Abstract
Purpose of review
Most children with cancer can be cured with combination regimens of chemotherapy, radiation, and/or surgery. However, standard therapies are toxic to normal tissues, cancer cells commonly develop resistance to chemotherapy, and relapsed malignancy is a leading cause of mortality in pediatrics. Elucidation of the principles of the normal immune response and tumor biology, coupled with technological developments, have led to important advances in the field of cancer immunotherapy. This review summarizes the biologic basis of cancer immunotherapy and highlights recent examples of progress in the application of novel humoral and cellular immunotherapies to children and adolescents with malignancy.
Recent Findings
Clinical trials of immunotherapy for pediatric cancer have recently been initiated. To date, most immune-based therapies have been well tolerated and some have shown clinically significant activity against specific refractory high-risk malignancies.
Summary
Recent clinical trial results provide proof-of-principle that cancer immunotherapy has the capacity to overcome chemotherapy resistance without the usual toxicities associated with cytotoxic regimens. Immunotherapy holds promise in the treatment of children and adolescents with cancer and has the potential to improve both survival and quality of life.
Keywords: cancer, childhood, immunotherapy, monoclonal antibodies, tumor vaccines
Introduction
Although most children with cancer are cured, the limits of standard cytotoxic therapies appear to have been reached for many pediatric malignancies.1 Non-specific side effects of treatment are substantial,2 and death from relapse remains a leading cause of mortality in pediatrics.3 Scientific discovery in immunology and tumor biology and related technological advances have facilitated dramatic developments in the field of cancer immunotherapy. This review summarizes biologic principles that underlie immunotherapy and highlights recent examples of the application of novel immunotherapeutic approaches to the treatment of cancer in children and adolescents.
The biology of immune responses
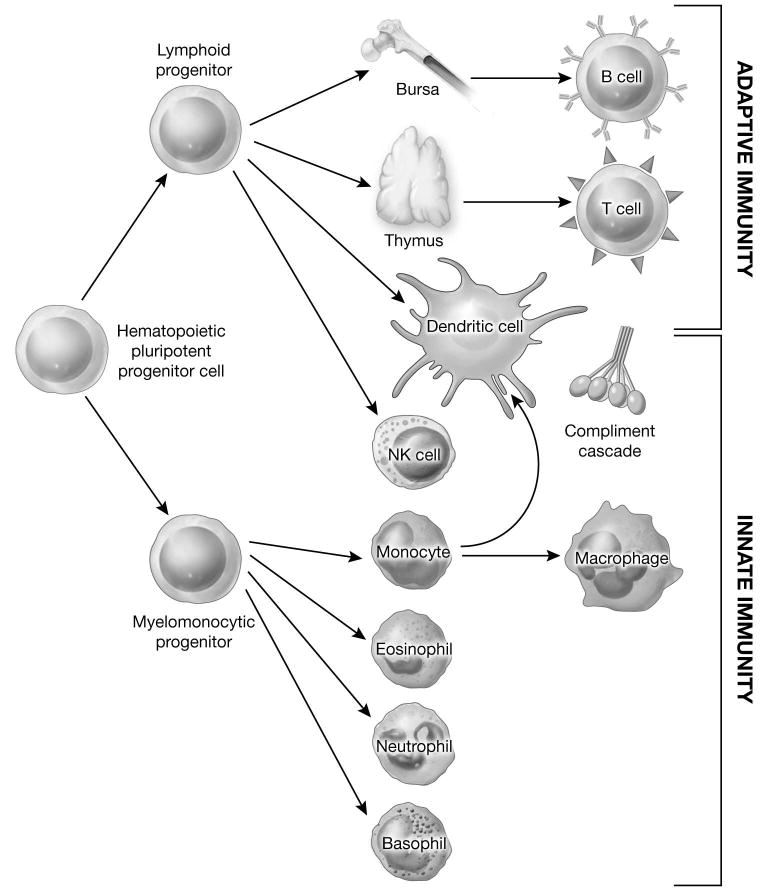
The normal human immune system is composed of two primary components: innate and adaptive immunity.4,5 Adaptive immunity is further subdivided into humoral and cellular arms. Importantly, these elements of the immune system are highly interdependent and interconnected.6 [Figure 1] Innate immune responses do not require prior exposure to target antigens. Effector cells, including phagocytic and cytotoxic leukocytes and cytokines play important roles in the first line of defense against microorganisms and in the activation of the adaptive immune response. There is evidence to indicate that the innate immune system can be directed against malignant cells.7 However, this approach to cancer immunotherapy has lagged behind the application of adaptive immune mechanisms. Clinical trials of activators of innate immunity in pediatric cancers have only recently begun and these will not be reviewed here. The adaptive immune system represents a complex network of afferent and efferent signals and effectors responsible for maintaining long-term immunity against infectious pathogens and foreign antigens. The humoral arm is constituted by B-lymphocytes responsible for the production of antibodies, while cellular immunity is mediated primarily by CD4+ and CD8+ T cells. Both components of the adaptive immune system have been successfully exploited in the treatment of cancer, and each will be considered separately.
Figure 1. Components of the innate and adaptive immune system.
Adapted from: Mackall CL, Sondel PM: Tumor immunology and pediatric cancer. In Pizzo, P.A. and Poplack, D.G. (eds) Principles and Practice of Pediatric Oncology, 6th edition. Philadelphia, PA, Lippincott Raven Publishers, 2009 (in press).6
Cancer-associated antigen targets for immunotherapy
A wide array of antigens can serve as targets for immune responses against cancer in experimental systems and in humans. These include specific chromosomal translocation fusion proteins, tissue- or cell- lineage-specific differentiation antigens, gene products that are over-expressed by malignant cells, and histocompatibility antigens.8,9,10 At the same time, cancer cells can elude immune responses in a variety of ways. Because the kinetics of immune-mediated killing might be inadequate to control rapidly proliferating cancer, reducing tumor burden to a state of minimal residual disease (MRD) prior to the initiation of immunotherapy is often utilized in attempt to overcome this disparity. Cancer cells can also evade immunologic recognition by a number of well-described mechanisms. Malignant cells may have diminished or absent expression of cancer-associated antigens and/or critically required immune co-stimulatory molecules (see below),11,12 produce immunosuppressive soluble factors or stimulate the production of immune suppressor cells, and express antigens that induce cell death (apoptosis) of immune effectors. Furthermore, cancer-associated antigens are often weakly immunogenic or overexpressed self-antigens, leading to weak immune responses due to selection events in the thymus early in life, and peripheral anergy. To augment anti-cancer immune responses, malignant cells can be modified to increase their immunogenicity, the immune system can be activated towards cancer-associated antigen targets, and tumor-associated suppressor cells can be depleted. All of these strategies are currently undergoing study in cancer immunotherapy trials.
Humoral Immunity and Antibody-Based Therapeutics of Cancer
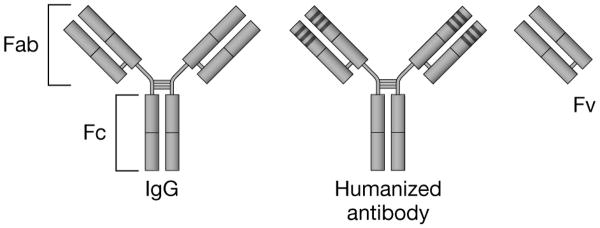
B-lymphocytes produce five classes of antibodies, or immunoglobulin (Ig) molecules (IgA, IgD, IgE, IgG, IgM). IgG secreted by memory B cells is the antibody with the highest concentration in circulation. This molecule is composed of two longer (heavy) chains and two shorter (light) chains. [Figure 2] The specificity of antigen binding is determined by the amino acid sequence of the variable region of the IgG molecule. After initial exposure to the cognate antigen, B cells produce IgM, which is followed by class switch and production of IgG of the same specificity.4
Figure 2. Structure of immunoglobin and monoclonal antibody fragments.
The immunoglobulin-G (IgG) molecule is composed of two longer (heavy) chains and two shorter (light) chains that are connected by disulfide bonds. The amino acid sequence of the variable (v) ends of each of the four chains determines the specificity of antigen binding. Humanized monoclonal antibody constructs consist predominantly of human amino acid sequences, with the exception of the three hypervariable complementarity-determining regions (CDR), which retain the foreign-specie sequences critical for antigen binding. The CDR domains are indicated by the dark bands at the end of the antigen-binding portion of the molecule.
Fab: Antigen binding fragment; Fc: Crystallizable or complement fixing fragment; Fv: Variable fragment
Monoclonal antibodies against human differentiation antigens
There has been dramatic progress in the clinical development of MoAb-based cancer therapeutics over the past 2 decades. Köhler and Milstein first demonstrated that monoclonal antibodies (MoAb) directed against specific human differentiation antigens could be generated from hybridomas derived from immunized mice,13 which made possible the large-scale production of such reagents for therapeutic use in humans. Subsequently, it was reported that fragments of antibody variable domains (Fv) could be linked together to make recombinant proteins capable of antigen binding.14 Methodologies have since been developed to produce fully human MoAbs and their fragments for clinical use and to generate humanized constructs with reduced immunogenicity. [Figure 2]
Monoclonal antibody-based therapies for cancer
For effective MoAb-targeting, the cognate antigen should be expressed in relatively high levels on the surface of the malignant cells and there should be limited-to-no expression on normal tissues. Ideally, there should also be minimal shedding of antigen from the cell surface, since high levels of free antigen could serve as a decoy, thus diminishing MoAb binding to the target.
MoAbs have the potential to kill cancer cells through direct and indirect effector pathways. Certain antibody-receptor binding interactions directly inhibit cell growth or induce cell death (apoptosis) through effects on intracellular signaling pathways. Indirect killing can occur by antibody-dependent cellular cytotoxicity (ADCC), complement-activation, and/or cell-mediated cytokine production. The mechanism involved, or whether killing occurs at all, varies with the specific MoAb, antigen and cancer. Importantly, immune-mediated cytotoxicity requires functional immune effector mechanisms, which are commonly deficient in patients with cancer.15,16
Monoclonal antibodies for hematologic malignancies
Hematologic malignancies, the most common pediatric cancers, are excellent candidates for MoAb-based therapeutics.17 Malignant blasts from patients with leukemia and lymphoma express lineage-specific human differentiation antigens with otherwise limited tissue distribution, and numerous MoAbs have been developed that effectively target such antigens. In 1997, the U.S. Food and Drug Administration (FDA) approved the first MoAb for the treatment of cancer, rituximab (Genentech, Inc. San Francisco, CA, U.S.A.). This agent is a MoAb directed against the B-lymphoid lineage antigen CD20. Rituximab combined with chemotherapy has been demonstrated to improve disease-free survival in adults with non-Hodgkin's lymphoma (NHL).18 Results of a recent Children's Oncology Group (COG) trial of rituximab with chemotherapy indicate that this agent can be safely administered to pediatric patients with relapsed CD20+ lymphoma and leukemia.19* The COG and the Berlin-Frankfurt-Muenster cooperative group (BFM) are currently conducting clinical trials of rituximab with chemotherapy for children and adolescents with newly diagnosed CD20+ hematologic malignancies. A COG study of a MoAb that targets the B-lineage antigen CD22, epratuzumab (Immunomedics, Inc., Morris Plains, NJ, U.S.A.), in combination with standard chemotherapy for children with acute lymphoblastic leukemia (ALL) is also in progress. Results in the initial cohort treated with an upfront epratuzumab monotherapy phase were recently published.20* The clinical activity of epratzumab as a single agent was limited and no complete or partial remissions were observed. In this preliminary analysis, the feasibility of giving epratuzumab in combination with standard chemotherapy was demonstrated and the rates of complete remission and clearance of MRD appeared favorable in comparison to historical results with chemotherapy. It is unlikely that MoAbs will have adequate single agent activity to be effective for pediatric hematologic malignancies when used alone. However, rare cases of complete remissions in adults and children with ALL have been reported with MoAbs targeting CD20, CD33, and CD52.21,22,23
Monoclonal antibodies for solid tumors
Recent success has also been achieved using MoAbs to target antigens expressed on pediatric solid tumors. The ganglioside GD2 is a neuroectodermal-restricted antigen expressed by neuroblastoma. A number of MoAbs targeting GD2 have been studied in children with high-risk neuroblastoma.24,25,26,27,28,29 The anti-GD2 MoAb 3F8 cleared MRD after autologous SCT in children with stage IV neuroblastoma.30 The anti-GD2 MoAb ch14.18, in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-2 (IL-2) after autologous SCT, enhanced event-free and overall survival in comparison to standard therapy.31** Importantly, this regimen had limited activity in patients with bulky neuroblastoma, consistent with a model wherein immune-based therapies are more effective when administered in the setting of MRD.
The insulin-like growth factor (IGF) signaling pathway plays a role in cell survival in certain pediatric sarcomas.32,33* A number of MoAbs against the IGF-1 receptor are undergoing clinical trial in pediatric and adult patients with sarcomas and dramatic responses have been observed suggesting that IGF-1 receptor blockade can interrupt critical cell survival signals.32
The tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor is expressed on a variety of cancers, and anti-TRAIL receptor MoAbs have shown activity against pediatric sarcomas in pre-clinical models.34 A phase I trial of an anti-TRAIL MoAb for children with solid tumors is in progress at the National Cancer Institute (NCI).
CTLA4 is a receptor on the surface of T cells that diminishes immune responses. Blockade of CTLA4 signaling through anti-CTLA4 MoAbs inhibits this suppressive signal and augments T cell mediated immune reactivity. This agent has activity against melanoma and other tumors in adults,35 and a pediatric Phase I trial is being performed at the NCI.
Conjugated monoclonal antibodies
The cytotoxicity of MoAbs can be dramatically increased by linkage to toxic moieties such as chemotherapeutic agents, bacterial and plant toxins, and radioisotopes. Conjugating highly cytotoxic agents to MoAbs should improve the therapeutic index since the MoAb directs killing to those cells that express the target antigen, thus limiting non-specific damage to normal tissues. Importantly, MoAb-based agents armed with potently cytotoxic compounds do not require active immune response mechanisms for activity. As a result, they can be effective even in profoundly immunocompromised patients.
Chemotherapeutic conjugates
MoAbs have been conjugated to a number of active chemotherapeutic agents. The first MoAb conjugate to receive FDA approval in the treatment of cancer was gemtuzumab ozogamicin (Pfizer, New York, NY, U.S.A.). This agent targets the myeloid antigen CD33 and is linked to calicheamicin, a potent antitumor antibiotic. Clinical trials of gemtuzumab ozogamicin have been conducted for children with acute myelogenous leukemia (AML).36,37** Approximately 30% of pediatric patients with relapsed AML respond to gemtuzumab ozogamicin as a single agent. Clinical trials designed to assess the efficacy of gemtuzumab ozogamicin in combination with chemotherapy are being conducted by the COG,38 while the Nordic Society of Paediatric Haematology and Oncology is studying its role as consolidation prior to SCT in children with high-risk AML. This agent has also been used to treat occasional cases of ALL with CD33 expression and anecdotal cases of successful remission induction have been reported.39
Toxin Conjugates
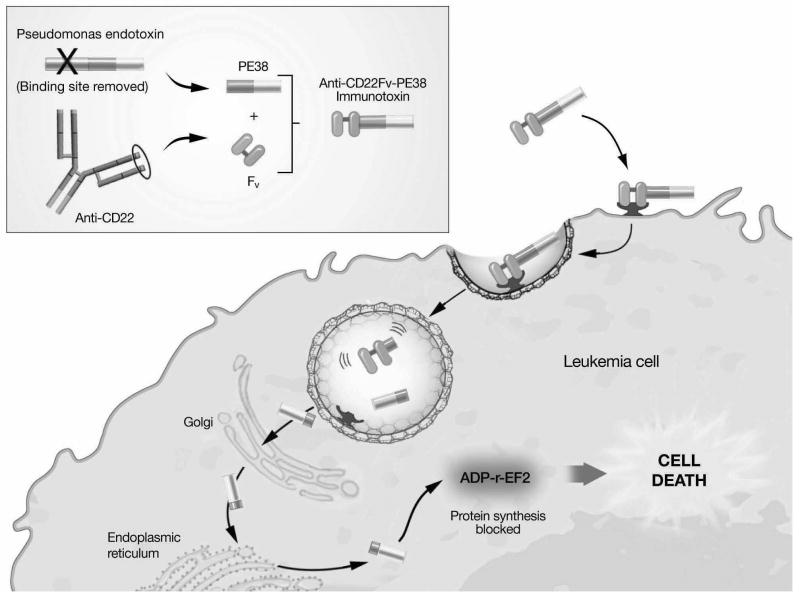
Immunotoxins are engineered proteins consisting of a MoAb-based targeting moiety that mediates cell binding and a toxin that induces cell death upon internalization. [Figure 3] A 38 kD truncated derivative of Pseudomonas exotoxin A (PE38) has been used at the NCI to develop recombinant immunotoxins that target human differentiation antigens.40 A recombinant immunotoxin that targets the B-lineage antigen CD22, CAT-3888 or BL22 (MedImmune LLC, Gaithersburg, MD, U.S.A.), is highly active in adults with B-lymphoid malignancies.41,42 A recently completed pediatric Phase I study demonstrated an acceptable toxicity profile and clinical activity in children with CD22+ ALL and NHL.43 A follow-up pediatric trial of a second-generation agent with higher CD22 binding affinity and increased pre-clinical activity,44 CAT-8015 or HA22, (MedImmune LLC, Gaithersburg, MD, U.S.A.), is in progress.45* Anti-CD22 immunotoxins appear to have synergistic in vitro cytotoxicity against childhood ALL blasts when combined with chemotherapy.46
Figure 3. Pseudomonas-based immunotoxins: structure and mechanism of cytotoxicity Inset:
Full-length Pseudomonas exotoxin A (PE) protein contains three functional domains that mediate antigen binding, cytosolic translocation, and cytotoxicity. The recombinant anti-CD22 immunotoxin RFB4(dsFv)-PE38 consists of cloned disulfide-stabilized single chain variable fragment (Fv) from the anti-CD22 monoclonal antibody RFB4 and the PE translocation and killing domains (PE38).
Immunotoxin binding to surface CD22 is followed by internalization through endocytosis. Immunotoxin cleavage occurs in the endosome and the C-terminal fragment is transported to the cytosol. ADP ribosylation (ADP-r) inactivates elongation factor-2 (EF2), which inhibits protein synthesis causing cell death.
Adapted from: Wayne AS. Application of immunotherapy in pediatric leukemias. Curr Hematol Malig Reports 2009;4(3):159-166.17
Radioisotope conjugates
MoAbs that target leukemia-associated antigens have been linked to radioactive isotopes, most commonly β-emitters (e.g., 90Yttrium, 131Iodine, 186Rhenium) and less frequently α-emitters (e.g., 213Bismuth). These agents are concentrated in the bone marrow and consequently cause severe myelosuppression. Thus, the application of radioimmunotherapy for hematologic malignancies is limited primarily to myeloablative conditioning prior to SCT,47 and there have been very few trials in the pediatric age group.48,49 Studies are being conducted with a 3F8-131Iodine conjugate for children with GD2-expressing brain tumors.50
Potential side effects and limitations of monoclonal antibody-based therapeutics
In general, most of the MoAb-based therapeutics currently being advanced for clinical use have been well tolerated with markedly reduced risks of organ toxicity in comparison to chemotherapy and radiation. Nonetheless, there are a variety of specific side effects associated with individual MoAbs and immunotoxins. Acute infusion reactions are relatively common, although these can usually be managed or prevented by treatment with antipyretics, antihistamines, and/or corticosteroids. Depletion of normal blood cells that express the target antigen can be expected. For example, rituximab is associated with B cell depletion, humoral immunosuppression, and risk of certain viral infections. Immunotoxins are associated with a number of unique toxicities, for example, vascular leak syndrome.
Immunogenicity
Despite the creation of human/murine (“humanized”) antibodies, some components of many MoAbs are derived from foreign species. Similarly, toxins represent foreign proteins. Thus, patients may develop antibodies to foreign protein epitopes (i.e., human anti-mouse and/or human anti-toxin antibodies) that bind and diminish or neutralize therapeutic activity. Notably, there may be pre-existing antibodies due to prior vaccination (e.g., Diphtheria) or infection (e.g., Pseudomonas).
Cellular Immunity and Cell-Based Therapeutics of Cancer
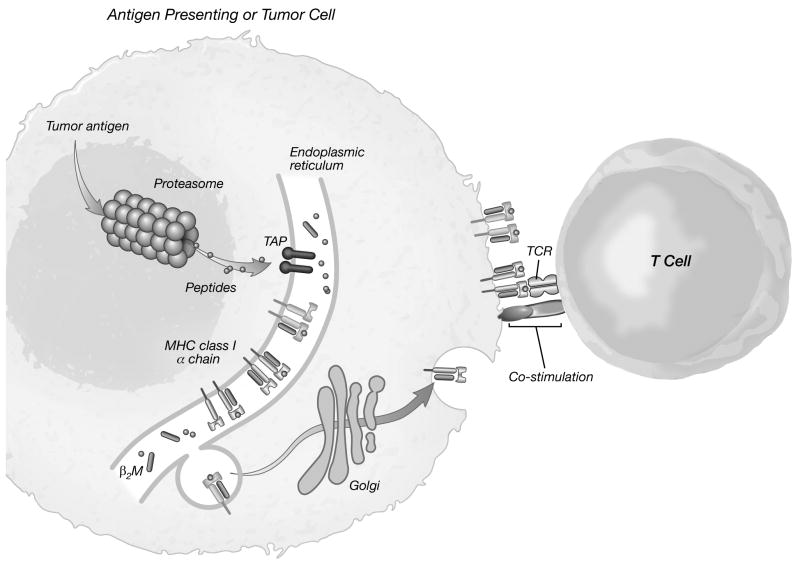
Cellular immunity is mediated primarily by CD4+ and CD8+ T cells. [Figure 1] T cells recognize antigen as peptides derived from the breakdown of intracellular proteins displayed on the surface of antigen presenting cells (APCs) in the context of major histocompatibility (MHC) antigens.8,12,51 [Figure 4] The generation of a successful immune response by a naïve T cell requires the interaction of an appropriate T cell receptor and MHC-presented peptide antigen, known as Signal #1, along with a co-stimulatory signal, known as Signal #2. [Figure 4] This second signal can be via an appropriate cytokine or through by cell-to-cell interaction through one of a variety a co-stimulatory molecule/ligand pairs (e.g., CD40/CD40L, B7 family/CD28). Activation of the T cell receptor in the absence of appropriate co-stimulation results in antigen-specific tolerance.5
Figure 4. The T cell immune response.
T cells recognize antigen as small peptides derived from the breakdown of intracellular proteins displayed on the surface of antigen presenting cells in the context of major histocompatibility (MHC) antigens. Tumor antigens are cleaved by the proteasome into fragment peptides. The peptides transported by the transporter associated with antigen processing (TAP) into the endoplasmic reticulum, where they are loaded onto MHC molecules that are assembled and transported to the cell surface.
Activation of naïve T cells requires the interaction of an appropriate T cell receptor (TCR) and MHC-presented peptide antigen (Signal #1) and a co-stimulatory signal (Signal #2).
β2M: beta-2 microglobulin
Adapted from: Kong HT, Restifo NP: Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nature Immunol 2002;3:999-1005.12
Allogeneic stem cell transplantation and the graft-versus-leukemia effect
Allogeneic SCT represents the most commonly employed and well-proven form of cellular immunotherapy for childhood cancer. SCT can be curative for most subtypes of pediatric hematologic malignancies.52,53 A T cell-mediated allogeneic immunologic reaction, the graft-versus-leukemia (GVL) effect, reduces the risk of relapse after SCT.54,55,56,57,58,59,60 Among the most dramatic demonstrations of the potency of T cell immunotherapy in the treatment of cancer is the effect of donor lymphocyte infusions (DLI) for relapsed leukemia after SCT.61*,62,63,64,65 [Supplemental Figure 1] DLI induces complete remissions in more than 70% of adults and children with chronic phase chronic myelogenous leukemia (CML). This approach, however, is far less effective in other subtypes of leukemia, especially ALL. There are a number of possible mechanisms for the resistance of ALL to DLI. For example, unlike myeloid cells, B-lineage lymphoblasts have very low expression of T cell co-stimulatory molecules (e.g., B7 family). Consequently, ALL blasts present antigens poorly and they may induce T cell anergy.66 Importantly, a major complication of DLI, and SCT in general, is graft-versus-host disease (GVHD), which represents an allogeneic reaction of donor T cells against normal host tissues. Thus, a key aim of cellular immunotherapy after SCT is to implement strategies that induce GVL in the absence of GVHD.
Cancer vaccines
Cancer vaccines are designed to generate sustained responses to a specific antigen through the formation of immunologic memory. A variety of approaches have been utilized in attempt to stimulate T cell immune responses toward cancer-associated antigens in vivo.
Peptide vaccines
Peptides can be readily synthesized for clinical use as a source of tumor-associated antigens for cancer vaccines. Immunologic and clinical responses, including complete remissions, have been observed in adults with leukemia and solid tumors treated with peptide vaccines targeting the myeloid antigen proteinase67,68 and the Wilms tumor-1 (WT1) protein, a transcription factor expressed by a wide array of malignancies.69,70 Importantly however, applicability of peptide vaccines is restricted by the specific antigenic epitopes and HLA binding motifs. This is particularly limiting in pediatric cancers, which are far less common than malignancies in adults.
Dendritic cells and artificial antigen presenting cells for cancer vaccines
Dendritic cells are specialized APCs that play a critical role in the adaptive immune response, [Figure 1] and can be generated from peripheral blood monocytes ex vivo for use in cancer vaccines.71 Additionally, artificial APCs can be engineered to express key co-stimulatory molecules for immunotherapy trials.72 Peptides, nucleic acids, proteins, and tumor lysates can all be used to prime APCs to present cancer-associated antigens, which can then be administered as a vaccine to direct a T cell response towards cancer-associated targets. [Supplemental Figure 2] Clinical responses to APC-based vaccines have been reported in pediatric trials, with one patient with fibrosarcoma achieving a complete response to a tumor lysate-pulsed dendritic cell vaccine.73,74 Favorable overall survival was reported in a recent study of autologous T cell infusion and peptide-pulsed dendritic cell vaccine targeting the translocation breakpoints in pediatric sarcomas.75* A follow-up trial using autologous tumor lysate as the antigenic source is in progress at the NCI. A novel allogeneic vaccine trial that utilizes WT1 peptide-loaded dendritic cells generated from healthy SCT donors is being conducted at the NCI for children and adults with WT1-expressing hematologic malignancies. Vaccines are administered along with DLI in attempt to augment the GVL effect for individuals with persistent or relapsed disease after allogeneic SCT. [Supplemental Figure 2]
Autologous and allogeneic cancer cells can also be modified to function directly as APCs. As an example, blasts from children with B-precursor ALL can be rendered capable of presenting antigens by incubation with the co-stimulatory molecule CD40 ligand and the cytokine IL-4. Thus, ALL blasts can be transformed into APCs, overcoming their ability to induce T cell anergy.66 Similarly, autologous and allogeneic neuroblastoma cells transduced with T cell activating cytokines have been utilized in vaccine trials in pediatric patients with neuroblastoma and response rates of 10 to 20% have been reported.76,77,78
Adoptive immunotherapy with ex vivo expanded T cells
As noted above, the efficacy of T cell-based immunotherapeutic approaches may be limited by the inability to rapidly generate large numbers of antigen-specific T cells in vivo. Effective approaches to generate, expand, and activate large quantities of activated target-directed T cells ex vivo for use in adoptive immunotherapy have been developed,79 and durable complete responses have been observed in adults with chemotherapy-resistant metastatic melanoma.80** Studies of genetically engineered cytotoxic T cells with specificity against antigens expressed by pediatric solid tumors are underway at the NCI, and dramatic responses in pediatric patients have recently been observed (unpublished results).
Potential side effects and limitations of cellular immunotherapies
To date, vaccine therapies have been well tolerated, with primarily only minimal injection site pain, erythema and/or induration observed. Immunotherapies that induce a strong T cell response may be associated with autoimmunity.81 Allogeneic lymphocyte infusions carry the risk of GVHD and transfusion-related side effects.
Combined Approaches to Cancer Immunotherapy
Immunotherapy trials commonly incorporate multiple components designed to maximize the immunologic response.
Chimeric Antigen Receptors
The effectors employed in adoptive therapy can be specifically directed using MoAb-based targeting to engage cancer cells via gene therapy techniques. Genetically engineered chimeric antigen receptors (CARs) have been designed to enable immune effectors to bind to and induce direct cytotoxicity against malignant cells that express differentiation antigens.82,83,84,85 T cells and NK cells engineered with CARs directed to leukemia-targets (e.g., B-lineage antigen CD19)86 and solid tumor antigens (e.g., GD2)87 are currently undergoing study at a number of pediatric centers including the NCI, St. Jude Children's Research Hospital, and the Baylor College of Medicine. EBV-specific cytotoxic lymphocytes engineered to target GD2 were shown to be active against refractory neuroblastoma.88**
Bi-specific monoclonal antibodies
MoAb constructs with dual specificity for a leukemia-associated antigen and a surface receptor on immune effector cells (e.g., CD3) can serve to direct cell-mediated cytotoxicity in a fashion similar to CARs. A recombinant anti-CD19/anti-CD3ε bi-specific antibody (blinatumomab, Micromet, Inc., Munich, Germany) has recently been shown to be active in adults with hematologic malignancies.89* Importantly, these agents require functional T cells for activity and therefore might be expected to have increased utility after allogeneic SCT.
Immunotherapy combined with standard treatment regimens
As noted above, clinical trials that integrate immunotherapy with standard cytoreductive regimens are undergoing study. The concept of “consolidative immunotherapy” holds particular promise in pediatric oncology, where the most common malignancies are usually responsive to front line therapies, and patients with high-risk disease can often be reduced to a state of MRD.
Conclusions
The majority of pediatric patients with cancer are cured with current standard treatment regimens. However, progress appears to have plateaued and the late effects associated with cytoxoxic agents are substantial.1,2 New approaches that can augment standard therapies and reduce non-specific toxicities are highly sought-after. Discoveries in immunology and cancer biology, along with technological advances, have led to the development of novel immune-based therapies. Immunologically-based cancer therapies have shown promise in pre-clinical models and have been translated into clinical trials. Benefits of immunotherapy in pediatric oncology have recently been realized in specific diseases and numerous approaches hold great promise.90** Future studies will help define the optimal approaches and combination regimens. In addition, the effectiveness of immune-based therapies for childhood cancer is expected to increase with the implementation of strategies that can hasten immune reconstitution after standard treatment.
Supplementary Material
Supplemental Figure 1: Donor lymphocyte infusion induces complete remission of chronic myelogenous leukemia relapsing after allogeneic stem cell transplant.
A 17-year-old developed recurrent chronic phase CML after myeloablative stem cell transplantation from a matched sibling donor.
A. Bone marrow biopsy 24 months after transplant reveals increased cellularity due to recurrent CML that was confirmed by cytogenetic demonstration of the Philadelphia chromosome. The patient received donor lymphocyte infusion only.
B. Bone marrow biopsy 6 months after donor lymphocyte infusion reveals hypocellularity. Complete remission (morphologic and molecular genetic) was confirmed by cytogenetic and molecular genetic analyses. The patient has remained in remission for 15 years without further anti-leukemic therapy.
[100× magnification; Hematoxylin and eosin stain]
Supplemental Figure 2: Allogeneic dendritic cell vaccine strategy targeting WT1 for post-transplant relapse of hematologic malignancy
Monocyte-derived dendritic cells are generated from the original allogeneic stem cell transplant donor and loaded with three HLA-A2 binding WT1 peptides that were synthesized linked to the TAT protein transduction domain designed to improve antigen presentation. Vaccines are administered along with donor lymphocytes.
IL-4: interleukin-4; GM-CSF: granulocyte-macrophage colony-stimulating factor; IFN-γ: interferon-gamma; LPS: lipopolysaccharide
Acknowledgments
The authors acknowledge Dr. Steven Rosenberg, Dr. Ira Pastan, Dr. Kristin Baird, Dr. Terry Fry, and Dr. Melinda Merchant, colleagues in the Center for Cancer Research, National Cancer Institute. This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. The authors declare no potential conflicts of interest.
Footnotes
This paper was prepared by employees of the U.S. Government making this a “work of the U.S. Government” and therefore copyright is not transferable.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wayne AS, Reaman GH, Helman LJ. Progress in the curative treatment of childhood hematologic malignancies. J Natl Cancer Inst. 2008;100(18):1271–1273. doi: 10.1093/jnci/djn306. [DOI] [PubMed] [Google Scholar]
- 2.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 3.Gloeckler Ries LA. Childhood cancer mortality. In: Reis LAG, Smith MA, Gurney JG, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995. National Cancer Institute, SEER Program; Bethesda: 1999. pp. 165–170. NIH Pub. No. 99-4649. http://www-seer.ims.nci.nih.gov. [Google Scholar]
- 4.Delves PJ, Roitt IM. The immune system. First of two parts. New Engl J Med. 2000;343(1):37–49. doi: 10.1056/NEJM200007063430107. [DOI] [PubMed] [Google Scholar]
- 5.Delves PJ, Roitt IM. The immune system. Second of two parts. New Engl J Med. 2000;343(2):108–117. doi: 10.1056/NEJM200007133430207. [DOI] [PubMed] [Google Scholar]
- 6.Mackall CL, Sondel PM. Tumor immunology and pediatric cancer. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 6th. Philadelphia, PA: Lippincott Raven Publishers; 2009. in press. [Google Scholar]
- 7.Capitini CM, Wayne AS, Mackall CL. Immune-based therapeutics for pediatric cancer. Expert Opin Biol Ther. 2008 doi: 10.1517/14712590903431022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berzofsky JA, Terabe M, Oh S, et al. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest. 2004;113:1515–1525. doi: 10.1172/JCI21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother. 2005;54(3):187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finn OJ. Cancer immunology. N Engl J Med. 2008;358(25):2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 11.Mapara MY, Sykes M. Tolerance and cancer: mechanisms of tumor evasion and strategies for breaking tolerance. J Clin Oncol. 2004;22:1136–1151. doi: 10.1200/JCO.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 12.Kong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nature Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 14.Huston JS, McCartney J, Tai MS, Mottola-Hartshorn C, Jin D, Warren F, Keck P, Oppermann H. Medical applications of single-chain antibodies. Int Rev Immunol. 1993;10(23):195–217. doi: 10.3109/08830189309061696. [DOI] [PubMed] [Google Scholar]
- 15.Haining WN, Cardoso AA, Keczkemethy HL, et al. Failure to define window of time for autologous tumor vaccination in patients with newly diagnosed or relapsed acute lymphoblastic leukemia. Exp Hematol. 2005;33:286–294. doi: 10.1016/j.exphem.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. New Engl J Med. 1995;332(3):143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 17.Wayne AS. Application of immunotherapy in pediatric leukemias. Curr Hematol Malig Reports. 2009;4(3):159–166. doi: 10.1007/s11899-009-0022-5. [DOI] [PubMed] [Google Scholar]
- 18.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 19.Griffin TC, Weitzman S, Weinstein H, et al. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20+) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic leukemia: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2009;52:177–181. doi: 10.1002/pbc.21753. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This cooperative group clinical trial combined the anti-CD20 monoclonal antibody rituximab with ifosfamide, carboplatin, and etoposide chemotherapy in children with relapsed and refractory lymphoma and leukemia that expressed the CD20 antigen. Toxicity was acceptable, with relatively common but manageable rituximab infusion reactions. The overall response rate was 60% (12/20), including 7 complete and 5 partial responses, which is comparable to other published active chemotherapy regimens in this patient population.
- 20.Raetz EA, Cairo MS, Borowitz MJ, et al. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children's Oncology Group Pilot Study. J Clin Oncol. 2008;26:3756–3762. doi: 10.1200/JCO.2007.15.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This represents a preliminary report of the ongoing COG trial with the anti-CD20 MoAb epratuzumab. Limited activity was observed in the initial patient cohort treated with epratuzumab alone. This study demonstrates the feasibility of combining MoAbs with standard chemotherapy for childhood cancer.
- 21.Corbacioglu S, Eber S, Gungor T, Hummerjohann J, Niggli F. Induction of long-term remission of a relapsed childhood B-acute lymphoblastic leukemia with rituximab chimeric anti-CD20 monoclonal antibody and autologous stem cell transplantation. J Pediatr Hematol Oncol. 2003;25:327–329. doi: 10.1097/00043426-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Laporte JP, Isnard F, Garderet L, Fouillard L, Gorin NC. Remission of adult acute lymphocytic leukaemia with alemtuzumab. Leukemia. 2004;18:1557–1558. doi: 10.1038/sj.leu.2403422. [DOI] [PubMed] [Google Scholar]
- 23.Feldman F, Kalaycio M, Weiner G, et al. Treatment of relapsed or refractory acute myeloid leukaemia with humanized anti-CD33 monoclonal antibody HuM195. Leukemia. 2003;17:314–318. doi: 10.1038/sj.leu.2402803. [DOI] [PubMed] [Google Scholar]
- 24.Yu AL, Uttenreuther-Fischer MM, Huang CS, Tsui CC, Gillies SD, Reisfeld RA, et al. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. J Clin Oncol. 1998;16(6):2169–80. doi: 10.1200/JCO.1998.16.6.2169. [DOI] [PubMed] [Google Scholar]
- 25.Kushner BH, Kramer K, Cheung NKV. Phase II Trial of the Anti-GD2 Monoclonal Antibody 3F8 and Granulocyte-Macrophage Colony-Stimulating Factor for Neuroblastoma. J Clin Oncol. 2001 November 15;19(22):4189–4194. doi: 10.1200/JCO.2001.19.22.4189. [DOI] [PubMed] [Google Scholar]
- 26.Osenga KL, Hank JA, Albertini MR, Gan J, Sternberg AG, Eickhoff J, et al. A Phase I Clinical Trial of the hu14.18-IL2 (EMD 273063) as a Treatment for Children with Refractory or Recurrent Neuroblastoma and Melanoma: a Study of the Children's Oncology Group. Clin Cancer Res. 2006 March 15;12(6):1750–1759. doi: 10.1158/1078-0432.CCR-05-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Modak S, Cheung NK. Disialoganglioside directed immunotherapy of neuroblastoma. Cancer investigation. 2007;25(1):67–77. doi: 10.1080/07357900601130763. [DOI] [PubMed] [Google Scholar]
- 28.Ozkaynak MF, Sondel PM, Krailo MD, Gan J, Javorsky B, Reisfeld RA, et al. Phase I study of chimeric human/murine anti-ganglioside GD2 monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after hematopoietic stem-cell transplantation: a Children's Cancer Group Study. J Clin Oncol. 2000;18(24):4077–4085. doi: 10.1200/JCO.2000.18.24.4077. [DOI] [PubMed] [Google Scholar]
- 29.Schusterman S, London W, Gillies S, Hank J, Voss S, Seeger R, et al. Anti-neuroblastoma activity of hu14.18-IL2 against minimal residual disease in a Children's Oncology Group (COG) phase II study. J Clin Oncol. 2008;26(suppl) doi: 10.1200/JCO.2009.27.8861. abstr 3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung NK, Kushner BH, Cheung IY, Kramer K, Canete A, Gerald W, et al. Anti-G(D2) antibody treatment of minimal residual stage 4 neuroblastoma diagnosed at more than 1 year of age. J Clin Oncol. 1998 September 1;16(9):3053–60. doi: 10.1200/JCO.1998.16.9.3053. [DOI] [PubMed] [Google Scholar]
- 31.Yu A, Gilman A, Ozkaynak M, London W, Kreissman S, Chen H, et al. A phase III randomized trial of the chimeric anti-GD2 antibody ch14.18 with GM-CSF and IL-2 as immunotherapy following dose intensive chemotherapy for high-risk neuroblastoma: Children's Oncology Group (COG) study ANBL0032. J Clin Oncol. 2009;27(15s) abstr 10067. [Google Scholar]; **This randomized Phase III trial was stopped prematurely when a survival benefit was observed in the cohort of patients who received the anti-GD2 MoAb after autologous SCT. This represents the first study to clearly demonstrate a survival benefit of MoAb-based therapy for children with cancer, and it paves the way for the incorporation of MoAbs into frontline regimens for high-risk neuroblastoma.
- 32.Kim SY, Wan X, Helman LJ. Targeting IGF-1R in the treatment of sarcomas: past, present and future. Bull Cancer. 2009;96(7):E52–60. doi: 10.1684/bdc.2009.0915. [DOI] [PubMed] [Google Scholar]
- 33.Kolb EAEA, Gorlick RR, Houghton PJPJ, Morton CLCL, Lock RR, Carol HH, et al. Initial testing (stage 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatr Blood Canc. 2008;50(6):1190–1197. doi: 10.1002/pbc.21450. [DOI] [PubMed] [Google Scholar]; * In this Pediatric Preclinical Testing Program study, a MoAb against the IGF-1 receptor increased event-free survival in 57% of pediatric solid tumor xenografts and showed activity against rhabdoid tumor, Ewing, rhabdomyosarcoma, glioblastoma, neuroblastoma, and osteosarcoma.
- 34.Merchant MS, Yang X, Melchionda F, Romero M, Klein R, Thiele CJ, Tsokos M, Kontny HU, Mackall CL. Interferon gamma enhances the effectiveness of tumor necrosis factor-related apoptosis-inducing ligand receptor agonists in a xenograft model of Ewing's sarcoma. Cancer Res. 2004;64(22):8349–56. doi: 10.1158/0008-5472.CAN-04-1705. [DOI] [PubMed] [Google Scholar]
- 35.Movva S, Verschraegen C. The monoclonal antibody to cytotoxic T lymphocyte antigen 4, ipilimumab (MDX-010), a novel treatment strategy in cancer management. Expert Opinion on Biological Therapy. 2009;9(2):231–241. doi: 10.1517/14712590802643347. [DOI] [PubMed] [Google Scholar]
- 36.Arceci RJ, Sande J, Lange B, et al. Safety and efficacy of gemtuzumab ozogamicin in pediatric patients with advanced CD33+ acute myeloid leukemia. Blood. 2005;106:1183–1188. doi: 10.1182/blood-2004-10-3821. [DOI] [PubMed] [Google Scholar]
- 37.Aplenc R, Alonzo TA, Gerbing RB, et al. Safety and efficacy of gemtuzumab ozogamicin in combination with chemotherapy for pediatric acute myeloid leukemia: a report from the Children's Oncology Group. J Clin Oncol. 2008;26:2390–3295. doi: 10.1200/JCO.2007.13.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study was the first to establish the maximum tolerated dose of gemtuzumab ozogamicin in combination with chemotherapy for children with AML.
- 38.Franklin J, Alonzo T, Hurwitz CA, et al. COG AAML03P1: efficacy and safety in a pilot study of intensive chemotherapy including gemtuzumab in children newly diagnosed with acute myeloid leukemia. Blood. 2008;112:136a. abstract. [Google Scholar]
- 39.Zwaan CM, Reinhardt D, Jürgens H. Gemtuzumab ozogamicin in pediatric CD33-positive acute lymphoblastic leukemia: first clinical experiences and relation with cellular sensitivity to single agent calicheamicin. Leukemia. 2003;17:468–470. doi: 10.1038/sj.leu.2402749. [DOI] [PubMed] [Google Scholar]
- 40.Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nature Rev. 2006;6:559–565. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 41.Kreitman RJ, Squires DR, Steler-Stevenson M, et al. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J Clin Oncol. 2005;23:6719–6729. doi: 10.1200/JCO.2005.11.437. [DOI] [PubMed] [Google Scholar]
- 42.Kreitman RJ, Stetler-Stevenson M, Margulies M, et al. Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J Clin Oncol. 2009;27:2983–2990. doi: 10.1200/JCO.2008.20.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wayne AS, Findley HW, Lew G, et al. Targeting CD22 in childhood B-precursor acute lymphoblastic leukemia: Pre-clinical studies and Phase I trial of the anti-CD22 immunotoxin CAT-3888 (BL22) Blood. 2007;110:262a. abstract. [Google Scholar]
- 44.Salvatore G, Beers R, Margulies I, Kreitman RJ, Pastan I. Improved cytotoxic activity toward cell lines and fresh leukemia cells of a mutant anti-CD22 immunotoxin obtained by antibody phage display. Clin Cancer Res. 2002;8:995–1002. [PubMed] [Google Scholar]
- 45.Wayne AS, Bhojwani D, Jeha S, Stetler-Stevenson M, Pui CH, McDevitt J, Fitzgerald DJ, Kreitman RJ, Kaucic K, Pastan I. Phase I clinical trial of the Anti-CD22 immunotoxin CAT-8015 (HA22) for pediatric acute lymphoblastic leukemia. Blood. 2009 abstract in press. [Google Scholar]; *Additional details can be provided prior to press, after presentation on 12/8/09
- 46.Ahuja Y, Stetler-Stevenson M, Kreitman RJ, Pastan I, Wayne AS. Pre-clinical evaluation of the anti-CD22 immunotoxin CAT-8015 in combination with chemotherapy agents for childhood B-precursor acute lymphoblastic leukemia (Pre-B ALL) Blood. 2007;110:265a. abstract. [Google Scholar]
- 47.Matthews DC, Appelbaum FR, Eary JF, Fisher DR, Durack LD, Bush SA, Hui TE, Martin PJ, Mitchell D, Press OW, et al. Development of a marrow transplant regimen for acute leukemia using targeted hematopoietic irradiation delivered by 131 I-labeled anti-CD45 antibody, combined with cyclophosphamide and total body irradiation. Blood. 1995;85(4):1122–1131. 15. [PubMed] [Google Scholar]
- 48.Cooney-Qualter E, Krailo M, Angiolillo A, Fawwaz RA, Wiseman G, Harrison L, et al. A Phase I Study of 90Yttrium-Ibritumomab-Tiuxetan in Children and Adolescents with Relapsed/Refractory CD20-Positive Non Hodgkin's Lymphoma: A Children's Oncology Group Study. Clin Cancer Res. 2007;13(18):5652–5660s. doi: 10.1158/1078-0432.CCR-07-1060. [DOI] [PubMed] [Google Scholar]
- 49.Modak S, Cheung N. Antibody-based targeted radiation to pediatric tumors. J Nucl Med. 2005;46 1:157S–63S. [PubMed] [Google Scholar]
- 50.Kramer K, Humm JL, Souweidane MM, Zanzonico PB, Dunkel IJ, Gerald WL, et al. Phase I Study of Targeted Radioimmunotherapy for Leptomeningeal Cancers Using Intra-Ommaya 131-I-3F8. J Clin Oncol. 2007 December 1;25(34):5465–5470. doi: 10.1200/JCO.2007.11.1807. [DOI] [PubMed] [Google Scholar]
- 51.Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Ann Rev Immunol. 1993;7:601–624. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- 52.Hahn T, Wall D, Camitta B, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute lymphoblastic leukemia in children: an evidence-based review. Biol Blood Marrow Transplant. 2005;11:823–861. doi: 10.1016/j.bbmt.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 53.Oliansky DM, Rizzo JD, Aplan PD, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute myeloid leukemia in children: an evidence-based review. Biol Blood Marrow Transplant. 2007;13:1–25. doi: 10.1016/j.bbmt.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 54.Porter DL, Antin JH. The graft-versus-leukemia effects of allogeneic cell therapy. Ann Rev Med. 1999;50:369–386. doi: 10.1146/annurev.med.50.1.369. [DOI] [PubMed] [Google Scholar]
- 55.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 56.Sullivan KM, Weiden PL, Storb R, et al. Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood. 1989;73:1720–1728. [PubMed] [Google Scholar]
- 57.Locatelli F, Zexxa M, Rondelli R, et al. Graft-versus-host disease prophylaxis with low dose cyclosporine-A reduces the risk of relapse in children with acute leukemia given HLA-identical sibling bone marrow transplantation: results of a randomized trial. Blood. 2000;95:1572–1579. [PubMed] [Google Scholar]
- 58.Rassam S, Katz F, Chessells J, M G. Successful allogeneic bone marrow transplantation in juvenile CML: conditioning or graft-versus-leukaemia effect? Bone Marrow Transplant. 1993;11(3):247–250. [PubMed] [Google Scholar]
- 59.Passweg JJR, Tiberghien PP, Cahn JJY, Vowels MMR, Camitta BBM, Gale RRP, et al. Graft-versus-leukemia effects in T lineage and B lineage acute lymphoblastic leukemia. Bone marrow transplantation. 1998;21(2):153–158. doi: 10.1038/sj.bmt.1701064. [DOI] [PubMed] [Google Scholar]
- 60.Zecca M, Prete A, Rondelli R, Lanino E, Balduzzi A, Messina C, et al. Chronic graft-versus-host disease in children: incidence, risk factors, and impact on outcome. Blood. 2002;100(4):1192–200. doi: 10.1182/blood-2001-11-0059. [DOI] [PubMed] [Google Scholar]
- 61.Tomblyn M, Lazarus HM. Donor lymphocyte infusions: the long and winding road: how should it be travelled. Bone Marr Transplant. 2008;42:569–579. doi: 10.1038/bmt.2008.259. [DOI] [PubMed] [Google Scholar]; * This recent review includes reports of DLI for hematologic malignancies published from 1995 to 2008. DLI in combination with withdrawal of immunosuppression and often other therapies induces leukemia remissions in more than 70% of individuals with chronic myelogenous leukemia in chronic phase and approximately 25% of those with other hematologic malignancies.
- 62.Yoshimi A, Bader P, Matthes-Martin S, et al. Donor leukocyte infusion after hematopoietic stem cell transplantation in patients with juvenile myelomonocytic leukemia. Leukemia. 2005;19:971–7. doi: 10.1038/sj.leu.2403721. [DOI] [PubMed] [Google Scholar]
- 63.Loren AW, Porter DL. Donor leukocyte infusions for the treatment of relapsed acute leukemia after allogeneic stem cell transplantation. Bone Marrow Transplant. 2008;41(5):483–493. doi: 10.1038/sj.bmt.1705898. [DOI] [PubMed] [Google Scholar]
- 64.Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia. Blood. 1995;86(5):2041–2050. see comments. [PubMed] [Google Scholar]
- 65.Collins RH, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. Journal of clinical oncology. 1997;15(2):433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 66.Cardosa AA, Schultze JL, Boussiotis VA, et al. Pre-B acute lymphoblastic leukemia may induce T-cell anergy to alloantigen. Blood. 1996;88:41–48. [PubMed] [Google Scholar]
- 67.Qazilbash MH, Wieder E, Rios R, et al. Vaccination with the PR1 leukemia-associated antigen can induce complete remission in patients with myeloid leukemia. Blood. 2004;104:77a. abstract. [Google Scholar]
- 68.Molldrem JJ, Kant S, Lu S, et al. Peptide vaccination with PR1 elicits active T-cell immunity that induces cytogenetic remission in acute myelogenous leukemia. Blood. 2002;100:6a. abstract. [Google Scholar]
- 69.Mailander V, Scheibenbogen C, Thiel E, et al. Complete remission in a patient with recurrent acute myeloid leukemia induced by vaccination with WT1 peptide in the absence of hematological or renal toxicity. Leukemia. 2004;18:165–166. doi: 10.1038/sj.leu.2403186. [DOI] [PubMed] [Google Scholar]
- 70.Oka Y, Tsuboi A, Taguchi T, et al. Induction of WT1 (Wilms' tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Nat Acad Sci. 2004;101:13885–13890. doi: 10.1073/pnas.0405884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong ECC, Maher VE, Hines K, et al. Development of a clinical-scale method for generation of dendritic cells from PBMC for us in cancer immunotherapy. Cytotherapy. 2001;3:19–29. doi: 10.1080/146532401753156377. [DOI] [PubMed] [Google Scholar]
- 72.Suhoski MM, Golovina TN, Aqui NA, et al. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol Ther. 2007;15:981–988. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geiger JD, Hutchinson RJ, Hohenkirk LF, McKenna EA, Yanik GA, Levine JE, et al. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001;61(23):8513–8519. [PubMed] [Google Scholar]
- 74.Geiger JD, Hutchinson RR, Hohenkirk LL, McKenna EE, Chang AA, Mulé JJ. Treatment of solid tumours in children with tumour-lysate-pulsed dendritic cells. Lancet. 2000;356(9236):1163–1165. doi: 10.1016/S0140-6736(00)02762-8. [DOI] [PubMed] [Google Scholar]
- 75.Mackall CL, Rhee EH, Read EJ, Khuu HM, Leitman SF, Bernstein D, et al. A pilot study of consolidative immunotherapy in patients with high-risk pediatric sarcomas. Clin Cancer Res. 2008;14(15):4850–4858. doi: 10.1158/1078-0432.CCR-07-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Autologous T cells and dendritic cells pulsed with peptides derived from the specific translocation breakpoints in Ewing sarcoma and alveolar rhabdomyosarcoma were administered after completion of standard therapy to children at high-risk of relapse. Using an intent-to-treat analysis of all patients entered onto the study, irrespective of whether immunotherapy was administered or not, a 5-year overall survival rate of 31% was observed. For those who received immunotherapy, the 5-year overall survival was 43%, which was favorable compared to historical controls.
- 76.Bowman L, Grossmann M, Rill D, Brown M, Zhong Wy, Alexander B, et al. IL-2 Adenovector-transduced autologous tumor cells induce antitumor immune responses in patients with neuroblastoma. Blood. 1998;92(6):1941–1949. [PubMed] [Google Scholar]
- 77.Rousseau RF, Haight AE, Hirschmann-Jax C, Yvon ES, Rill DR, Mei Z, et al. Local and systemic effects of an allogeneic tumor cell vaccine combining transgenic human lymphotactin with interleukin-2 in patients with advanced or refractory neuroblastoma. Blood. 2003;101(5):1718–1726. doi: 10.1182/blood-2002-08-2493. [DOI] [PubMed] [Google Scholar]
- 78.Russell HV, Strother D, Mei Z, Rill D, Popek E, Biagi E, et al. Phase I trial of vaccination with autologous neuroblastoma tumor cells genetically modified to secrete IL-2 and lymphotactin. J Immunother. 2007;30(2):227–233. doi: 10.1097/01.cji.0000211335.14385.57. [DOI] [PubMed] [Google Scholar]
- 79.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Autologous tumor-infiltrating lymphocytes and IL-2 administered to patients with refractory metastatic melanoma after lymphodepleting chemotherapy and radiation resulted in objective response rates of 50% to 70%. Responses included durable complete remissions in individuals with bulky disease.
- 81.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cooper LJ, Topp MS, Serrano LM, et al. T-cell clones can be rendered specific for CD19:toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101:1637–1644. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 83.Rossig C, Pscherer S, Landmeier S, Altvater B, Jurgens H, Vormoor J. Adoptive cellular immunotherapy with CD19-specific T cells. Klin Padiatr. 2005;217:351–356. doi: 10.1055/s-2005-872521. [DOI] [PubMed] [Google Scholar]
- 84.Kershaw MH, Teng MWL, Smyth MJ, Darcy PK. Supernatural T cells: genetic modification of T cells for cancer therapy. Nat Rev Immunol. 2005;5(12):928–940. doi: 10.1038/nri1729. [DOI] [PubMed] [Google Scholar]
- 85.Sadelain M, Brentjens R, Rivière I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21(2):215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kochenderfer JN, Feldman SA, Zhao Y, Xu H, Black MA, Morgan RA, Wilson WH, Rosenberg SA. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009;32(7):689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Molecular therapy. 2007;15(4):825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 88.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** In this study EBV-specific cytotoxic lymphocytes engineered to target GD2 were administered to children with neuroblastoma. Clinical responses were seen observed in 50% of patients including one complete remission that was sustained for more than 1 year without further therapy.
- 89.Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]; * Four complete and 7 partial responses were observed in this Phase I study of a bi-specific MoAb targeting CD19 and CD3 in adults with NHL and leukemia.
- 90.Capitini CM, Cooper LJN, Egeler RM, Handgretinger R, Locatelli F, Sondel PM, et al. Highlights of the First International “Immunotherapy in Pediatric Oncology: Progress and Challenges” Meeting. Journal of pediatric hematology/oncology. 2009;31(4):227–244. doi: 10.1097/MPH.0b013e31819a5d8d. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This report includes summary abstracts from an NIH symposium on pediatric immunotherapy, and includes recent data on pre-clinical and clinical studies in this area. Progress with and key obstacles to cancer immunotherapy in children and adolescents are highlighted.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Donor lymphocyte infusion induces complete remission of chronic myelogenous leukemia relapsing after allogeneic stem cell transplant.
A 17-year-old developed recurrent chronic phase CML after myeloablative stem cell transplantation from a matched sibling donor.
A. Bone marrow biopsy 24 months after transplant reveals increased cellularity due to recurrent CML that was confirmed by cytogenetic demonstration of the Philadelphia chromosome. The patient received donor lymphocyte infusion only.
B. Bone marrow biopsy 6 months after donor lymphocyte infusion reveals hypocellularity. Complete remission (morphologic and molecular genetic) was confirmed by cytogenetic and molecular genetic analyses. The patient has remained in remission for 15 years without further anti-leukemic therapy.
[100× magnification; Hematoxylin and eosin stain]
Supplemental Figure 2: Allogeneic dendritic cell vaccine strategy targeting WT1 for post-transplant relapse of hematologic malignancy
Monocyte-derived dendritic cells are generated from the original allogeneic stem cell transplant donor and loaded with three HLA-A2 binding WT1 peptides that were synthesized linked to the TAT protein transduction domain designed to improve antigen presentation. Vaccines are administered along with donor lymphocytes.
IL-4: interleukin-4; GM-CSF: granulocyte-macrophage colony-stimulating factor; IFN-γ: interferon-gamma; LPS: lipopolysaccharide