Since March, 1971, cyclophosphamide (Cytoxan) has had a prominent role in the immunosuppression administered to recipients of whole organs at our center. This report will be concerned with the results in these patients, about two-thirds of whom now have a potential follow-up of more than 1 yr.
Materials and Methods
Renal Homotransplantation
From Related Donors
Donors for 32 recipients included 16 siblings (six were double haplotype HL-A identical), 14 parents, one aunt, and one grandmother. Except for the search for HL-A-identical siblings, the results of HL-A matching were not given weight in donor selection. An attempt was made to avoid transplantation when the crossmatch was positive for cytotoxic antibodies. However, in one case, such antibodies developed in the period between testing and operation with a resulting hyperacute rejection.
Cyclophosphamide, prednisone, and horse anti-lymphocyte globulin (ALG) were started several days in advance of operation and continued afterwards in various modifications of the general regimen described in detail elsewhere1,2 and shown in Figs. 1 and 2. Postoperatively, ALG was usually continued for 2–4 mo.
Fig. 1.
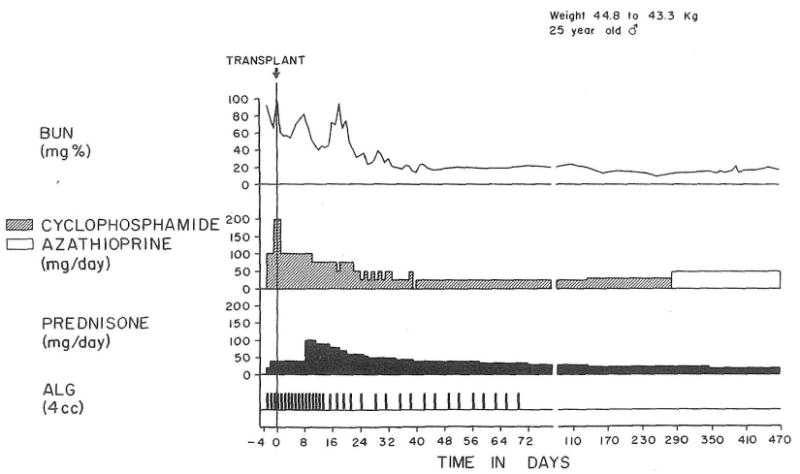
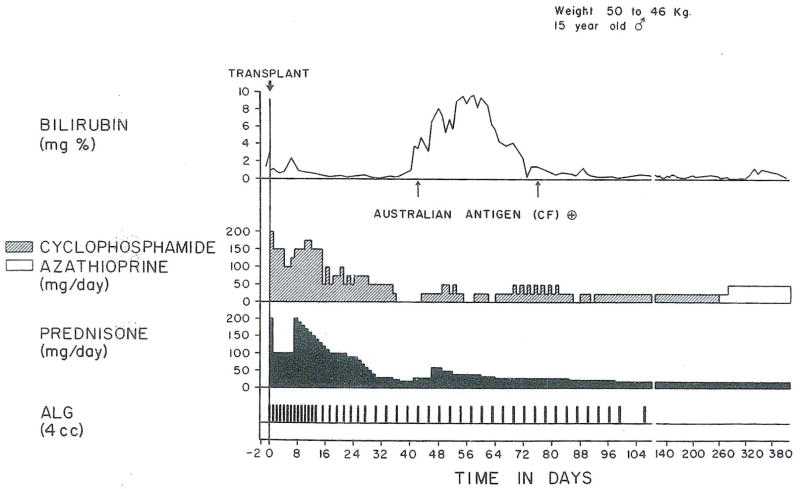
The chronic use of cyclophosphamide in conjunction with prednisone and early ALG in the recipient of a parental renal homograft. Treatment was changed to azathioprine after 10 mo, with an increase in the milligram per day dose. The patient has a perfect result after 1½ yr.
Fig. 2.
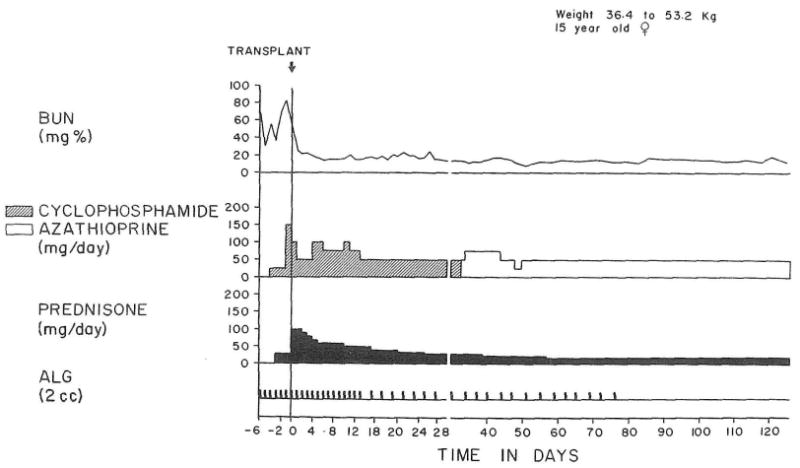
The use of cyclophosphamide for the first postoperative month with subsequent azathioprine treatment. The result was excellent after almost a year.
Before September 1971 most patients were treated with cyclophosphamide for many months after which a change was eventually made in almost every case to maintenance therapy with azathioprine (Fig. 1). In contrast, patients treated in the last 3 mo of the study (October to December 1971) had a shorter course of cyclophosphamide therapy (1–2 mo) before switching to azathioprine (Fig. 2). Doses of cyclophosphamide in milligram per kilogram body weight were usually one-half to two-thirds those later employed with azathioprine. With either of these potentially radiomimetic agents, an effort was made to avoid leukopenia.
Cadaveric Transplantation
The crossmatch test for cytotoxic antibodies was negative in all cases. HL-A typing was performed, but since the results were not taken into consideration for donor-recipient pairing, all recipients were given more or less badly matched kidneys (5 C, 10 D, and 18 E)*.
Therapy after cadaveric transplantation was similar to that after consanguineous transplantation except that immunosuppressive pretreatment was not feasible. The cadaveric recipients, by and large, presented more complicated problems of management than did related cases. Their average age was 33 ± 12.7 (SD) yr as opposed to 27 ± 11.2 (SD) yr for the consanguineous recipients. In addition whereas only one of the 32 recipients of related kidneys was undergoing retransplantation, nine of the 29 cadaveric recipients had already rejected one or more homografts at some previous time.
Hepatic Homotransplantation
There were 16 patients, aged 1–53 yr, who received orthotopic hepatic homografts from 2–19 mo ago and who were treated with a cyclophosphamide-containing, triple drug regimen similar to that used for recipients of cadaveric kidneys (Fig. 3). The indications for liver replacement were biliary atresia (seven cases), chronic aggressive hepatitis (four cases), biliary cirrhosis (three cases), Wilson's disease (one case) and hepatic hemangioendothelial sarcoma (one case).
Fig. 3.
Triple drug treatment, including cyclophosphamide, in an orthotopic liver recipient. No rejection was diagnosed, but serum hepatitis in the second and third postoperative months caused jaundice. After 9 mo, azathioprine was substituted for cyclophosphamide.
Results
From Related Donors (Table 1)
Table 1.
Results with Renal Homotransplantation: Follow-up 9–18 Months
| Donor | Total Number | Lost Per cent | |
|---|---|---|---|
| Consanguineous | |||
| Patients | 32 | 3 | 9.4 |
| Grafts | 32 | 6 | 18.8 |
| Cadaveric | |||
| Patients | 29 | 7* | 24.1 |
| Grafts | 33 | 11* | 33.3 |
Four of the seven deaths and seven of the 11 graft losses were in nine patients undergoing retransplantation.
Three (9.4%) of the 32 recipients died, 2 from Pneumocystis carinii pneumonia, and the third from perforation of a sigmoid colonic diverticulum. Three additional grafts were lost, two from hyperacute rejection and one from a technical surgical accident, for a total kidney loss rate of 6 in 32 (18.8%).
From 9–18 mo post-transplantation, the remaining 26 consanguineous renal homografts have average functions as follows: BUN 25.6 ± 10.3 (SD) mg/ml, and creatinine clearance 70.9 ± 20.4 (SD) ml/min. The clinical result is presently considered excellent with 24 of these 26 kidneys and fair in the other two.
Cadaveric Transplantation (Table 1)
Seven (24%) of the 29 recipients died; all but 1 from causes that were apparently directly related to immunosuppression. These included brain tumor (glioblastoma multiforme), hindquarter gangrene due to Pseudomonas, a fungal brain abscess (Aspergillus), Pneumocystis carinii pneumonitis, perforated sigmoid diverticulitis and giant cecal ulcer. The seventh patient died of a recently described complication of hyperparathyroidism—consisting of widespread skin and muscle gangrene apparently due to calcium plugging of multiple small arteries.3 There were four deaths (44%) among the 9 patients undergoing ertransplantation (after a previous graft had been in residence for 1 hr to 5 yr, average 18 mo) compared to three (15%) among the 20 receiving primary homografts.
The 29 recipients of cadaver kidneys were given 33 transplants. Two of the four extra organs were used in the classic sequence after the initial grafts had been rejected and removed but in the other 2 cases second transplants were inserted 1½ and 5 wk after the first because of poor initial function. In these latter recipients, both the first and second homografts eventually functioned for long periods.
Twenty-two of the 33 grafts (67%) are still functioning after 9–18 mo. The 11 lost organs failed for the following reasons: Seven from recipient death; two from uncontrolled rejection; one from hyperacute rejection; and one because it was removed for wound pain 14 mo post-transplantation (one of the double homografts described in the preceding paragraph).
Twenty-one of the 22 surviving patients have life-supporting urine excretion, the exceptional patient being back on dialysis. The homografts have average function as follows: BUN 26.1 ± 10.2 (SD) mg/ml and creatinine clearance 67.9 ± 23.3 (SD) ml/min. The clinical result is currently considered excellent or good in 19 of these 21 survivors who bear functioning grafts, fair in one, and poor in one.
Liver Homotransplantation
Nine of the 16 recipients of hepatic homografts died after 2–87 days. Causes of failure were biliary tract obstruction (three cases), biliary fistula (two cases), homograft rejection (two cases), acute liver infarction (one case), and tumor recurrence (one case). All five patients with biliary tract complications succumbed, four of sepsis in spite of surgical correction of the lesion and/or drainage, and the fifth after an attempt at retransplantation.
Seven patients are alive 18, 14, 7, 5, 5, 4, and 2 mo after transplantation for Wilson's disease (one case), biliary atresia (one case), congenital biliary cirrhosis (two cases), and chronic aggressive hepatitis (three cases). One of the patients has moderate hepatic dysfunction due to chronic rejection at 14 months (bilirubin 2 mg%) and the other six have normal or near normal liver function.
Discussion
In this communication, most of the case material consisted of patients who received cyclophosphamide as the initial cytotoxic agent in a triple drug program with the frequent later substitution of azathioprine. In previous publications,1,2 considerable experience was reported with the reverse order of administration, using cyclophosphamide to replace azathioprine. Under both circumstances, cyclophosphamide has seemed equivalent to azathioprine in its toxicity as well as in its immunosuppressive effectiveness.
It is of interest that in kidney recipients two drugs belonging to such different chemical families and having such fundamentally different modes of action as cyclophosphamide and azathioprine can be substituted freely for each other. It remains to be seen if switching agents will have real benefits such as, for example, if the depletion of sensitized immunocompetent cells was more effective. At the moment, this remains a possibility, but one which has by no means been proven.
In the therapeutic program that has evolved during the last 18 months and is now in routine use, cyclophosphamide has been used as the first-line agent for 2 mo or for whatever fraction of that time during which doses of at least 1 mg/kg/day can be tolerated without bone marrow depression. Even in these small quantities, cyclophosphamide may cause leukopenia, in which case the substitution of azathioprine is made prematurely.
Using this manipulation of the cyclophosphamide-azathioprine complex in a system that also includes prednisone and ALG, patient and graft-survival in renal cases have been essentially the same as with the precyclophosphamide triple-drug combination of azathioprine, prednisone, and ALG.2 The quality of graft function in the cyclophosphamide-treated patients has been at least as good and possibly even superior.
In analyzing results with immunosuppressive programs in the modern era of transplantation, more than passing consideration must be given to the increasing complexity of case material, which is going to make it difficult to achieve results as good as those obtained in highly selected series of a few years ago. More and more, candidates are being accepted who, until recently, would have been excluded because of age, associated diseases, or other reasons. In addition, a third of all cadaveric renal homografts in the present report were used for retransplantation under conditions which proved to have a very high risk (44% mortality). In contrast, patients receiving primary cadaveric homografts faced only a 15% mortality, approximately the same with 9–18-mo follow-ups as with consanguineous transplantation.
The essential equivalency of results after renal transplantation under primary cyclophosphamide, as opposed to azathioprine therapy, will not be a strong inducement for other groups, mainly interested in the kidney, to change their present regimen of azathioprine management, except for specific indications such as drug hepatoxicity. But with liver transplantation, future possibilities are more expansive. The disadvantages of azathioprine for liver transplantation have been well recognized, including its potential hepatotoxicity, as well as its dependence upon liver function for both activation and degradation.4,5 Recently, Bach and Dardenne6,7 have provided striking evidence, with the rosette inhibition bioassay, that azathioprine loses its immunosuppressive qualities in human beings if there is significant liver malfunction. Their work has been confirmed in human liver recipients by Mitchell et al.8
If, as seems likely from Bach's data, the rosette inhibition test is a discriminating bioassay of immunosuppression, hepatic damage from ischemia, rejection, or other causes could cancel the efficacy of azathioprine at a crucial time, especially early postoperatively. This could explain why rejection has been so difficult to control in human liver recipients, particularly when ALG has been omitted,5 in spite of impressive evidence from animal research4,5,9-11 that the liver should be “easier” than other organs. It is possible that the potency of cyclophosphamide is similarly turned off during hepatic dysfunction, since cyclophosphamide is also activated in the liver. Studies of rosette inhibition during cyclophosphamide treatment, to settle this question, will be of great interest.
Our experience with cyclophosphamide is consistent with the hypothesis that this drug retains sufficient potency throughout variations in postoperative hepatic function. During the learning period, mistakes in cyclophosphamide dosage, technical surgical complications, and recurrence of malignancy caused several deaths. Nevertheless, seven of 16 patients (44%) are still alive, including five of the six recipients treated in the present year. This last group is the best consecutive series of liver cases we have ever seen and may herald the transition of hepatic transplantation from clinical investigation to true patient service.
Summary
Cyclophosphamide was given as the initial cytotoxic drug in combination with prednisone and ALG to 61 renal recipients. After 1 mo or longer, azathioprine was substituted for cyclophosphamide for maintenance therapy. After 9–18 mo, the patient and kidney survivals with consanguineous transplantation are 91% and 81%, respectively, and after cadaveric renal transplantation the figures are 76% and 67%, respectively. These results are similar to those obtained in the past with azathioprine alone.
Sixteen recipients of orthotopic cadaveric livers were similarly treated with seven (44%) living after 2–18 mo. With avoidance of technical accidents and increased experience with cyclophosphamide, the record since January 1972 is five survivors of six. In these patients, little difficulty was encountered with control of rejection. This observation, plus recent evidence that azathioprine may not be immunosuppressive in patients with significant hepatic dysfunction, suggests that cyclophosphamide is the preferable primary cytotoxic drug for the early treatment of liver recipients.
Acknowledgments
Supported by research grants from the Veterans Administration, by grants RR-00051 and RR-00069 from the general clinical research centers program of the Division of Research Resources, National Institutes of Health, and by grants AI-10176-01, AI-AM-08898, and AM-07772.
Footnotes
C = one mismatched HL-A antigen; D = one mismatch of each HL-A locus; E = double mismatch at one HL-A locus, usually with mismatches at other locus as well.
References
- 1.Starzl TE, Halgrimson CG, Penn I, Martineau G, Schroter G, Amemiya H, Putnam CW, Groth CG. Lancet. 1971;2:70. doi: 10.1016/s0140-6736(71)92046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Halgrimson CG, Penn I, Martineau G, Schroter G, Amemiya H, Putnam CW, Groth CG, Putnam CW, Halgrimson CG, Schroter GP, Martineau G, Launois B, Corman JL, Penn I, Booth AS, Jr, Porter KA, Groth CG. Surg Gynecol Obstet. 1971;133:981. [PMC free article] [PubMed] [Google Scholar]
- 3.Massry SG, Gordon A, Coburn JW, Kaplan L, Franklin SS, Maxwell MH, Kleeman CR. J Med. 1970;49:416. doi: 10.1016/s0002-9343(70)80034-1. [DOI] [PubMed] [Google Scholar]
- 4.Starzl TE, Marchioro TL, Porter KA. In: Advances in Surgery Chicago, Year Book. Welch C, editor. 1966. p. 295. [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Marchioro TL, Porter KA, Putnam CW. Experience in Hepatic Transplantation. Philadelphia: Saunders; 1969. [Google Scholar]
- 6.Bach JF, Dardenne M. Paper presented at the Fourth International Congress of Nephrology; Stockholm. June, 1969; Program abstract quoted by Mitchell et al., see Ref 8. [Google Scholar]
- 7.Bach JF, Dardenne M. Proc Roy Soc Med. 1972;65:260. doi: 10.1177/003591577206500316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell CG, Eddleston ALWF, Smith MGM, Williams R. Lancet. 1970;1:1196. doi: 10.1016/s0140-6736(70)91786-1. [DOI] [PubMed] [Google Scholar]
- 9.Calne RY, White HJO, Yoffa DE, Binns RM, Maginn RR, Herbertson RM, Millard PR, Molina VP, Davis DR. Br Med J. 1967;4:645. doi: 10.1136/bmj.4.5580.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terblanche J, Peacock JH, Bowes J, Davies R, Tierris EJ, Palmer DB, Hunt AC. Br J Surg. 1967;54:231. [Google Scholar]
- 11.Garnier H, Clot JP, Bertrand M, Camplez P, Kunlin A, Gorin JP, Goaziou FL, Levy R, Cordier G. C R Acad Sci (Paris) 1965;260:5621. [PubMed] [Google Scholar]