Abstract
Objective
The balance between apoptosis susceptibility and efferocytosis of macrophages is central to plaque remodeling and inflammation. LRP1 and its ligand, apoE, have been implicated in efferocytosis and apoptosis in some cell types. We investigated the involvement of the macrophage LRP1/apoE axis in controlling plaque apoptosis and efferocytosis.
Method and Results
LRP1-/- macrophages displayed nearly 2-fold more TUNEL positivity compared to WT cells in the presence of DMEM alone or with either LPS or oxidized LDL. The survival kinase, pAkt, was barely detectable in LRP1-/- cells, causing decreased pBad and increased cleaved caspase-3. Regardless of the apoptotic stimulation and degree of cell death, LRP1-/- macrophages displayed enhanced inflammation with increased IL-1β, IL-6, and TNFα expression. Efferocytosis of apoptotic macrophages was reduced by 60% in LRP1-/- versus WT macrophages despite increased apoE expression by both LRP1-/- phagocytes and WT apoptotic cells. Compared to WT macrophage lesions, LRP1-/- lesions had 5.7-fold more necrotic core with more dead cells not associated with macrophages.
Conclusion
Macrophage LRP1 deficiency increases cell death and inflammation by impairing pAkt activation and efferocytosis. Increased apoE expression in LRP1-/- macrophages suggests that the LRP1/apoE axis regulates the balance between apoptosis and efferocytosis thereby preventing necrotic core formation.
Keywords: LRP1, apoptosis, efferocytosis, apolipoprotein E, necrosis, inflammation
LRP1 is a ubiquitous multifunctional receptor, and its systemic expression is essential for embryonic development1. LRP1 is critical for the clearance of plasma remnants, as conditional hepatic LRP1 deletion results in increased plasma triglyceride and chylomicron levels2. We have shown that deletion of macrophage LRP1 increases atherosclerotic lesion formation in the proximal aorta of LDLR-/- mice without affecting serum lipoprotein levels and despite decreased macrophage uptake of apoE-containing VLDL3. The enhanced atherosclerotic lesion formation was associated with increased formation of breaks in the elastic lamina, activated inflammation, and increased macrophage cellularity in the plaque3.
Macrophages in atherosclerotic lesions undergo apoptosis and necrosis due to cholesterol toxicity, oxidative stress, and signaling from cytokines and other molecules4-6. A determining factor in lesion formation, remodeling, and progression is the balance between the generation of apoptotic cells and their phagocytosis (efferocytosis), as non-internalized apoptotic cells secrete inflammatory cytokines, thus driving uncontrolled cell death and inducing plaque instability7. LRP1 has been linked to the efferocytosis of apoptotic cells by both macrophages and nonprofessional phagocytes through co-localization with ABCA7 and activation of extracellular signal-regulated kinases (ERK)8, 9. However, it remains to be determined whether macrophage LRP1 plays a significant role in efferocytosis of lesional apoptotic macrophages. Indeed, studies have shown that the Mertk receptor, not LRP14, mediates the efferocytosis of macrophages made apoptotic by free cholesterol burden, whereas other studies suggest that the Mertk receptor plays a significant role in efferocytosis of lesional apoptotic macrophages10. Besides mediating efferocytosis, it is possible that LRP1 regulates macrophage susceptibility to apoptosis. Studies with cell types including fibroblasts and neurons have demonstrated that blockage of LRP1/ligand interaction via receptor-associated protein (RAP) enhances cell death under serum-free conditions11, 12. In addition, studies have shown that incubation of macrophages with antibodies directed against the ligand binding domain of LRP1 results in increased diacylglycerol, cAMP, and intracellular calcium mobilization13, 14 raising the possibility that LRP1 signaling regulates levels of phosphorylated Akt (pAkt)15, 16, a critical player in promoting macrophage survival15, 17, 18.
Macrophage apolipoprotein E (apoE) is a ligand for LRP1 and a major determinant of atherosclerosis susceptibility19-21 by regulating cholesterol trafficking22, and reducing oxidative stress23. Besides these atheroprotective functions, studies suggest that apoE may reduce inflammation by mediating efficient efferocytosis of apoptotic cells24. Compared to wild-type (WT) mice, apoE-/- mice show increased numbers of apoptotic cells and enhanced inflammatory responses in different tissues24. Furthermore, in vitro studies have demonstrated that the efferocytosis of apoE-/- apoptotic cells is impaired24. Consistent with the notion that apoE plays a role in efferocytosis, the synthesis of apoE is markedly enhanced in macrophages undergoing apoptosis,25, 26 perhaps as a mechanism to increase recognition and internalization by neighboring phagocytes through LRP1. In addition, studies have shown that apoE is linked to cell survival in other cell types including ovarian27 and neuronal cells.28 In neurons, apoE signaling reduces susceptibility to apoptosis via interaction with a number of receptors including apoE receptor 229, LRP430, and LRP128.
Our previous studies have demonstrated that deletion of macrophage LRP1 increases atherosclerosis, plaque instability, inflammation, and macrophage cellularity3. Because apoE is a ligand for LRP1, we postulated that a functional axis exists between macrophage apoE and LRP1 to minimize inflammation and uncontrolled cell death by maintaining an optimal balance between macrophage survival and efferocytosis of apoptotic cells. To study the effects of macrophage LRP1 on cellular apoptosis, we used a macrophage specific LRP1-/- mouse we developed using a Cre/lox-based approach3, 31. Through assessment of annexin V binding of membrane-exposed phosphatidylserine (PS), TUNEL staining, and analyses of activated caspase 3, we show that deletion of LRP1 increases cellular apoptosis with and without exogenous stimulation of cell death and in the absence or presence of cholesterol overload. The enhanced induction of apoptosis was associated with enhanced inflammation. Furthermore, our studies demonstrate that deletion of macrophage LRP1 impairs efferocytosis of apoptotic cells and increases susceptibility to apoptosis by suppressing the pAkt pathway. All events occurred under conditions of enhanced apoE expression, thus suggesting that the anti-inflammatory, pro-survival, and efferocytosis effects mediated by apoE depend on its interaction with LRP1. More importantly, we show that macrophage LRP1 deficient lesions have excessive accumulation of TUNEL positive cells, more dead cells not associated with macrophages, and markedly increased necrotic core formation compared to lesions containing WT macrophages. Taken together, our studies demonstrate an atheroprotective role for the LRP1/apoE axis in preventing necrotic core formation by regulating the balance between apoptosis susceptibility and efferocytosis.
Materials and Methods
A detailed description of all methods is available in the supplemental materials (available online at http://atvb.ahajournals.org).
In vivo and In vitro Analysis of Macrophage Apoptosis
For in vivo analysis, cells taken directly from the peritoneal cavity were subjected to flow cytometry analysis of AnnexinV/7aaD (BD Biosciences) and CD11b. For in vitro analysis, WT and LRP1-/- peritoneal macrophages were incubated for 24h in DMEM alone or containing either lipopolysaccharide (LPS, 50ng/ml) or copper oxidized LDL (50μg protein/ml). Cell death was determined by TUNEL (Roche). Activated caspase 3 was done using NucView Caspase Detection kit (Biotium).
In Vitro Measurement of the Efferocytosis of Apoptotic Macrophages
WT, LRP1-/-, or apoE-/- peritoneal macrophages were labeled with carboxy-fluorescein diacetate, succinimidyl ester (CFDA-SE, Molecular Probes) cell tracer and made apoptotic by incubation with either staurosporine (5μg/mL) for 24h or BAY11-7082 (20μM) for 2h. Apoptotic cells were then incubated for 2h with fresh phagocyte. After vigorous washing with PBS, the phagocytes were fixed in 4% paraformaldehyde, counterstained with DAPI, and efferocytosis of apoptotic cells was visualized using fluorescence microscopy.
Analysis of Efferocytosis in the Peritoneal Cavity
CFDA SE Cell Tracer Green-labeled WT macrophages were made apoptotic by the addition of 20μM Bay 11-7082 in serum-free DMEM for 2h. Then, 20×106 cells in 1ml of PBS were injected into WT or MΦLRP-/- mice that had been injected two days prior with 1ml of 3.0% thioglycollate. One hour later the peritoneal cells were harvested, and viable cells were labeled by incubation with nonfluorescent C12-resazurin which metabolizes to red fluorescent C12-resorufin (Invitrogen). The macrophage phagocytes were then labeled using rat anti-mouse CD68-biotin antibody (Serotec) and streptavidin-Alexa Fluor 647 conjugate. Flow cytometry was then performed on a 5-laser BD LSRII using FACSDiva 6.0 software (BD Biosciences). Cells positive for CFDA SE + CD68 + C12-resorufin versus CD68 + C12-resorufin only were considered to be phagocytes positive for uptake of apoptotic cells.
Analysis of Atherosclerotic Lesion Apoptosis, Efferocytosis, and Necrosis
Recipient LDLR-/- mice (female, 6-weeks old) were lethally irradiated and transplanted with bone marrow cells (BM) from female WT or MΦLRP-/- mice. Four weeks later, the mice were placed on a western-type diet for 16 weeks. Apoptotic cells were detected in five-micron proximal aortic cryosections by TUNEL using the in situ cell death detection kit, TMR red (Roche). Nuclei were counterstained with Hoechst, and images of 5 serial sections from each mouse were taken using fluorescence microscopy. The efferocytosis in lesions following the procedure as described by Schrijvers et al32 and as modified by Thorp and colleagues10. The same sections that were stained with TUNEL and Hoechst were stained for macrophages using a rabbit antimacrophage antibody (AIA31240, Accurate Chemical and Scientific Corp.) goat anti-rabbit biotinylated conjugated secondary antibody, and Alexa Fluor 488 (Molecular Probes, Inc.). The free versus macrophage associated apoptotic cells or bodies were then counted. Lesion necrosis was detected by staining with Harris's hematoxylin and eosin (H&E), and quantitated by measuring the H&E negative acellular area in the intima versus total intimal area.
Macrophage Survival and Apoptotic Proteins
Total Akt, phosphorylated Akt (pAkt), and phosphorylated Bad (pBad) were detected by western blot using rabbit polyclonal antibodies to Akt, pAkt(serine 473), and pBad(serine 136) (Cell Signaling Technology, Inc.). Protein signal was detected using goat anti-rabbit IgG secondary antibody conjugated to horseradish peroxidase and the ECL plus chemiluminescence kit.
ApoE Secretion and Immunocytochemistry
Medium apoE was detected by western blot using rabbit anti-serum against mouse apoE and goat anti-rabbit horseradish peroxidase conjugated IgG. Immunocytochemistry of cell apoE was done using rabbit anti-serum against mouse apoE and FITC-conjugated goat anti-rabbit IgG. Cells were counterstained using Vectashield with DAPI to visualize nuclei (Vector Labs).
Statistical Analysis
In vitro data are expressed as mean ± standard deviation of triplicate determinations. In vivo data are expressed as mean ± SEM. Differences between two mean values were determined by two-tailed Student's t-test, one-way ANOVA (Bonferroni's post test), and Mann-Whitney test. p<0.05 was considered to be significant.
Results
Effects of macrophage LRP1 deletion on apoE synthesis and secretion
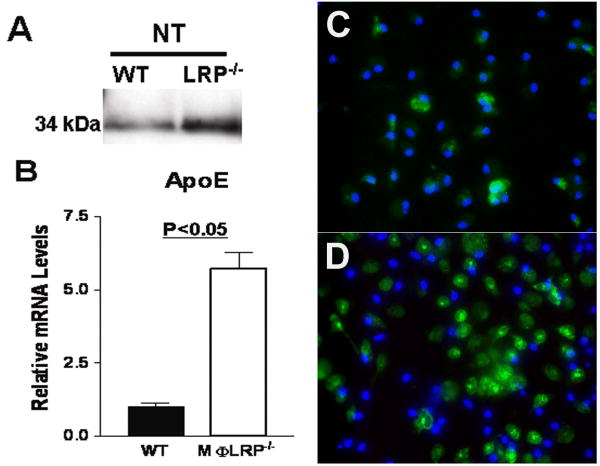
As apoE is a ligand for LRP1 and may function in inflammatory signaling, regulation of efferocytosis, and apoptosis susceptibility, we first examined the effects of LRP1 deletion on macrophage apoE synthesis and secretion. Western blot of 24h conditioned media from unstimulated LRP1-/- macrophages (serum-free DMEM) showed a 3-fold increase in apoE accumulation compared to WT macrophages (Figure 1A). Because LRP1 is an internalizing apoE receptor, the accumulation of apoE could simply represent inefficient local clearance. However, our findings that apoE mRNA levels increased by 5-fold in the absence of LRP1 actually suggests the presence of a counter-regulatory loop (Figure 1B). Furthermore, immunohistochemical analyses showed that intracellular apoE was also increased in LRP1-/- macrophages after 24h incubation in DMEM alone (Figure 1C).
Figure 1.
Detection of apoE protein in the medium (A), apoE mRNA levels in cells (B), and immunohistochemistry for apoE (green) and nuclei (DAPI, blue) in WT (C) or LRP1-/- (D) macrophages after 24h in serum-free DMEM. *p<0.05, Student's t test.
Deletion of LRP1 increases macrophage death and inflammation
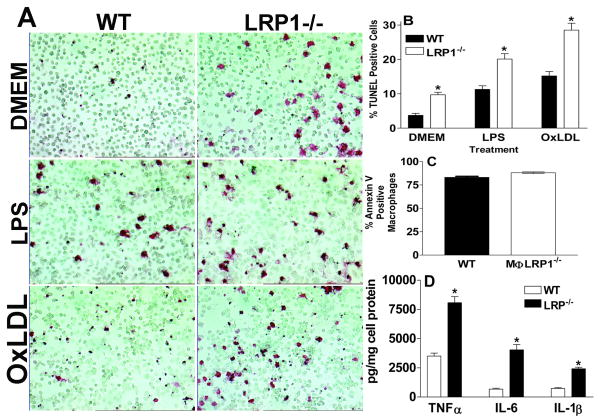
To identify the influence of LRP1 deletion on apoptosis susceptibility in vitro, WT or LRP1-/- macrophages were exposed to three apoptotic stimuli (nutrient deprivation with serum-free DMEM, inflammatory LPS, and cholesterol burden with oxidized LDL) and cell death was determined by TUNEL staining (Figures 2A and 2B). After 24h of incubation in serum-free DMEM the number of TUNEL positive cells was 2.6-fold greater in cultures of LRP1-/- versus WT macrophages. In agreement with studies demonstrating that LPS enhances apoptosis of WT macrophages 33, 34, stimulation for 24h with LPS versus DMEM alone resulted in increased numbers of TUNEL positive cells in cultures of both WT and LRP1-/- cells. However, positivity for TUNEL staining was 1.8-fold more in LRP1-/- macrophages compared to WT cells (Figures 2A and 2B). Similarly, incubation with oxidized LDL increased the TUNEL staining of both cell types, but the number of TUNEL positive cells was 1.9-fold more in LRP1-/- macrophages. Under pro-apoptotic conditions, the inflammatory status of the LRP1-/- macrophages was heightened compared to WT cells. Incubation with serum-free DMEM resulted in 6.1- and 2.3-fold higher IL-1β and IL-6 mRNA levels in LRP1-/- macrophages compared to WT cells (Supplemental Figure I). Whereas incubation with LPS increased the IL-1β and IL-6 mRNA levels in both cell types, this effect was enhanced in LRP1-/- macrophages (by 3- and 1.7-fold, respectively) compared to WT cells (Supplemental Figure I). Similar to our previous results, 3 TNFα, iNOS, and MMP-9 mRNA levels were also increased in LRP1-/- macrophages with and without stimulation with LPS (data not shown). To investigate the relationship between activated apoptosis and inflammation in LRP1 deficiency, we next examined the inflammatory response under conditions that induced similar numbers of apoptotic cells between the two cell types. To accomplish this goal, the cells were incubated with the non-specific protein kinase inhibitor, staurosporine, which stimulates a high degree apoptosis in macrophage cultures35. At a dose of 5μg/ml, staurosporine inhibits a broad range of cell survival kinases including PKC and PKA36, and prevents the activation of Akt37. Staurosporine treatment led to similar levels of apoptotic macrophages in cultures of WT and LRP1-/- cells, as determined by annexin V binding (Figure 2C). Despite similar numbers of apoptotic cells under these conditions, LRP1-/- secreted 2-, 6- and 3-fold more TNFα, IL-6, and IL-1β, respectively compared to WT macrophages (Figure 2D).
Figure 2.
TUNEL positive macrophages (A and B) after 24h in DMEM alone or with LPS (50ng/ml) or oxidized LDL (50μg/ml). Annexin V positive cells (C) and medium cytokine levels (D) after 24h in DMEM with staurosporine (50μg/ml). *p<0.05 between the two groups, Student's t test (A-D). Data represent 2 (C-D) or 3 (A-B) experiments.
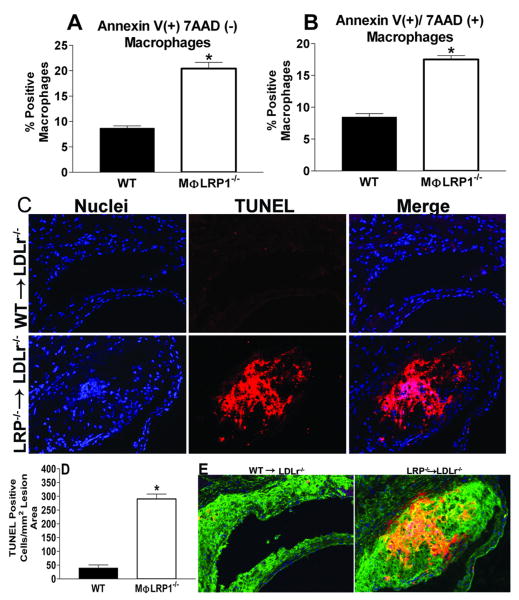
We next confirmed that LRP1 deletion increased macrophage apoptosis in vivo. First, we performed flow cytometry to examine annexin V binding to membrane phosphatidylserine (PS) and 7aaD binding to exposed cellular DNA in macrophages (CD11b+ cells) from the peritoneal lavage cells of WT (n=3) and MΦLRP1-/- (n=3) mice 4 days after stimulation with thioglycollate (Figures 3A and 3B). The deletion of macrophage LRP1 induced a 2.3-fold increase in apoptosis (annexin V positive cells (Figure 3A), and a doubling of nonviable macrophages (annexin V and 7aaD positive cells; Figure 3B). We also examined the effects of macrophage LRP1 deletion on the accumulation of TUNEL positive cells in atherosclerotic lesions in WT and MΦLRP1-/- BM recipient LDLr-/- mice fed a western diet for 16 weeks (Figures 3C and 3D). The lesions of MΦLRP1-/- (n=4) recipient LDLr-/- mice contained 7.3-fold more TUNEL positive cells compared to mice transplanted with WT (n=5) BM (Figures 3C and 3D). In the lesions of both WT and MΦLRP1-/- recipient LDLr-/- mice, the TUNEL positive cells were localized to macrophage enriched areas (Figure 3E).
Figure 3.
Peritoneal macrophages positive for annexinV alone(A) or annexinV and 7AAD (B). TUNEL analyses of aortic sections from WT (n=5) and MΦLRP1-/- (n=4) BM recipient LDLr-/- mice fed western diet for 16 weeks (C). Micrographs show nuclei (DAPI, blue), TUNEL positive staining (red) and merged images. Quantitation of TUNEL results (D) and co-localization of macrophage stain (green) in TUNEL-positive regions of arteries from mice with LRP1-/- BM (E). *p<0.05, Mann-Whitney test
Macrophage LRP1 deletion increases susceptibility to apoptosis by impairing the pAkt survival pathway
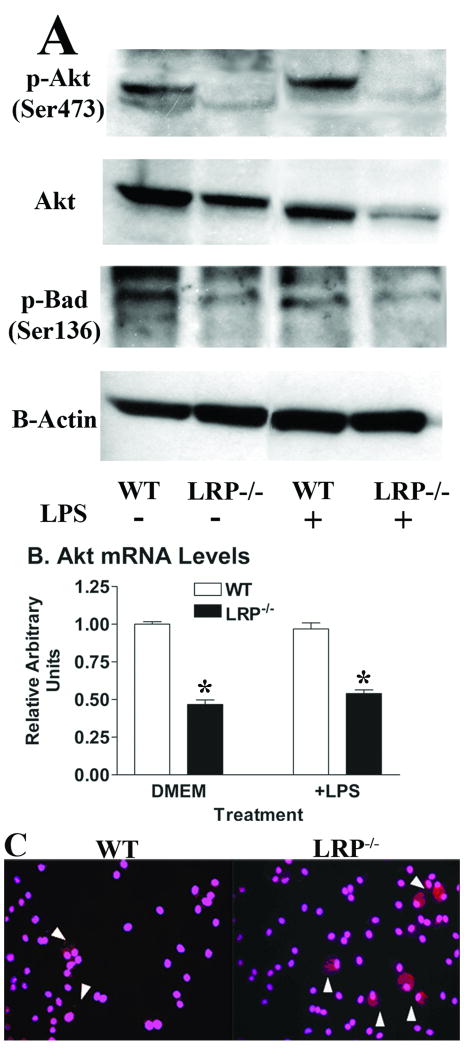
A number of studies including ours have demonstrated that the pAkt pathway is fundamental in reducing WT macrophage susceptibility to apoptosis15, 17, 18. As earlier studies demonstrated that antibody ligation to macrophage LRP1 increases intracellular calcium mobilization, cAMP, and diacylglycerol13, 14, we next determined whether deletion of macrophage LRP1 affects activation of Akt. Compared to WT macrophages, LRP1-/- cells had markedly decreased levels of pAkt(serine 473) when incubated with either serum-free DMEM alone or with LPS (Figure 4A). Similar differences were observed with pAkt(threonine 308) levels (data not shown). Interestingly, total Akt levels were also decreased in LRP1-/- macrophages versus WT cells in the presence of DMEM alone or with LPS (Figure 4A). This effect was likely due to decreased levels of Akt1 mRNA (Figure 4B). One mechanism by which pAkt prevents apoptosis is by phosphorylating Bad38. When Bad is phosphorylated, it loses its ability to complex with both Bcl-2 and Bcl-xL, thus allowing these two antiapoptotic proteins to prevent the activation of caspase 9 and caspase 338, 39. In keeping with the decreased pAkt, levels of pBad(serine 136) were decreased in LRP1-/- macrophages treated with either DMEM alone or with LPS when compared to WT cells (Figure 4A). Consistent with the changes in pAkt and pBad(serine 136), immunohistochemical analyses showed increased activated caspase 3 by LRP1-/- macrophages, even in the absence of exogenous cell death stimulation (Figure 4C). Thus, suppression of the pAkt survival pathway likely contributes to the increased numbers of apoptotic cells in cultures of LRP1-/- macrophages (Figure 2) and in lesions of MΦLRP1-/- recipient LDLr-/- mice (Figure 3D).
Figure 4.
(A) Western blot of cell pAkt(serine 473), total Akt, pBad(serine 136), and β-actin and (B) Akt1 mRNA levels in WT or LRP1-/- macrophages after 24h in DMEM alone or with LPS (50ng/ml). (C) Immunohistochemical detection of activated caspase-3 (red) after 24h in DMEM. DAPI (pink) staining identifies nuclei. *p<0.05, Student's t test (B) and all data represent 2 experiments.
Effects of macrophage LRP1 deletion on efferocytosis of apoptotic cells
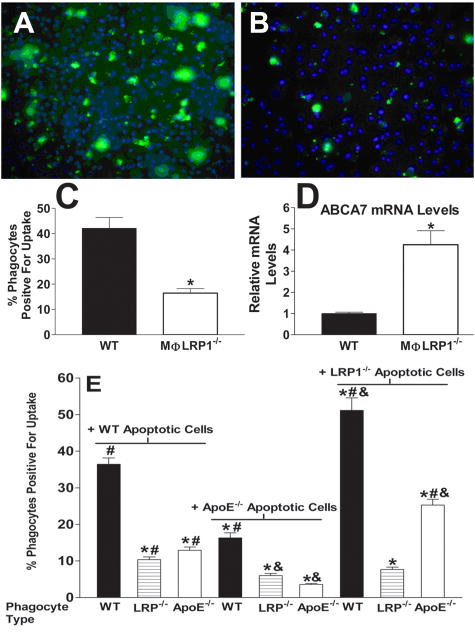
As LRP1 has been suggested to play a role in the uptake of apoptotic cells, we next determined whether deletion of macrophage LRP1 reduced the uptake of apoptotic WT macrophages. To examine this possibility, CFDA-SE labeled WT cells were made apoptotic by incubation with staurosporine and then added to WT or LRP1-/- macrophages (Figures 5A and 5B). Eighty-three percent of the WT cells treated with staurosporine were apoptotic as determined by annexin V binding. Deletion of macrophage LRP1 decreased the uptake of WT apoptotic cells by 60% (Figure 5C) suggesting that the increased numbers of apoptotic/nonviable cells in LRP1-/- cultures (Figure 2) results in part from impaired efferocytosis. Because ABCA7 is linked to efficient phagocytosis of apoptotic bodies via LRP1 signaling8 and interacts with the amphipathic helical apoproteins40, we also determined the effects of LRP1 deletion on ABCA7 synthesis. Deletion of macrophage LRP1 resulted in a >4-fold increase in ABCA7 mRNA synthesis compared to WT cells (Figure 5D) suggesting that the lack of efferocytosis via LRP1 results in compensatory upregulation of its partner, ABCA7.
Figure 5.
Efferocytosis of apoptotic (CFDA-SE/green label) WT cells by WT (A) or LRP1-/- (B) macrophages. DAPI (blue) staining identifies nuclei. (C) CFDA-SE positive phagocytes as percentage of total macrophages. (D) ABCA7 mRNA after 24h in DMEM. *p<0.05, Student's t test. (E) Phagocytosis of CFDA-SE labeled WT, apoE-/-, or LRP1-/- apoptotic macrophages by WT, apoE-/-, or LRP1-/- efferocytes. Differences (p<0.05) were marked as follows: * versus the WT/WT condition; # versus the apoE-/-/apoE-/- condition; & versus the apoE-/-/WT condition. ANOVA with Bonferroni's post test. Data represent two (D, E) or four (C) experiments.
Interestingly, the impaired efferocytosis occurs under conditions of a marked increase in apoE secretion by LRP1-/- macrophages (Figure 1A) and despite a 9-fold increase in apoE synthesis in WT cells made apoptotic by treatment with staurosporine (data not shown). This suggests that apoE depends on LRP1 for its effects on efferocytosis. To more directly examine a role for the LRP1/apoE in efferocytosis, we compared the phagocytosis of WT, apoE-/-, and LRP1-/- apoptotic macrophages by WT, apoE-/-, and LRP1-/- efferocytes (Figure 5E). Compared to the uptake by WT phagocytes, phagocytosis of WT apoptotic cells by LRP1-/- phagocytes was decreased by 64% and that by apoE-/- phagocytes was decreased by 70%. These data demonstrate that phagocyte-derived apoE facilitates phagocytosis, and is consistent with studies suggesting that LRP1 interaction with cell surface apoE is enhanced via an endogenous secretion capture mechanism41. Compared to the phagocytosis of WT apoptotic cells, the uptake of apoE-/- apoptotic macrophages by WT phagocytes was decreased by 56%, demonstrating that apoptotic cell apoE is also important in efferocytosis. The uptake of apoE-/- apoptotic cells by LRP1-/- phagocytes was also decreased but was not significantly different compared to uptake of WT apoptotic cells. The complete absence of apoE caused the largest reduction in efferocytosis, which was significantly different compared to uptake of WT apoptotic cells by LRP1-/- suggesting that other receptors besides LRP1 contribute in small part to apoE-mediated efferocytosis. Consistent with a critical role for the LRP1/apoE axis, the phagocytosis of LRP1-/- apoptotic macrophages, which express more apoE than WT cells, was significantly enhanced in both WT and apoE-/- phagocytes but not in LRP1-/- efferocytes (Figure 5E).
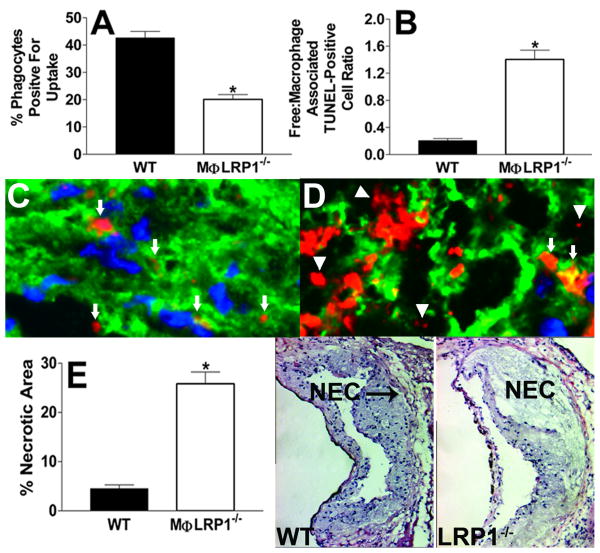
Deletion of macrophage LRP1 impairs efferocytosis and promotes necrosis in vivo
We examined the in vivo phagocytosis of WT apoptotic cells by peritoneal macrophages of both WT (n=4) and MΦLRP1-/- (n=5) mice (Figure 6A). Compared to the efferocytosis of WT apoptotic macrophages by peritoneal phagocytes in WT mice, the phagocytosis in MΦLRP1-/- mice was decreased by 53%. We next determined whether lesional phagocytosis is defective in MΦLRP1-/- (n=4) versus WT (n=5) BM recipient LDLr-/- mice fed a western diet for 16 weeks by following established procedures by Schrijvers et al32 as modified by Thorp and colleagues10. The number of TUNEL positive cells that were associated with viable macrophages versus the free TUNEL positive cells (Figures 6C and 6D) was quantitated (Figure 6B). As depicted in the images (Figures 6C and 6D), apoptotic cells or bodies were counted as free when they were not associated with or found in close proximity to viable macrophages (detected as Alexa Fluor 488 stained macrophage cytoplasm surrounding a Hoeschst-stained nucleus). Apoptotic cells or bodies that were associated with macrophage cytoplasmic debris, but not in contact or close proximity with viable macrophages were counted as free. Compared to lesions in WT BM recipient LDLr-/- mice, the ratio of free to macrophage associated TUNEL positive cells was 6.8-fold greater in lesions of MΦLRP1-/- recipient mice (Figure 6B). Consistent with the defective lesional efferocytosis, the percent necrotic area was 5.7-fold greater in lesions of MΦLRP1-/- (n=4) versus WT (n=5) BM recipient LDLr-/- mice (Figure 6E).
Figure 6.
Percent of macrophages positive for efferocytosis of apoptotic CFDA-SE/labeled WT cells (A) 1h post injection into the peritoneal cavity of WT (n=3) or MΦLRP1-/- (n=4) mice as determined by flow cytometry. Quantitation of the ratio of free versus macrophage associated TUNEL positive cells (B) in aortic sections from WT (n=5) and MΦLRP1-/- (n=4) BM recipient LDLr-/- mice fed western diet for 16 weeks. (C, D) TUNEL positive cells in aortic sections from WT (C) or MΦLRP1-/- (D) BM recipient LDLr-/- mice. Cells are either free (triangles) or associated with viable macrophages (arrows). (E) Quantitation of necrotic area in aortic sections from WT (n=5) and MΦLRP1-/- (n=4) BM recipient LDLr-/- mice. Images show hematoxylin and eosin staining of aortic root sections. NEC denotes necrotic area (E). *p<0.05, Mann Whitney test.
Discussion
Our previous studies demonstrated that macrophage LRP1 expression paradoxically slows the development of atherosclerosis even though it increases uptake of remnant lipoproteins3. The enhanced atherosclerotic lesion formation that occurs with macrophage LRP1 deletion is associated with disruption of the elastic lamina, heightened inflammation, and increased macrophage cellularity3. An imbalance between the rate of apoptosis and efferocytosis of dying cells by macrophages accelerates atherosclerosis by promoting post-apoptotic necrosis, inflammation, and plaque instability7. The present studies show that deletion of macrophage LRP1 increases cell death in vitro by impairing efferocytosis and by increasing susceptibility to apoptosis via suppression of Akt activation. More importantly, a critical in vivo role for LRP1 is demonstrated by the findings that deletion of macrophage LRP1 promoted marked increases in lesional TUNEL positive cells, free apoptotic cells, and necrotic area. In addition to causing an imbalance between efferocytosis and apoptosis susceptibility, absence of LRP1 induced inflammation and increased apoE secretion. The increased apoE secretion by LRP1-/- macrophages is apparently at odds with the finding of increased atherosclerosis that occurs with macrophage LRP1 deficiency, as increased expression of macrophage apoE in the vessel wall has consistently been linked to reduced atherogenesis in the mouse19-21. This suggests that some or most of the beneficial effects of apoE in the artery wall involve interaction with LRP1. The current studies provide evidence that atheroprotective functions of the LRP1/apoE axis include regulation of macrophage inflammation, apoptosis susceptibility, and efferocytosis.
Role of the LRP1/apoE axis in efferocytosis
The present studies demonstrate that deletion of macrophage LRP causes marked reductions in efferocytosis of apoptotic cells in cultures of macrophages (Figure 5C) and in vivo in both the peritoneal cavity (Figure 6A) and atherosclerotic lesions (Figure 6B), suggesting that the impaired efferocytosis by LRP1-/- macrophages contributes to the increased inflammation (Figure 2D and Supplemental Figure 1) 3 and apoptosis seen in culture (Figures 2A and 2B) and in atherosclerotic lesions (Figures 3C and 3D). It is also likely that defective efferocytosis contributes to the enhanced plaque necrosis (Figure 6E) and progression seen in vivo in the setting of macrophage LRP1 deficiency3. The observation of impaired efferocytosis by LRP1-/- macrophages is consistent with other studies demonstrating decreased uptake of apoptotic cells by LRP1-/- non-phagocytic cells (i.e. fibroblasts)9. However, other studies have suggested that macrophage Mertk receptor, and not LRP1, is required to mediate the phagocytosis of WT macrophages made apoptotic by free cholesterol burden4. In this regard, our findings that macrophage LRP1 deletion promotes lesional apoptotic cell accumulation and necrosis demonstrates a critical role for LRP1 in the efferocytosis of macrophages made apoptotic by stimuli that normally occur in atherosclerotic lesions.
Studies have shown that ABCA7 binds to amphipathic helical apoproteins (apoE, apoAI)40. In macrophages, ABCA7 translocates to the cell membrane and co-localizes with LRP1 to optimize signaling via LRP1 and the efferocytosis of apoptotic cells8. Our demonstration that LRP1-/- macrophages upregulate ABCA7 mRNA synthesis by >4-fold (Figure 5D), suggests that impaired phagocytosis due to loss of LRP1 results in compensatory upregulation of its partner, ABCA7, and that the function of ABCA7 in efferocytosis is specific to LRP1.
Studies suggest that apoE plays a role in mediating efficient efferocytosis of apoptotic cells. ApoE-/- mice show increased numbers of apoptotic cells in different tissues as well as increased levels of inflammatory cytokines. Also, efferocytosis of apoE-/- apoptotic cells is impaired24. Furthermore, apoE avidly binds PS42, and the synthesis of apoE is markedly enhanced in macrophages undergoing apoptosis25, 26. The present studies show that the LRP1-/- macrophages have markedly impaired efferocytosis despite increased apoE expression, strongly suggesting that apoE largely depends on interaction with LRP1 for its role in efferocytosis. Although we cannot exclude the possibility that apoE interacts with other receptors on lesion phagocytes besides LRP1, or that other ligands interact with LRP1, we propose that the LRP1/apoE axis is a main mechanism for regulation of efferocytosis and therefore, plaque cell integrity, necrosis, and inflammation. Consistent with this possibility the uptake of LRP1-/- apoptotic macrophages, which express more apoE than WT cells, was enhanced in both WT and apoE-/- macrophages, but not in LRP1-/- phagocytes (Figure 5E). It can also be postulated that the endogenous synthesis of apoE in the lesion, where plasma apoE has limited access43, provides a means for optimal cooperation with LRP1 on neighboring phagocytes for efficient efferocytosis.
Role of the LRP1/apoE axis in Akt Activation
Our studies demonstrate that deletion of macrophage LRP1 suppresses activation of Akt resulting in decreased phosphorylation of Bad and increased caspase 3 activation (Figure 4). Thus, suppression of the pAkt pathway likely contributes to the increased cell death in LRP1-/- macrophage cultures and in LRP1-/- lesions which is substantiated by studies showing that Akt activation is a critical determinant of macrophage survival15, 17, 18. The impairment in Akt activation is consistent with studies showing that macrophage LRP1 signaling increases intracellular cAMP, diacylglycerol, and calcium mobilization, which are important second messengers leading to Akt phosphorylation15, 16. Furthermore, recent studies demonstrated that knockdown of LRP1 in Schwann cells decreases pAkt levels44. Decreased synthesis of Akt1 mRNA contributed to the reduction in Akt activation in LRP1-/- macrophages (Figure 4), suggesting that the multiple downstream effects of LRP1 signaling also regulate transcription of Akt1. A critical determinant of Akt1 transcription in macrophages is the transcription factor CREB which is activated downstream from cAMP production45. It is of interest to note that consistent with LRP signaling stimulating cAMP production, recent studies demonstrated that antibodies to LRP increased the levels of pCREB in neuronal cells46. Like LRP1, apoE has been implicated in cell survival. ApoE stimulates activation of Akt in neuronal cells in a calcium and cAMP dependent manner28. In addition, apoE interaction with macrophages increases intracellular calcium mobilization and diacylglycerol production. Interestingly, this effect is inhibited by RAP14. Thus, it is likely that interaction of endogenous apoE with macrophage LRP1 promotes activation of Akt and reduces apoptosis susceptibility. Although a number of LDL receptor family members have been implicated in the survival effects of apoE on neuronal cells28-30, our studies suggest that in macrophages, the pro-survival effects of apoE depend on interaction with LRP1.
Role of the LRP1/apoE axis in macrophage inflammation
Studies show that macrophage apoptosis due to either oxidative stress47 or free cholesterol stimulation48 is associated with increased inflammation. Our results show that heightened inflammation occurs in apoptotic LRP1-/- macrophages even without exogenous stimulation or cholesterol burden (Supplemental Figures 1A, 1B and 3, 49). Furthermore, even when comparable numbers of apoptotic cells are induced in cultures of WT versus LRP1-/- macrophages by incubation with a non-specific protein kinase inhibitor (Figure 2C), the LRP1-/- macrophages exhibit markedly increased secretion of inflammatory cytokines compared to WT cells (Figure 2D), thus demonstrating that regardless of either the degree or mode of apoptosis, the deletion of LRP results in enhanced inflammation. This suggests that LRP1 is a critical player in controlling macrophage inflammation. Besides impaired efferocytosis, other mechanisms may mediate the LRP1 effects on inflammation. Addition of exogenous apoE50, 51 or expression of endogenous apoE52, 53 reduces inflammation in macrophages stimulated with LPS and IFNγ. Furthermore, our previous studies demonstrated that deletion of macrophage LRP increases NF-қβ activation49. Interestingly, the anti-inflammatory effects of apoE involve decreased NF-қβ activation during LPS stimulation of macrophages52.
It is also likely that the LRP1/apoE interaction regulates inflammation by impairing the tumor necrosis factor receptor-1 (TNFR1) pathway, as we previously showed that deletion of LRP1 results in increased cell surface TNFR1, which binds TNFα and causes enhanced NF-қβ activation and inflammation 49. As NF-қβ activation can also promote cell survival54, the enhanced NF-қβ activation that occurs with LRP1 deficiency is somewhat at odds with the simultaneous increase in cell death. However, in some cell types, NF-қβ activation can promote either cell survival or apoptosis via selective gene regulation depending upon the pathway of stimulation (i.e. growth factor versus etoposide)55, 56. Thus, it is plausible that NF-қβ activation via the TNFα/TNFR1 pathway is proapoptotic and contributes to the enhanced cell death in LRP1-/- macrophages. Consistent with this possibility are studies demonstrating that TNFα stimulates apoptosis via NO production6. In addition, we have obtained evidence that that the expression of pro-apoptotic death receptor-5 and its ligand TRAIL57, 58 is increased whereas that of anti-apoptotic Bcl-xL is decreased in LRP1-/- macrophages (Yancey et al., unpublished observations).
In summary, our studies demonstrate that deletion of macrophage LRP1 creates an imbalance between efferocytosis and apoptosis susceptibility resulting in enhanced inflammation, lesion cell death, and plaque necrosis. As these effects were accompanied by increased apoE secretion in LRP1-/- macrophages, a functional axis between these two proteins may play a unique and dominant role in atherogenesis.
Supplementary Material
Acknowledgments
Sources of Funding: This study was supported by grants from the NIH (HL57986 and HL65709 to SF, HL65405 and HL53989 to ML), and an American Heart Association Beginning Grant-in-Aid to PY. We also acknowledge the partial support from the Lipid, Lipoprotein, and Atherosclerosis Core of the Vanderbilt Mouse Metabolic Phenotyping Centers (NIH DK59637).
Footnotes
Disclosures: Sergio Fazio (≥$10 000), Patricia G. Yancey (≥$10 000), and MacRae F. Linton (≥$10 000) received research grant RO1 HL57986.
References
- 1.Herz J, Clouthier DE, Hammer RE. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 1992;71:411–421. doi: 10.1016/0092-8674(92)90511-a. [DOI] [PubMed] [Google Scholar]
- 2.Rohlmann A, Gotthardt M, Hammer RE, Herz J. Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. Journal of Clinical Investigation. 1998;101:689–695. doi: 10.1172/JCI1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overton CD, Yancey PG, Major AS, Linton MF, Fazio S. Deletion of Macrophage LDL Receptor-Related Protein Increases Atherogenesis in the Mouse. Circ Res. 2007;100:670–677. doi: 10.1161/01.RES.0000260204.40510.aa. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Gerbod-Giannone MC, Seitz H, Cui D, Thorp E, Tall AR, Matsushima GK, Tabas I. Cholesterol-induced apoptotic macrophages elicit an inflammatory response in phagocytes, which is partially attenuated by the Mer receptor. J Biol Chem. 2006;281:6707–6717. doi: 10.1074/jbc.M510579200. [DOI] [PubMed] [Google Scholar]
- 5.Boullier A, Li Y, Quehenberger O, Palinski W, Tabas I, Witztum JL, Miller YI. Minimally oxidized LDL offsets the apoptotic effects of extensively oxidized LDL and free cholesterol in macrophages. Arterioscler Thromb Vasc Biol. 2006;26:1169–1176. doi: 10.1161/01.ATV.0000210279.97308.9a. [DOI] [PubMed] [Google Scholar]
- 6.Choi KS, Song EK, Yim CY. Cytokines secreted by IL-2-activated lymphocytes induce endogenous nitric oxide synthesis and apoptosis in macrophages. J Leukoc Biol. 2008;83:1440–1450. doi: 10.1189/jlb.1007701. [DOI] [PubMed] [Google Scholar]
- 7.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 8.Jehle AW, Gardai SJ, Li S, Linsel-Nitschke P, Morimoto K, Janssen WJ, Vandivier RW, Wang N, Greenberg S, Dale BM, Qin C, Henson PM, Tall AR. ATP-binding cassette transporter A7 enhances phagocytosis of apoptotic cells and associated ERK signaling in macrophages. J Cell Biol. 2006;174:547–556. doi: 10.1083/jcb.200601030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe-/- mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi H, Campenot RB, Vance DE, Vance JE. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J Neurosci. 2007;27:1933–1941. doi: 10.1523/JNEUROSCI.5471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu K, Wu C, Mars WM, Liu Y. Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor-related protein 1-mediated integrin signaling. J Clin Invest. 2007;117:3821–3832. doi: 10.1172/JCI32301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misra UK, Gawdi G, Pizzo SV. Ligation of low-density lipoprotein receptor-related protein with antibodies elevates intracellular calcium and inositol 1,4, 5-trisphosphate in macrophages. Arch Biochem Biophys. 1999;372:238–247. doi: 10.1006/abbi.1999.1521. [DOI] [PubMed] [Google Scholar]
- 14.Misra UK, Adlakha CL, Gawdi G, McMillian MK, Pizzo SV, Laskowitz DT. Apolipoprotein E and mimetic peptide initiate a calcium-dependent signaling response in macrophages. J Leukoc Biol. 2001;70:677–683. [PubMed] [Google Scholar]
- 15.Misra UK, Pizzo SV. Coordinate regulation of forskolin-induced cellular proliferation in macrophages by protein kinase A/cAMP-response element-binding protein (CREB) and Epac1-Rap1 signaling: effects of silencing CREB gene expression on Akt activation. J Biol Chem. 2005;280:38276–38289. doi: 10.1074/jbc.M507332200. [DOI] [PubMed] [Google Scholar]
- 16.Rykx A, De Kimpe L, Mikhalap S, Vantus T, Seufferlein T, Vandenheede JR, Van Lint J. Protein kinase D: a family affair. FEBS Lett. 2003;546:81–86. doi: 10.1016/s0014-5793(03)00487-3. [DOI] [PubMed] [Google Scholar]
- 17.Babaev VR, Chew JD, Ding L, Davis S, Breyer MD, Breyer RM, Oates JA, Fazio S, Linton MF. Macrophage EP4 deficiency increases apoptosis and suppresses early atherosclerosis. Cell Metab. 2008;8:492–501. doi: 10.1016/j.cmet.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WC. Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab. 2007;6:446–457. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fazio S, Babaev V, Murray A, Hasty A, Carter K, Gleaves L, Atkinson J, Linton M. Increased atherosclerosis in C57BL/6 mice transplanted with apo E null bone marrow. Proc Natl Acad Sci USA. 1997;94:4647–4652. doi: 10.1073/pnas.94.9.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellosta S, Mahley RW, Sanan DA, Murata J, Newland DL, Taylor JM, Pitas RE. Macrophage-specific expression of human apolipoprotein E reduces atherosclerosis in hypercholesterolemic apolipoprotein E-null mice. J Clin Invest. 1995;96:2170–2179. doi: 10.1172/JCI118271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasty AH, Linton MF, Brandt SJ, Babaev VR, Gleaves LA, Fazio S. Retroviral gene therapy in ApoE-deficient mice: ApoE expression in the artery wall reduces early foam cell lesion formation. Circulation. 1999;99:2571–2576. doi: 10.1161/01.cir.99.19.2571. [DOI] [PubMed] [Google Scholar]
- 22.Yancey PG, Yu H, Linton MF, Fazio S. A Pathway-Dependent on ApoE, ApoAI, and ABCA1 Determines Formation of Buoyant High-Density Lipoprotein by Macrophage Foam Cells. Arterioscler Thromb Vasc Biol. 2007;27:1123–1131. doi: 10.1161/ATVBAHA.107.139592. [DOI] [PubMed] [Google Scholar]
- 23.Rosenblat M, Aviram M. Oxysterols-induced activation of macrophage NADPH oxidase enhances cell-mediated oxidation of LDL in the atherosclerotic apolipoprotein E deficient mouse: inhibitory role for vitamin E. Atherosclerosis. 2002;100:69–80. doi: 10.1016/s0021-9150(01)00563-9. [DOI] [PubMed] [Google Scholar]
- 24.Grainger DJ, Reckless J, McKilligin E. Apolipoprotein E modulates clearance of apoptotic bodies in vitro and in vivo, resulting in a systemic proinflammatory state in apolipoprotein E-deficient mice. J Immunol. 2004;173:6366–6375. doi: 10.4049/jimmunol.173.10.6366. [DOI] [PubMed] [Google Scholar]
- 25.Tedla N, Glaros E, Brunk U, Jessup W, Garner B. Heterogeneous expression of apolipoprotein-E by human macrophages. Immunology. 2004;113:338–347. doi: 10.1111/j.1365-2567.2004.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinn CM, Kagedal K, Terman A, Stroikin U, Brunk UT, Jessup W, Garner B. Induction of fibroblast apolipoprotein E expression during apoptosis, starvation-induced growth arrest and mitosis. Biochem J. 2004;378:753–761. doi: 10.1042/BJ20031352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YC, Pohl G, Wang TL, Morin PJ, Risberg B, Kristensen GB, Yu A, Davidson B, Shih Ie M. Apolipoprotein E is required for cell proliferation and survival in ovarian cancer. Cancer Res. 2005;65:331–337. [PubMed] [Google Scholar]
- 28.Laffont I, Takahashi M, Shibukawa Y, Honke K, Shuvaev VV, Siest G, Visvikis S, Taniguchi N. Apolipoprotein E activates Akt pathway in neuro-2a in an isoform-specific manner. Biochem Biophys Res Commun. 2002;292:83–87. doi: 10.1006/bbrc.2002.6586. [DOI] [PubMed] [Google Scholar]
- 29.Beffert U, Nematollah Farsian F, Masiulis I, Hammer RE, Yoon SO, Giehl KM, Herz J. ApoE receptor 2 controls neuronal survival in the adult brain. Curr Biol. 2006;16:2446–2452. doi: 10.1016/j.cub.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 30.Lu Y, Tian QB, Endo S, Suzuki T. A role for LRP4 in neuronal cell viability is related to apoE-binding. Brain Res. 2007;1177:19–28. doi: 10.1016/j.brainres.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 31.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 32.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 33.Chen YQ, Zhou YQ, Wang MH. Activation of the RON receptor tyrosine kinase protects murine macrophages from apoptotic death induced by bacterial lipopolysaccharide. J Leukoc Biol. 2002;71:359–366. [PubMed] [Google Scholar]
- 34.Comalada M, Xaus J, Valledor AF, Lopez-Lopez C, Pennington DJ, Celada A. PKC epsilon is involved in JNK activation that mediates LPS-induced TNF-alpha, which induces apoptosis in macrophages. Am J Physiol Cell Physiol. 2003;285:C1235–1245. doi: 10.1152/ajpcell.00228.2003. [DOI] [PubMed] [Google Scholar]
- 35.Namgaladze D, Kollas A, Brune B. Oxidized LDL attenuates apoptosis in monocytic cells by activating ERK signaling. J Lipid Res. 2008;49:58–65. doi: 10.1194/jlr.M700100-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Ruegg UT, Burgess GM. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci. 1989;10:218–220. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- 37.Sato S, Fujita N, Tsuruo T. Interference with PDK1-Akt survival signaling pathway by UCN-01 (7-hydroxystaurosporine) Oncogene. 2002;21:1727–1738. doi: 10.1038/sj.onc.1205225. [DOI] [PubMed] [Google Scholar]
- 38.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 39.Thorp E, Li Y, Bao L, Yao PM, Kuriakose G, Rong J, Fisher EA, Tabas I. Brief report: increased apoptosis in advanced atherosclerotic lesions of Apoe-/- mice lacking macrophage Bcl-2. Arterioscler Thromb Vasc Biol. 2009;29:169–172. doi: 10.1161/ATVBAHA.108.176495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang N, Lan D, Gerbod-Giannone M, Linsel-Nitschke P, Jehle AW, Chen W, Martinez LO, Tall AR. ATP-binding cassette transporter A7 (ABCA7) binds apolipoprotein A-I and mediates cellular phospholipid but not cholesterol efflux. J Biol Chem. 2003;278:42906–42912. doi: 10.1074/jbc.M307831200. [DOI] [PubMed] [Google Scholar]
- 41.Linton M, Hasty A, Babaev V, Fazio S. Hepatic apoE expression is required for remnant lipoprotein clearance in the absence of the low density lipoprotein receptor. J Clin Invest. 1998;101:1726–1736. doi: 10.1172/JCI2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee G, Pollard HB, Arispe N. Annexin 5 and apolipoprotein E2 protect against Alzheimer's amyloid-beta-peptide cytotoxicity by competitive inhibition at a common phosphatidylserine interaction site. Peptides. 2002;23:1249–1263. doi: 10.1016/s0196-9781(02)00060-8. [DOI] [PubMed] [Google Scholar]
- 43.Maiti SN, Balasubramanian K, Ramoth JA, Schroit AJ. Beta-2-glycoprotein 1-dependent macrophage uptake of apoptotic cells. Binding to lipoprotein receptor-related protein receptor family members. J Biol Chem. 2008;283:3761–3766. doi: 10.1074/jbc.M704990200. [DOI] [PubMed] [Google Scholar]
- 44.Campana WM, Li X, Dragojlovic N, Janes J, Gaultier A, Gonias SL. The low-density lipoprotein receptor-related protein is a pro-survival receptor in Schwann cells: possible implications in peripheral nerve injury. J Neurosci. 2006;26:11197–11207. doi: 10.1523/JNEUROSCI.2709-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Misra UK, Pizzo SV. Upregulation of AKT1 protein expression in forskolin-stimulated macrophage: evidence from ChIP analysis that CREB binds to and activates the AKT1 promoter. J Cell Biochem. 2007;100:1022–1033. doi: 10.1002/jcb.21086. [DOI] [PubMed] [Google Scholar]
- 46.Qiu Z, Hyman BT, Rebeck GW. Apolipoprotein E receptors mediate neurite outgrowth through activation of p44/42 mitogen-activated protein kinase in primary neurons. J Biol Chem. 2004;279:34948–34956. doi: 10.1074/jbc.M401055200. [DOI] [PubMed] [Google Scholar]
- 47.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Schwabe R, Devries-Seimon T, Yao P, Gerbod-Giannone M, Tall A, Davies R, Flavell R, Brenner D, Tabas I. Free cholesterol-loaded macrophages are an abundant source of TNF-alpha and IL-6. Model of NF-kappa B- and MAP kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem. 2005;280:21763–21772. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- 49.Gaultier A, Arandjelovic S, Niessen S, Overton CD, Linton MF, Fazio S, Campana WM, Cravatt BF, 3rd, Gonias SL. Regulation of tumor necrosis factor receptor-1 and the IKK-NF-kappaB pathway by LDL receptor-related protein explains the antiinflammatory activity of this receptor. Blood. 2008;111:5316–5325. doi: 10.1182/blood-2007-12-127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li FQ, Sempowski GD, McKenna SE, Laskowitz DT, Colton CA, Vitek MP. Apolipoprotein E-derived peptides ameliorate clinical disability and inflammatory infiltrates into the spinal cord in a murine model of multiple sclerosis. J Pharmacol Exp Ther. 2006;318:956–965. doi: 10.1124/jpet.106.103671. [DOI] [PubMed] [Google Scholar]
- 51.Laskowitz DT, Thekdi AD, Thekdi SD, Han SK, Myers JK, Pizzo SV, Bennett ER. Downregulation of microglial activation by apolipoprotein E and apoE-mimetic peptides. Experimental Neurology. 2001;167:74–85. doi: 10.1006/exnr.2001.7541. [DOI] [PubMed] [Google Scholar]
- 52.Jofre-Monseny L, Loboda A, Wagner AE, Huebbe P, Boesch-Saadatmandi C, Jozkowicz A, Minihane AM, Dulak J, Rimbach G. Effects of apoE genotype on macrophage inflammation and heme oxygenase-1 expression. Biochem Biophys Res Commun. 2007;357:319–324. doi: 10.1016/j.bbrc.2007.03.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laskowitz DT, Matthew WD, Bennett ER, Schmechel D, Herbstreith MH, Goel S, McMillian MK. Endogenous apolipoprotein E suppresses LPS-stimulated microglial nitric oxide production. Neuroreport. 1998;9:615–618. doi: 10.1097/00001756-199803090-00010. [DOI] [PubMed] [Google Scholar]
- 54.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 55.Graham B, Gibson SB. The two faces of NFkappaB in cell survival responses. Cell Cycle. 2005;4:1342–1345. doi: 10.4161/cc.4.10.2047. [DOI] [PubMed] [Google Scholar]
- 56.Shetty S, Graham BA, Brown JG, Hu X, Vegh-Yarema N, Harding G, Paul JT, Gibson SB. Transcription factor NF-kappaB differentially regulates death receptor 5 expression involving histone deacetylase 1. Mol Cell Biol. 2005;25:5404–5416. doi: 10.1128/MCB.25.13.5404-5416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaplan MJ, Ray D, Mo RR, Yung RL, Richardson BC. TRAIL (Apo2 ligand) and TWEAK (Apo3 ligand) mediate CD4+ T cell killing of antigen-presenting macrophages. J Immunol. 2000;164:2897–2904. doi: 10.4049/jimmunol.164.6.2897. [DOI] [PubMed] [Google Scholar]
- 58.Secchiero P, Candido R, Corallini F, Zacchigna S, Toffoli B, Rimondi E, Fabris B, Giacca M, Zauli G. Systemic tumor necrosis factor-related apoptosis-inducing ligand delivery shows antiatherosclerotic activity in apolipoprotein E-null diabetic mice. Circulation. 2006;114:1522–1530. doi: 10.1161/CIRCULATIONAHA.106.643841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.