Abstract
Tissue engineering is a promising approach to implement endothelial cells as a cellular delivery therapy for vascular disease. We and others previously demonstrated that endothelial cells embedded in three-dimensional collagen-based matrices retain their full biosecretory spectrum, enabling them to serve as powerful regulators of vascular diseases. Fascinatingly, matrix embedding of endothelial cells not only allows for their implantation but also seems to provide protection from allo- and xenogeneic-triggered host immune responses. This is not an effect of simple physical shielding but a more fundamental influence of cell–matrix interconnectivity on the cellular immune phenotype. Reduced cytokine-induced levels of costimulatory and adhesion molecules associated with significantly lower expression levels of major histocompatibility class II expression on matrix-embedded human aortic endothelial cells when compared to the same cells cultured on two-dimensional polystyrene coated-tissue culture plates. Strikingly, the entire interferon-γ-dependent signaling cascade resulting in MHC class II molecule expression is markedly suppressed in endothelial cells grown to confluence within three-dimensional scaffolds. These findings might be of pivotal importance for designing endothelial cell-based therapies in general and might enhance our understanding of the underlying pathophysiology in a broad range of cardiovascular diseases (e.g., atherosclerosis, vasculitis, chronic allograft vasculopathy).
Keywords: Endothelial cell, Matrix, MHC class II molecule
INTRODUCTION
Endothelial cells (EC) grown within three-dimensional collagen-based scaffolds line the interstices of the sponge-like matrix. In this environment cells achieve a phenotype that is even more regulatory than EC in confluent monolayers and provide control that can only otherwise be provided by the endothelium of the intact blood vessel. Perivascular implants of these constructs reduced intimal thickening in animal models from rats to pigs after arterial denudation, stenting, or bypass grafting (3,17). Autologous cells offer the theoretical advantage of immune acceptance. As self-cells they should not be subject to the same immune reactivity as allogeneic or xenogeneic EC. Yet, endothelial dysfunction is often the first manifestation of vascular disease and host EC may be inadequate to provide the biochemical control needed and even subject to auto-antibodies generated with repeated injury and repair (2). Healthy nonautologous EC are phenotypically and biochemically far more desirable (4), but their implantation poses an immunologic challenge. Matrix embedding significantly reduces the immune reaction to allogeneic and even xenogeneic EC in vitro and in vivo (12,14,16). Matrix-embedded EC exhibit a muted expression pattern of adhesion molecules, chemokines, and costimulatory molecules and show markedly decreased expression of major histocompatibility complex (MHC) class II molecules (12). This finding is of special interest as the immunogenic capacity of EC is directly proportional to the extent of MHC class II expression on the cell surface (8) and therefore challenged the notion of further investigation.
ROLE OF MHC MOLECULES IN GRAFT REJECTION AND VASCULAR DISEASE
Rejection of whole organs or transplanted cells is driven by recognition of foreign molecules presented via molecules encoded by MHC molecules. Acute graft rejection is initiated by direct allo- or xenorecognition of foreign MHC molecules by T cells, triggering lymphatic activation and differentiation. In general, T lymphocytes fall into two major functional classes distinguished by the expression of cell surface proteins CD4 and CD8. Cytotoxic CD8+ T cells recognize antigens presented by MHC class I molecules and cause graft rejection by direct lysis. CD4+ T-helper cells display specificity for MHC class II molecules and upon recognition direct activation of macrophages and B cells to the site of infection by release of cytokines such as interleukins and interferon (IFN)-γ (11,27).
Numerous studies indicate the CD4+ T-cell-mediated recognition of MHC class II molecules as the main initiator of acute allograft rejection rather than recognition of MHC class I molecules. Utilization of anti-CD4 monoclonal antibodies or graft transplantation in CD4-deficient hosts resulted in long-term graft survival in allograft models of cardiac transplantation (9,28). Furthermore, matching for MHC class I antigens only minimally prolonged graft survival, while matching for MHC class II antigens allowed the indefinite survival of kidneys in about one third of the recipients (22). It has also been established that acute allograft rejection occurs via direct MHC class II presentation by the graft rather than via indirect donor antigen presentation by host-derived antigen-presenting cells (23). Traditionally, donor-derived professional antigen-presenting cells, such as dendritic cells, macrophages, and B cells, have been implicated as the main antigen-presenting cells in the setting of organ and cell transplantation (10). However, substantial in vitro and in vivo data demonstrated antigen presentation to T cells via donor-derived somatic cells, such as EC, to be of equally crucial importance for the initiation of graft rejection. Besides a role of MHC class II expression by engrafted EC for initiation of acute and chronic allorejection (6), aberrant endothelial MHC class II expression has been demonstrated in atherosclerosis, autoimmune diseases (e.g., vasculitis, lupus erythematosus), and myocarditis (29,31).
MATRIX EMBEDDING OF ENDOTHELIAL CELLS ALTERS EXPRESSION OF MHC II MOLECULES
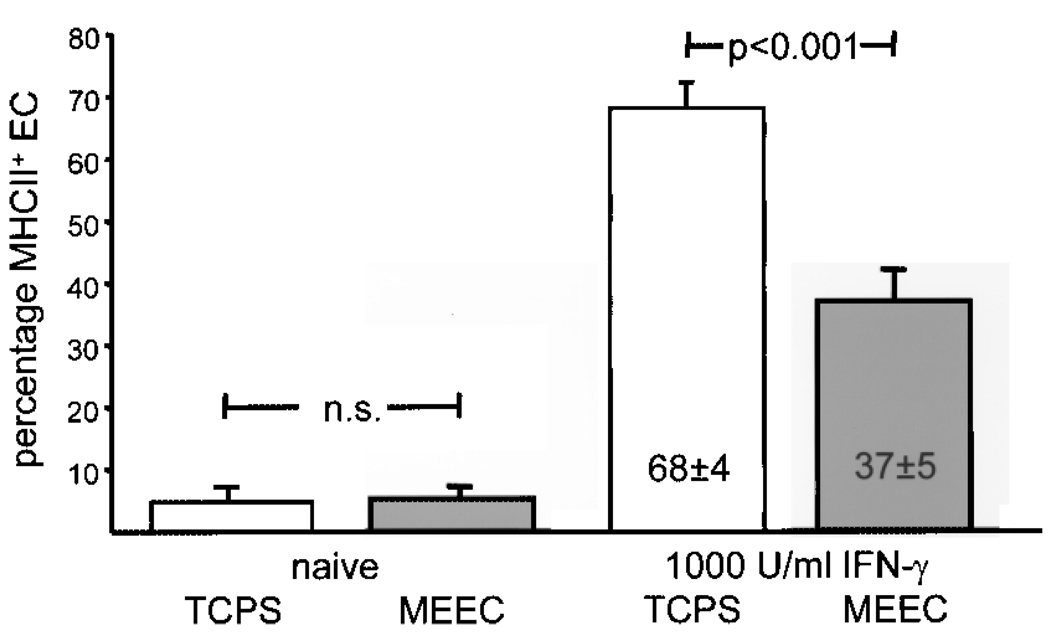
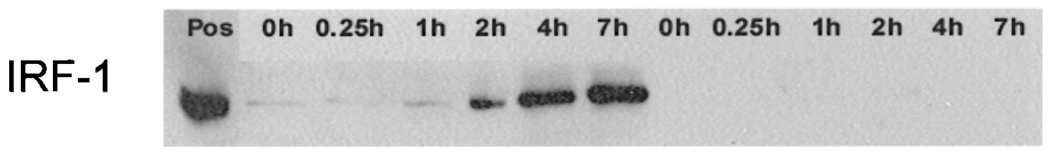
A fascinating aspect of matrix embedding of EC is that it not only allows for their implantation but also seemed to provide immunoprotection from the host immune system. Aside from a sparse infiltration with immune cells, three-dimensional allo- and xenogeneic EC implants induced only a weak humoral immune response in pigs, rats, and mice compared to free, nonembedded cells (14,16,18). In addition, in vitro data revealed decreased expression of costimulatory and adhesion molecules on human and porcine aortic matrix-embedded EC (16,19,20). Further analysis revealed a fundamental influence of matrix embedding and cell matrix contact on immunogenicity of EC: matrix-embedded human aortic EC (HAE) express significantly lower protein and transcript levels of MHC class II molecules upon stimulation with IFN-γ than HAE grown on tissue culture poly-styrene plates (13,15,16) (Fig. 1). Matrix-embedded HAE displayed a marked inhibition of intracellular IFN-γ-dependent downstream signaling pathway while no differences in surface expression of IFN-γ receptor subunit I and II between the two growing forms of EC could be demonstrated. Western blot analysis demonstrated IFN-γ-responsive phosphorylation of the Janus tyrosine kinases (JAK)1 and JAK2 in tissue culture-grown HAE but almost no phosphorylation of JAK proteins in matrix-embedded HAE upon cytokine stimulation. Further analysis of specific mediators in the signaling cascade transmitting IFN-γ signals from the cell surface to the nucleus demonstrated an attenuated and delayed phosphorylation of STAT-1 in matrix-embedded HAE when compared to extent and kinetics of STAT-1 phosphorylation in HAE grown to confluence on polystyrene-coated tissue culture plates (13). Significantly lower protein levels of IFN-γ-regulatory factor(IRF)-1 in matrix-embedded HAE paralleled this phenomenon (Fig. 2). DNA binding of phosphorylated STAT-1 and IRF-1 is required to initiate expression of CIITA, acting as main inducer for MHC II expression, which as well was significantly repressed in matrix-embedded HAE. Thus, all events of the IFN-γ signal transduction pathway downstream of the JAK phosphorylation appear inhibited in matrix-embedded HAE (13,15). Collagen coating of tissue culture plates was without effect on IFN-γ- induced STAT-1 phosphorylation, indicating a pivotal role of the microenvironment for endothelial immunogenicity (13). On a functional level, induction of allogeneic T-cell proliferation was significantly muted when HAE were presented in a matrix-embedded form when compared to HAE grown on two-dimensional culture plates (16).
Figure 1.
Three-dimensional matrix embedding attenuates IFN-γ-induced MHC class II expression by human aortic EC. EC (104) were analyzed for surface expression of MHC class II molecules by flow cytometry. EC either grown two-dimensional on tissue culture polystyrene plates or embedded within collagen matrices were left untreated (naive) or incubated with 1000 U/ml IFN-γ.
Figure 2.
Western blot demonstrated almost no protein expression of IRF-1 after IFN-γ stimulation for indicated time periods (hours) in matrix-embedded EC whereas IRF-1 expression in two-dimensional-grown human aortic EC followed a time dependency. First lane: positive control; lanes 2–7: protein expression of IRF-1 in two-dimensional-grown human aortic EC; lanes 8–13: IRF-1 expression of IFN-γ stimulation for indicated time periods (hours) in matrix-embedded EC.
MATRIX EMBEDDING OF ENDOTHELIAL CELLS ALTERS EXPRESSION PATTERNS OF INTEGRINS
Several cytokine signaling cascades are induced through adhesion, in particular integrin-mediated mechanisms, in various cell- and function-specific manners. Similar to the signal transduction induced by cytokine receptor binding, interaction of integrins with the extracellular matrix can induce tyrosine phosphorylation of many intracellular proteins (5).
A microarray focused on integrin expression levels upon stimulation HAE on with IFN-γ revealed significant differences in integrin expression between matrix-embedded and nonembedded EC. Interestingly, increased expression of integrins αv and β3 highly correlated with expression of MHC class II molecules (13,15). A role for these two integrins for cellular immune behavior may well explain the differences in proneness to cytokine-induced EC activation with respect to the matrix microenvironment (25). STAT-1, for instance, is activated via integrins in the context of regulation of cell adhesion and migration (30).
DISCUSSION
Previous studies demonstrated that the endothelial basement membrane controls aspects of cell adhesion, spreading, migration, contractility, differentiation, proliferation, protein synthesis, and secretion. Furthermore, this membrane is altered in many in vivo disease states, from diabetes to glomerulopathy to atherosclerosis (1,2, 7,24). Dysfunction of endothelial cells has been closely associated with changes in basement membrane composition, cumulating in the degree of attachment of EC. Our recent data suggest that the quality of basement membrane anchoring might play a pivotal role for EC immunobiology (21). Accordingly, the altered regulation of the IFN-γ signaling cascade in matrix-embedded EC likely derives from a direct interaction between EC and matrix rather than simple concomitant presence or contact of the two materials (15).
Besides muted upregulation of MHC class II molecules on matrix-embedded EC, we demonstrated reduced expression levels of costimulatory and adhesion molecules on embedded EC (16). This allowed for implantation of allogeneic and even xenogeneic matrix-embedded EC in various animal models without endangering a significant host immune response (16,18). As one result, perivascular implants of nonsyngeneic matrix-embedded EC were successfully used to influence vascular repair processes without the need for immune-suppressive therapy (17–20). Equally fascinating, EC– matrix compounds induced in vivo tolerance for subsequent implantation of allogeneic EC even when presented in a nonembedded form (14). This further emphasizes the pivotal role of MHC class II molecules in acute and chronic rejection of nonsyngeneic grafts (9,22,23,28).
Our data are of unique importance especially for designing EC-based therapies (26). Engineering of quasi-physiologic EC–matrix compounds that ensure the full spectrum of vasoregulative EC factors, thereby pertaining a healthy EC immune phenotype, might pave the way for future therapeutic schemes in treatment and prevention of a range of cardiovascular diseases. Furthermore, our findings might enhance our understanding on underlying causes for aberrant (endothelial) MHC class II molecule expression in various diseases.
ACKNOWLEDGMENTS
This work was supported in part by a Philip Morris External Research Program Postdoctoral Fellowship and a grant from the Deutsche Herzstiftung, Frankfurt, Germany to Heiko Methe and a grant from the National Institutes of Health to Elazer R. Edelman (HL 49039).
REFERENCES
- 1.Aumailley M, Smyth N. The role of laminins in basement membrane function. J. Anat. 1998;193(Pt 1):1–21. doi: 10.1046/j.1469-7580.1998.19310001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003;23(2):168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 3.Centra M, Ratych RE, Cao GL, Li J, Williams E, Taylor RM, Rosen GM. Culture of bovine pulmonary artery endothelial cells on Gelfoam blocks. FASEB J. 1992;6(12):3117–3121. doi: 10.1096/fasebj.6.12.1521742. [DOI] [PubMed] [Google Scholar]
- 4.Chang CC, Hoying JB. Directed three-dimensional growth of microvascular cells and isolated microvessel fragments. Cell Transplant. 2006;15(6):533–540. doi: 10.3727/000000006783981693. [DOI] [PubMed] [Google Scholar]
- 5.Clark EA, Brugge JS. Integrins and signal transduction pathways: The road taken. Science. 1995;268(5208):233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 6.Denton MD, Davis SF, Baum MA, Melter M, Reinders ME, Exeni A, Samsonov DV, Fang J, Ganz P, Briscoe DM. The role of the graft endothelium in transplant rejection: Evidence that endothelial activation may serve as a clinical marker for the development of chronic rejection. Pediatr. Transplant. 2000;4(4):252–260. doi: 10.1034/j.1399-3046.2000.00031.x. [DOI] [PubMed] [Google Scholar]
- 7.Erickson AC, Couchman JR. Still more complexity in mammalian basement membranes. J. Histochem. Cytochem. 2000;48(10):1291–1306. doi: 10.1177/002215540004801001. [DOI] [PubMed] [Google Scholar]
- 8.Ferry B, Leszczinski D, Halttunen J, Hayry P. Regulation by interferon-gamma and methylprednisolone of class II antigenicity and immunogenicity. Transplant. Proc. 1987;19(1 Pt 1):249–250. [PubMed] [Google Scholar]
- 9.Krieger NR, Yin DP, Fathman CG. CD4+ but not CD8+ cells are essential for allorejection. J. Exp. Med. 1996;184(5):2013–2018. doi: 10.1084/jem.184.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J. Exp. Med. 1990;171(1):307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation—how much of the promise has been realized? Nat. Med. 2005;11(6):605–613. doi: 10.1038/nm1251. [DOI] [PubMed] [Google Scholar]
- 12.Methe H, Edelman ER. Cell–matrix contact prevents recognition and damage of endothelial cells in states of heightened immunity. Circulation. 2006;114(1 Suppl):I233–I238. doi: 10.1161/CIRCULATIONAHA.105.000687. [DOI] [PubMed] [Google Scholar]
- 13.Methe H, Edelman ER. Tissue engineering of endothelial cells and the immune response. Transplant. Proc. 2006;38(10):3293–3299. doi: 10.1016/j.transproceed.2006.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Methe H, Groothuis A, Sayegh MH, Edelman ER. Matrix adherence of endothelial cells attenuates immune reactivity: Induction of hyporesponsiveness in allo- and xenogeneic models. FASEB J. 2007;21(7):1515–1526. doi: 10.1096/fj.06-7051com. [DOI] [PubMed] [Google Scholar]
- 15.Methe H, Hess S, Edelman ER. The effect of three-dimensional matrix-embedding of endothelial cells on the humoral and cellular immune response. Semin. Immunol. 2008;20(2):117–122. doi: 10.1016/j.smim.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Methe H, Nugent HM, Groothuis A, Seifert P, Sayegh MH, Edelman ER. Matrix embedding alters the immune response against endothelial cells in vitro and in vivo. Circulation. 2005;112(9 Suppl):I89–I95. doi: 10.1161/01.CIRCULATIONAHA.105.524991. [DOI] [PubMed] [Google Scholar]
- 17.Nathan A, Nugent MA, Edelman ER. Tissue engineered perivascular endothelial cell implants regulate vascular injury. Proc. Natl. Acad. Sci. USA. 1995;92(18):8130–8134. doi: 10.1073/pnas.92.18.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nugent HM, Edelman ER. Endothelial implants provide long-term control of vascular repair in a porcine model of arterial injury. J. Surg. Res. 2001;99(2):228–234. doi: 10.1006/jsre.2001.6198. [DOI] [PubMed] [Google Scholar]
- 19.Nugent HM, Edelman ER. Tissue engineering therapy for cardiovascular disease. Circ. Res. 2003;92(10):1068–1078. doi: 10.1161/01.RES.0000073844.41372.38. [DOI] [PubMed] [Google Scholar]
- 20.Nugent HM, Groothuis A, Seifert P, Guerraro JL, Nedelman M, Mohanakumar T, Edelman ER. Perivascular endothelial implants inhibit intimal hyperplasia in a model of arteriovenous fistulae: A safety and efficacy study in the pig. J. Vasc. Res. 2002;39(6):524–533. doi: 10.1159/000067207. [DOI] [PubMed] [Google Scholar]
- 21.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: A potential role in atherosclerosis. J. Cell Biol. 2005;169(1):191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pescovitz MD, Thistlethwaite JR, Jr, Auchincloss H, Jr, Ildstad ST, Sharp TG, Terrill R, Sachs DH. Effect of class II antigen matching on renal allograft survival in miniature swine. J. Exp. Med. 1984;160(5):1495–1508. doi: 10.1084/jem.160.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pietra BA, Wiseman A, Bolwerk A, Rizeq M, Gill RG. CD4 T cell-mediated cardiac allograft rejection requires donor but not host MHC class II. J. Clin. Invest. 2000;106(8):1003–1010. doi: 10.1172/JCI10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raines EW, Koyama H, Carragher NO. The extracellular matrix dynamically regulates smooth muscle cell responsiveness to PDGF. Ann. NY Acad. Sci. 2000;902:39–51. doi: 10.1111/j.1749-6632.2000.tb06299.x. [DOI] [PubMed] [Google Scholar]
- 25.Sahni A, Sahni SK, Francis CW. Endothelial cell activation by IL-1beta in the presence of fibrinogen requires alphavbeta3. Arterioscler. Thromb. Vasc. Biol. 2005;25(10):2222–2227. doi: 10.1161/01.ATV.0000183605.27125.6f. [DOI] [PubMed] [Google Scholar]
- 26.SanMartin A, Borlongan CV. Cell transplantation: Toward cell therapy. Cell Transplant. 2006;15(7):665–673. doi: 10.3727/000000006783981666. [DOI] [PubMed] [Google Scholar]
- 27.Sayegh MH, Turka LA, Milford EL, Carpenter CB. Mixed lymphocyte response-generated suppressor cell specificity in humans is major histocompatibility complex-restricted. Hum. Immunol. 1989;26(4):281–287. doi: 10.1016/0198-8859(89)90006-2. [DOI] [PubMed] [Google Scholar]
- 28.Shizuru JA, Seydel KB, Flavin TF, Wu AP, Kong CC, Hoyt EG, Fujimoto N, Billingham ME, Starnes VA, Fathman CG. Induction of donor-specific unresponsiveness to cardiac allografts in rats by pretransplant anti-CD4 monoclonal antibody therapy. Transplantation. 1990;50(3):366–373. doi: 10.1097/00007890-199009000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Turesson C. Endothelial expression of MHC class II molecules in autoimmune disease. Curr. Pharm. Des. 2004;10(2):129–143. doi: 10.2174/1381612043453414. [DOI] [PubMed] [Google Scholar]
- 30.Xie B, Zhao J, Kitagawa M, Durbin J, Madri JA, Guan JL, Fu XY. Focal adhesion kinase activates Stat1 in integrin-mediated cell migration and adhesion. J. Biol. Chem. 2001;276(22):19512–19523. doi: 10.1074/jbc.M009063200. [DOI] [PubMed] [Google Scholar]
- 31.Xu QB, Oberhuber G, Gruschwitz M, Wick G. Immunology of atherosclerosis: Cellular composition and major histocompatibility complex class II antigen expression in aortic intima, fatty streaks, and atherosclerotic plaques in young and aged human specimens. Clin. Immunol. Immunopathol. 1990;56(3):344–359. doi: 10.1016/0090-1229(90)90155-j. [DOI] [PubMed] [Google Scholar]