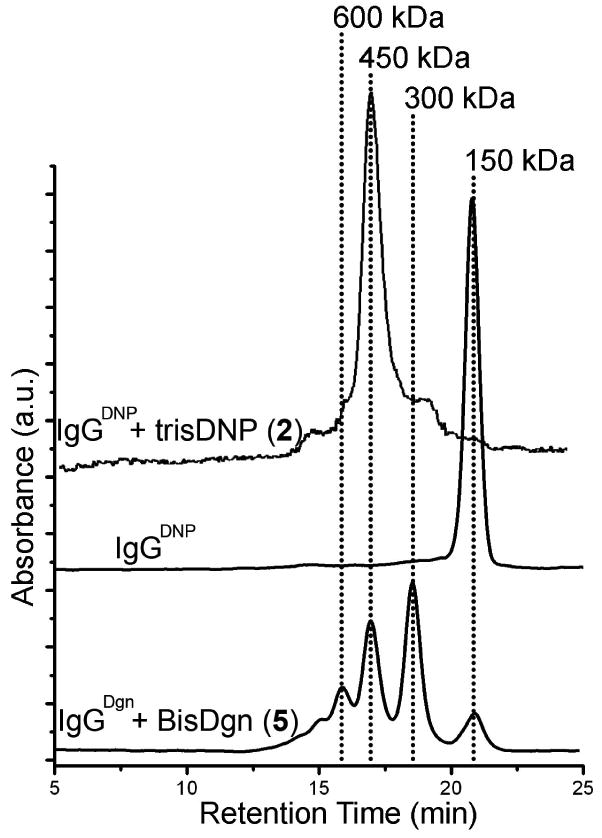
The bottom SE-HPLC trace is the IgGDgn purified using the described procedure in Figure 5. IgGDgn was present as a mixture of cyclic complexes (monomer, dimer, trimer, and tetramer), as we did not have a weaker-binding monomeric ligand to dissociate its complexes. The middle trace is of the IgGDNP purified using our protocol and the top trace is after mixing purified IgGDNP with trisDNP (ligand 2).
This procedure yielded a final product that was >95% pure by HPLC and, we believe, has two active Fab binding sites. The most significant experiment to establish the bivalency of the purified IgGDNP was to affirm its capacity to form the kinetically stable bicyclic complex ((IgGDNP)322) upon addition of tris-DNP 2 (Figure 6b). Conversion of the monomeric IgGDNP purified with this method to the complex proceeded with >95% yield. This value was slightly greater than that observed for the commercially available, affinity-purified IgGDNP (>90%).
This procedure can selectively isolate one IgG from a mixture of ascites fluids—one containing IgGDNP, and the second IgGDgn. The procedure described above separated IgGDNP from this mixture, and gave results similar to those we have described in detail in the preceding section. Bivalent digoxin ligand 5 could also purify IgGDgn from this mixture. We conclude that the procedure is capable of selective precipitation of a target IgG from a mixture containing multiple IgG molecules with different specificities. (The experiment summarized in Figure 4 implies the same conclusion).
Discussion
The thermodynamic stability of the complexes, rather than their kinetic stability, is critical to the effectiveness of this protocol. Theoretical studies predict that this stability is directly related to the monovalent affinity of the antibody for the hapten and the concentration of antibody. 28, 32, 33 SE-HPLC established that isolation of IgGDNP using multivalent ligands of 4-NP (Kdaffinity ∼ 0.5 μM) or of DNP (Kdaffinity ∼ 0.8 nM) gave comparable yields. We believe, based on the results, that this procedure is applicable for the purification of monoclonal antibodies with affinities in the range from μM to nM for their haptens/antigens, provided that bi- and/or trivalent analogues of these haptens/antigens are synthetically available.
We have not explored the application of this technique to other antibody isotypes. The majority of previous studies of cyclic complexes have used bivalent (IgG or IgE) antibodies. 13, 23, 25, 29 Further study of the aggregating behavior of IgAs and IgMs is required before this procedure can be applied to their purification. We believe, however, that the bivalent IgE class of antibodies will be amenable to the purification approach described here, because the only difference between IgG and IgE antibodies is in the Fc region.
AMS precipitation of cyclic complexes of antibodies provides a convenient, non-chromatographic method to purify antibodies from complex solutions, and to purify bivalently active antibody from inactive antibody and/or monovalently active antibody. This method is, to our knowledge, the only purification procedure for monoclonal antibodies that selectively isolates monoclonal IgGs with two active Fab binding sites, and is able to start from a crude biological source of antibodies.
The logic of the method is straightforward and the procedures are easy to execute experimentally. They can be applied to small quantities of solutions and antibodies; although we have not worked with large volumes or quantities, this procedure should be scalable to large quantities. We believe that the antibodies isolated using this procedure will exclusively have two fully active Fab binding sites, since both sites are required to form the cyclic complexes.
The primary limitation of this technique is its requirement that appropriate bi- and trivalent haptens be synthetically (or naturally) accessible. The antibodies that we used in this study were directed against small-molecule haptens. The requirement for a synthetically accessible bivalent derivative of the hapten may limit the application of this technique to purify antibodies directed towards a recognition site created by the tertiary structure of a protein, although oligopeptides sometimes can be developed that bind such proteins (by random combinatorial methods, if necessary). 34-36 For some antibodies directed against proteins, the bivalent hapten could, in principle, be a dimer of the antigenic protein. For antibodies raised against large or membrane bound proteins, mimotopes (short peptide sequences that mimic the binding site), or peptidomimetics (organic molecules that mimic the function of mimotopes) could serve as multivalent molecules. 37-39 Discovery of mimotopes usually requires high throughput screening of peptide libraries, or phage display. 40-42
We believe that this technique has the potential to be useful in many applications that require purifying substantial quantities of antibodies for common biological and clinical analyses, and perhaps for human therapeutics.43 This technique may also be useful for fractionating mixtures of polyclonal antibodies from serum based on their affinity for a given hapten and/or their specificity.
Methods
Synthesis and Purification of Multivalent Ligands
We used straightforward synthetic strategies (See Supplementary Methods) based on previously reported syntheses to prepare and purify multivalent ligands 1–5. 13
SE-HPLC
SE- HPLC measurements were carried out on a Tosoh TSK-GEL G3000SWXL and Tosoh TSK-GEL G4000SWXL size-exclusion columns using a Varian ProStar 400 HPLC system with autosampler. HPLC runs were performed with an isocratic solvent system that was 50 mM phosphate buffer and 370 mM NaCl (to adjust the ionic strength to 0.475 M) at pH 6.8, with a 0.5 mL/min flow rate. The sample peaks were analyzed by UV-Vis detector, as monitored at λ=214 nm. The chromatograms of the cyclic complexes of commercial IgG were obtained from injection where we kept the concentration of antibody constant while carrying out serial dilutions of the bi- and trivalent DNP haptens 1 and 2. We determined the concentrations of our samples using the reported extinction coefficients for IgGs and DNP. We incubated all samples for 12 h at 4 °C prior to injection onto the SE-HPLC column. Samples from purified IgGs from ascites fluid were run after 1/3 dilution into running buffer.
ELISA
The wells of a 96-well ELISA plate were incubated with DNP-BSA conjugate to adsorb it on the well surface. After treating the plate with the sample to be assayed and washing, we incubated the plate with a secondary antibody (anti-rat IgG from goat) conjugated to Horse Radish Peroxidase (HRP). We treated the wells with Amplex Red, and monitored the HRP-catalyzed hydrolysis of this substrate using fluorescence (ex: 545nm, em: 590nm). To determine the concentration of IgGDNP in each well, we compared the fluorescence results from the wells that contain samples to that of a well that contains known concentration of pure IgGDNP. We averaged four independent sets of ELISA results obtained from purified antibodies using our purification procedure. We also measured the enzymatic activity of a known concentration of commercially available IgGDNP in parallel to quantify the amount of antibody obtained from precipitation using each multivalent ligand listed in Figure 2.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health (GM 30367). The American Cancer Society (S.W.T.) and the NIH (B.F.S. and L.A.E.) provided postdoctoral fellowships. The authors also thank Dr. David Wood of the Department of Chemical Engineering at Princeton University for stimulating discussions.
References
- 1.Brekke OH, Sandlie I. Therapeutic antibodies for human diseases at the dawn of the twenty-first century. Nat Rev Drug Discov. 2003;2:52–62. doi: 10.1038/nrd984. [DOI] [PubMed] [Google Scholar]
- 2.Drews J. Drug discovery: A historical perspective. Science. 2000;287:1960–1964. doi: 10.1126/science.287.5460.1960. [DOI] [PubMed] [Google Scholar]
- 3.Safarik I, Safarikova M. Use of magnetic techniques for the isolation of cells. J Chromatogr B. 1999;722:33–53. [PubMed] [Google Scholar]
- 4.Hoogenboom HR, et al. Antibody phage display technology and its applications. Immunotechnology. 1998;4:1–20. doi: 10.1016/s1380-2933(98)00007-4. [DOI] [PubMed] [Google Scholar]
- 5.Perico N, Remuzzi G. Prevention of transplant rejection - Current treatment guidelines and future developments. Drugs. 1997;54:533–570. doi: 10.2165/00003495-199754040-00003. [DOI] [PubMed] [Google Scholar]
- 6.Casadevall A, Scharff MD. Return to the Past - the Case for Antibody-Based Therapies in Infectious-Diseases. Clin Infect Dis. 1995;21:150–161. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minunni M, Mascini M. Detection of Pesticide in Drinking-Water Using Real-Time Biospecific Interaction Analysis (Bia) Anal Lett. 1993;26:1441–1460. [Google Scholar]
- 8.Lerner RA. Manufacturing immunity to disease in a test tube: The magic bullet realized. Angew Chem -Int Edit. 2006;45:8106–8125. doi: 10.1002/anie.200603381. [DOI] [PubMed] [Google Scholar]
- 9.Kelley B. Very large scale monoclonal antibody purification: The case for conventional unit operations. Biotechnol Prog. 2007;23:995–1008. doi: 10.1021/bp070117s. [DOI] [PubMed] [Google Scholar]
- 10.Farid SS. Process economics of industrial monoclonal antibody manufacture. J Chromatogr B. 2007;848:8–18. doi: 10.1016/j.jchromb.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 11.Farid SS. Economic drivers and trade-offs in antibody purification processes. Biopharm Int. 2008:37–42. [Google Scholar]
- 12.Shukla AA, Hubbard B, Tressel T, Guhan S, Low D. Downstream processing of monoclonal antibodies - Application of platform approaches. J Chromatogr B. 2007;848:28–39. doi: 10.1016/j.jchromb.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Bilgiçer B, Moustakas DT, Whitesides GM. A synthetic trivalent hapten that aggregates anti-2,4-DNP IgG into bicyclic trimers. J Am Chem Soc. 2007;129:3722–3728. doi: 10.1021/ja067159h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hlavacek WS, Posner RG, Perelson AS. Steric effects on multivalent ligand-receptor binding: Exclusion of ligand sites by bound cell surface receptors. Biophys J. 1999;76:3031–3043. doi: 10.1016/S0006-3495(99)77456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Posner RG, Erickson JW, Holowka D, Baird B, Goldstein B. Dissociation Kinetics of Bivalent Ligand Immunoglobulin-E Aggregates in Solution. Biochemistry. 1991;30:2348–2356. doi: 10.1021/bi00223a008. [DOI] [PubMed] [Google Scholar]
- 16.Erickson JW, Posner RG, Goldstein B, Holowka D, Baird B. Bivalent Ligand Dissociation Kinetics from Receptor-Bound Immunoglobulin-E - Evidence for a Time-Dependent Increase in Ligand Rebinding at the Cell-Surface. Biochemistry. 1991;30:2357–2363. doi: 10.1021/bi00223a009. [DOI] [PubMed] [Google Scholar]
- 17.Gagnon B. Purification Tools for Monoclonal Antibodies. Validated Biosystems; Tucson, AZ: 2007. [Google Scholar]
- 18.Fahrner RL, Iyer HV, Blank GS. The optimal flow rate and column length for maximum production rate of protein A affinity chromatography. Bioprocess Eng. 1999;21:287–292. [Google Scholar]
- 19.Fahrner RL, Whitney DH, Vanderlaan M, Blank GS. Performance comparison of Protein A affinity-chromatography sorbents for purifying recombinant monoclonal antibodies. Biotechnol Appl Biochem. 1999;30:121–128. [PubMed] [Google Scholar]
- 20.O'Leary RM, Feuerhelm D, Peers D, Xu Y, Blank GS. Determining the useful lifetime chromatography resins - Prospective small-scale studies. Biopharm-Appl Technol Biopharm Dev. 2001;14:10–+. [Google Scholar]
- 21.Martin TD. IgIV: Contents, properties, and methods of industrial production - evolving closer to a more physiologic product. Int Immunopharmacol. 2006;6:517–522. doi: 10.1016/j.intimp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Johnston A, Adcock W. Biotechnology & Genetic Engineering Reviews. Vol. 17. 2000. pp. 37–70. [DOI] [PubMed] [Google Scholar]
- 23.Schweitzerstenner R, Licht A, Luscher I, Pecht I. Oligomerization and Ring-Closure of Immunoglobulin-E Class Antibodies by Divalent Haptens. Biochemistry. 1987;26:3602–3612. doi: 10.1021/bi00386a053. [DOI] [PubMed] [Google Scholar]
- 24.Hlavacek WS, et al. Quantifying aggregation of IgE-Fc epsilon RI by multivalent antigen. Biophys J. 1999;76:2421–2431. doi: 10.1016/s0006-3495(99)77397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schweitzerstenner R, Licht A, Pecht I. Dimerization Kinetics of the Ige-Class Antibodies by Divalent Haptens. 1. the Fab-Hapten Interactions. Biophys J. 1992;63:551–562. doi: 10.1016/S0006-3495(92)81609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aldington S, Bonnerjea J. Scale-up of monoclonal antibody purification processes. J Chromatogr B. 2007;848:64–78. doi: 10.1016/j.jchromb.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 27.Baird B, Posner R, Goldstein B, Holowka D. Studies on the Binding of a Bivalent Ligand to Bivalent Immunoglobulin E-Receptor Complexes at the Cell-Surface. FASEB J. 1991;5:A652–A652. [Google Scholar]
- 28.Dembo M, Goldstein B. Theory of Equilibrium Binding of Symmetric Bivalent Haptens to Cell-Surface Antibody - Application to Histamine-Release from Basophils. J Immunol. 1978;121:345–353. [PubMed] [Google Scholar]
- 29.Posner R, Goldstein B, Holowka D, Baird B. Dissociation Kinetics of Bivalent Haptens Bound to Immunoglobulin-E Antibodies. Biophys J. 1990;57:A295–A295. [Google Scholar]
- 30.Subramanian K, Holowka D, Baird B, Goldstein B. The Fc segment of IgE influences the kinetics of dissociation of a symmetrical bivalent ligand from cyclic dimeric complexes. Biochemistry. 1996;35:5518–5527. doi: 10.1021/bi9523522. [DOI] [PubMed] [Google Scholar]
- 31.Xu KL, Goldstein B, Holowka D, Baird B. Kinetics of multivalent antigen DNP-BSA binding to IgE-Fc epsilon RI in relationship to RBL-2H3 cell activation. Faseb J. 1996;10:1254–1254. [PubMed] [Google Scholar]
- 32.Whitesides GM, Krishnamurthy VM. Designing Ligands to Bind Proteins. Quarterly Reviews of Biophysics. 2005;38:385–395. doi: 10.1017/S0033583506004240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hornick CL, Karush F. Antibody Affinity-3: The Role of Multivalence. Immunochemistry. 1972;9:325–340. doi: 10.1016/0019-2791(72)90096-1. [DOI] [PubMed] [Google Scholar]
- 34.Meloen RH, Puijk WC, Slootstra JW. Mimotopes: realization of an unlikely concept. J Mol Recogn. 2000;13:352–359. doi: 10.1002/1099-1352(200011/12)13:6<352::AID-JMR509>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 35.Olson GL, et al. Concepts and Progress in the Development of Peptide Mimetics. J Med Chem. 1993;36:3039–3049. doi: 10.1021/jm00073a001. [DOI] [PubMed] [Google Scholar]
- 36.Giannis A. Peptidomimetics for Receptor Ligands Discovery, Development, and Medical Perspectives. Angew Chem -Int Edit Engl. 1993;32:1244–1267. [Google Scholar]
- 37.Scala G, et al. Selection of HIV-specific immunogenic epitopes by screening random peptide libraries with HIV-1-Positive sera. J Immunol. 1999;162:6155–6161. [PubMed] [Google Scholar]
- 38.Hanessian S, McNaughtonSmith G, Lombart HG, Lubell WD. Design and synthesis of conformationally constrained amino acids as versatile scaffolds and peptide mimetics. Tetrahedron. 1997;53:12789–12854. [Google Scholar]
- 39.Meola A, et al. Derivation of Vaccines from Mimotopes - Immunological Properties of Human Hepatitis-B Virus Surface-Antigen Mimotopes Displayed on Filamentous Phage. J Immunol. 1995;154:3162–3172. [PubMed] [Google Scholar]
- 40.Steward MW, Stanley CM, Obeid OE. A Mimotope from a Solid-Phase Peptide Library Induces a Measles Virus-Neutralizing and Protective Antibody-Response. J Virol. 1995;69:7668–7673. doi: 10.1128/jvi.69.12.7668-7673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motti C, et al. Recognition by Human Sera and Immunogenicity of Hbsag Mimotopes Selected from an M13 Phage Display Library. Gene. 1994;146:191–198. doi: 10.1016/0378-1119(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 42.Folgori A, et al. A General Strategy to Identify Mimotopes of Pathological Antigens Using Only Random Peptide Libraries and Human Sera. EMBO J. 1994;13:2236–2243. doi: 10.1002/j.1460-2075.1994.tb06501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.FDA. Guidelines for Monoclonal Antibodies for Human Use. http://www.fda.gov/cber/gdlns/ptc_mab.txt.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


