Abstract
Visual perception of the environment plays an important role in many mosquito behaviors. Characterization of the cellular and molecular components of mosquito vision will provide a basis for understanding these behaviors. A unique feature of the R7 photoreceptors in Aedes aegypti and Anopheles gambiae is the extreme apical projection of their rhabdomeric membrane. We show here that the compound eye of both mosquitoes is divided into specific regions based on nonoverlapping expression of specific rhodopsins in these R7 cells. The R7 cells of upper dorsal region of both mosquitoes express a long wavelength op2 rhodopsin family member. The lower dorsal hemisphere and upper ventral hemisphere of both mosquitoes express the UV-sensitive op8 rhodopsin. At the lower boundary of this second region, the R7 cells again express the op2 family rhodopsin. In Ae. aegypti, this third region is a horizontal stripe of one to three rows of ommatidia, and op8 is expressed in a fourth region in the lower ventral hemisphere. However, in An. gambiae, the op2 family member expression is expanded throughout the lower region in the ventral hemisphere. The overall conserved ommatidial organization and R7 retinal patterning show these two species retain similar visual capabilities. However, the differences within the ventral domain may facilitate species-specific visual behaviors.
Indexing terms: mosquito vision, visual pigments, UV sensitivity, retinal patterning
The mosquitoes Ae. aegypti and An. gambiae are vectors of prevalent tropical diseases (Hill et al., 2005). These mosquitoes are able to identify hosts for blood feeding in low light environments, yet visual cues contribute to feeding as well as other behaviors (Day, 2005; Kawada et al., 2007; Kawada et al., 2005). Genome sequencing projects revealed that both Ae. aegypti and An. gambiae possess ten different rhodopsin genes for light reception (Hill et al., 2002; Nene et al., 2007). Phylogenetic analysis shows that there are six rhodopsins in Ae. aegypti and five rhodopsins in An. gambiae likely to be long wavelength pigments (maximum absorbance more than 500 nm), one UV pigment (maximum absorbance less than 400 nm), one short wavelength pigment (maximum absorbance 400–500 nm), as well as two other uncharacterized rhodopsin groups containing one or two gene members (Nene et al., 2007; Spaethe and Briscoe, 2004).
Brammer (1970) characterized the ultrastructure of the Ae. aegypti eye. The Ae. aegypti eye, like many insects, is composed of ommatidial units containing eight photoreceptor cells. Seven of the eight photoreceptor cells possess a microvillar rhabdomere on the distal 50% of the cell body. These rhabdomeres are attached to the neighboring rhabdomeres to create a circular ring structure known as a fused rhabdom. The other photoreceptor cell, R7 cell, has a much smaller rhabdomere located at the distal surface of the fused rhabdom (Brammer, 1970). The critical advantage of the fused arrangement is increased light sensitivity, expected to be advantageous for species requiring visual function in low light situations. An open rhabdom arrangement, in which rhabdomeres are not attached together, is found in Drosophila as well as in certain diurnal mosquito species such as Toxorhynchites (Land et al., 1999). The open rhabdom structure decreases light sensitivity, but allows an opportunity for higher visual acuity when coupled with appropriate second order neural wiring connections such as found in Drosophila and other higher order flies (Clandinin and Zipursky, 2002; Kirschfeld, 1967).
Usually a photoreceptor cell expresses a single rhodopsin though a few exceptions are known (Mazzoni et al., 2008; Sison-Mangus et al., 2006). The one photoreceptor/one rhodopsin scheme serves as the basis for color vision and specialized roles of particular photoreceptor classes in vision-mediated behaviors. Determination of the rhodopsin expression pattern in different photoreceptor cells and the spectral sensitivities of these rhodopsins is required to understand the role of each photoreceptor cell in mosquito biology. The specialized R7 photoreceptor cell is the focus of this report. Here we show that the R7 cell of both Ae. aegypti and An. gambiae is uniquely positioned in each ommatidial unit, projecting its rhabdomere only at the most distal extreme of the fused rhabdom. Two different rhodopsins are expressed in nonoverlapping sets of R7 cells, specifying a retinal domain structure that is similar but not identical in the two species. The data suggest that R7 photoreceptors play an important role in visual behaviors of Ae. aegypti and An. gambiae.
MATERIALS AND METHODS
Electron microscopy
White-eyed Khw and the red-eyed Liverpool Ae. aegypti mosquitoes, and Mali NIH red eyed strain of An. gambiae, were used for transmission electron microscopy. The laboratory embedding and staining procedures were as previously described in the analysis for Drosophila photoreceptors (Ahmad et al., 2007).
Production of Antibodies
The sequence encoding the 49 amino acids of the C-terminal tail of the Aaop8 rhodopsin was amplified by PCR using the full length Aaop8 cDNA clone as template and primers were: 5'-GCGAATTCTACGTGTACGCCATCAGCCA-3' and 5'-ATGCGGCCGCATCGGTAGACGAGTCAGTAG-3'. Similarly, the sequence encoding the 46 amino acids of the C-terminal tail of the Aaop2 rhodopsin was cloned by using an Aaop2 cDNA clone and 5'-GCGAATTCTACGGTATCAGCCATCCGAA-3' and 5'-TAGCGGCCGCCGTTCTGTAGCGCATTTAGG-3' primers. The PCR products were confirmed by DNA sequencing and ligated into the pET32a(+) expression plasmid using introduced EcoR1 and Not1 sites. The plasmids were then transformed into BL21DE3-PlysE cells, and proteins were produced by purification from the cell lysate through affinity chromatography using His-Bind columns (Novagen). Purified Aaop8 fusion protein was injected into mice using Titer Max Gold Adjuvant (Sigma) for the production of polyclonal antibody. Polyclonal antibody to Aaop2 was raised in rabbits (Proteintech) and purified using the Immunopure (A plus) IgG Purification Kit (Pierce).
Immunolocalization in sectioned and whole mount retinal preparations
For sectioned head preparations, the heads of the Khw Ae. aegypti and Mali NIH An. gambiae mosquitoes were fixed in 4% paraformaldehyde/5% sucrose overnight at 4 °C, rinsed 3 times 10 min in 5% sucrose/1X PBS (pH 7.4), placed in 5% sucrose/1X PBS overnight at 4 °C, then placed in 30% sucrose/1X PBS overnight at 4 °C, and finally in 30% sucrose/1X PBS:Tissue Freezing Medium (1:1; Triangle Biomedical Sciences) for 4 h at RT. The tissue was then embedded and frozen in 100% Tissue Freezing Medium and sectioned at 10–12 µm. Slides were dried at 50 °C for 2 h, rehydrated in 1X PBS for 20 min, and blocked for 1 h in blocking buffer (1X PBS/5% normal goat serum/0.3% Triton X-100/1% DMSO). Sections were incubated overnight at 4°C in a 1:100 dilution of Aaop8 mouse antiserum and/or 1:50 dilution of purified Aaop2 rabbit antiserum in blocking buffer. The slides were then rinsed 3 times for 10 min in PBT (1X PBS/0.1% Tween-20) and incubated for 1 h in a 1:500 dilution of goat anti-mouse and/or goat anti-rabbit IgG Alexa Fluor® 488 secondary antibody and/or 1:40 phalloidin-Alexa Flour® 594 in blocking buffer. Secondary antibodies and phalloidin were purchased from Invitrogen. Slides were rinsed three times for 10 min in PBT and mounted with Vectashield (Vector Labs). Analysis was done on a BioRad1024 confocal microscope.
For preparation of retinas for whole mounted observation, adult mosquito heads were bisected, leaving one eye undamaged, and fixed for 30 min with 2% paraformaldehyde at RT, or up to 24 h at 4°C. Retinas were dissected, washed three times in PBT, and incubated with the primary antibodies (mouse anti-Aaop8: 1:100, purified Rabbit anti-Aaop2: 1:50) diluted in BNT (1X PBS/0.1% BSA/0.1% Tween-20/250 mM NaCl) overnight at 4°C. After two rinses and a 30 min wash with PBT, retinal tissues were incubated with secondary antibodies (Alexa Fluor® 488 goat anti-mouse IgG or Alexa Fluor® 488 goat anti-rabbit IgG: 1:500 in BNT) and Alexa Fluor® 594 phalloidin (1:40) for 2–4 h at RT. After 4 times 10 min washes with PBT, retinal tissues were mounted in Vectashield and imaged with a BioRad1024 confocal microscope. Digital photomicrographs were scaled and adjusted for contrast and brightness using Adobe Photoshop CS3 software (Adobe Systems, San Jose, CA).
RESULTS
Ae. aegypti and An. gambiae have similar photoreceptor organization
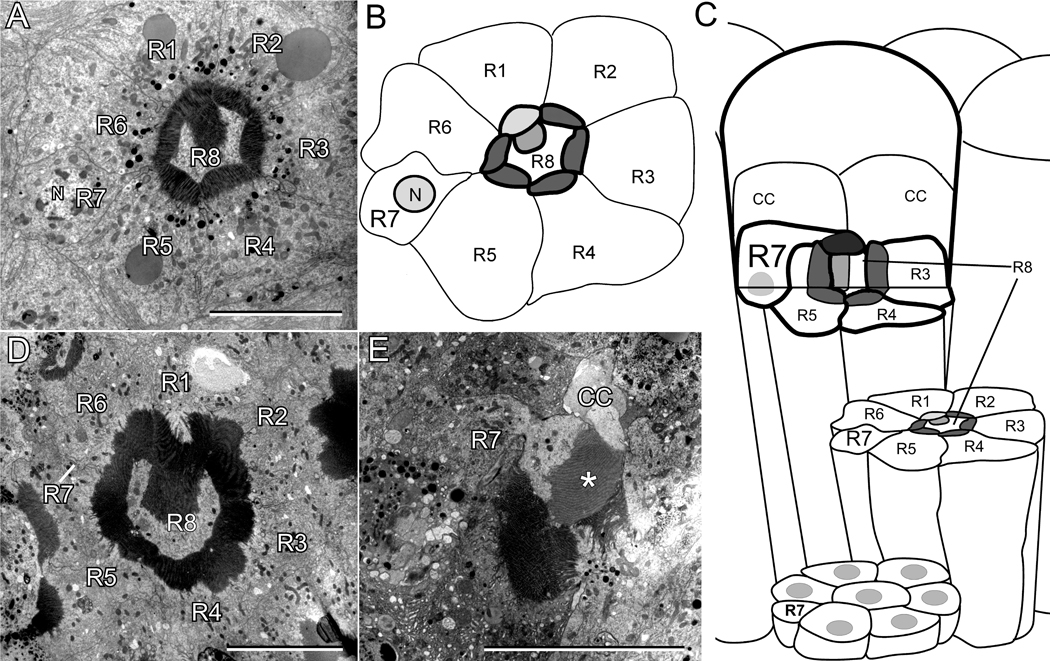
A previous study (Brammer, 1970) documented the arrangement of photoreceptors within an ommatidium in the adult Ae. aegypti eye. We confirmed this eye structure in both the pigmented and white-eyed strains of Ae. aegypti used in our study. Fig. 1A shows an electron micrograph cross-section through the middle of the rhabdomeric region of the pigmented Ae. aegypti eye. Fig. 1B depicts the cell and rhabdomeric membranes of the photoreceptors shown in the Fig. 1A micrograph, and Fig. 1C shows a diagram depicting the overall organization of the Ae. aegypti ommatidium. The rhabdomeric membranes of all photoreceptors are fused and form a ring structure such that the R1-6 cells project their rhabdomeres towards the interior. The R8 photoreceptor cell body fills the central space within the ring and contains an outward facing rhabdomere attached to the rhabdomere of the R1 cell. The remaining photoreceptor cell is the R7 cell. In the plane of the section shown in Fig. 1A, the R7 cell body and nucleus are visible, and located between the R5 and R6 photoreceptors. Fig. 1C shows that the rhabdomere of the R7 photoreceptor is positioned at the most distal rhabdom surface, lying on top of the fused rhabdom structure. The R7 photoreceptor cell is also distinguished by the location of its nucleus. Whereas the nuclei of all other photoreceptors are positioned proximally, near the basement membrane, the R7 cell’s nucleus is distal, positioned at the depth of the other photoreceptors’ rhabdomeres (Fig. 1A,B,C).
Figure 1.
Organization of photoreceptors in the ommatidium of Ae. aegypti and An. gambiae. A: Electron microscopy image of a single ommatidium in Ae. aegypti at the depth of the rhabdom showing the location of individual photoreceptors. B: Drawing based on A, showing the outline of the cell bodies and rhabdomeres of each of the photoreceptors. The R7 photoreceptor cell contains the nucleus (labeled N) but lacks a rhabdomere at this depth. C: Schematic illustration of the ultrastructure of an Ae. aegypti ommatidial unit. The diagram is based on the electron microscopic analyses of Brammer (1970) and of this report. D: Electron microscopy image of an An. gambiae ommatidial unit. The view is similar to that shown for Ae. aegypti in A. E: A distal retinal section of An. gambiae showing the extension of the R7 rhabdomere, marked with “*”, over the fused rhabdom. R1-R8, R1-R8 photoreceptor cells; N, nucleus; CC, cone cell. Scale bars in A, D, and E are approximately 10 µm.
Light microscopic analysis suggested that An. gambiae has a similar retinal organization as found in Ae. aegypti (Land et al., 1999). We confirmed this by carrying out an electron microscopic analysis. Fig. 1D shows an An. gambiae ommatidial unit at the depth of the rhabdom. The R8 cell fills the central space, projecting a rhabdomere outward that associated with the R1 cell’s rhabdomere, and the rhabdomeres of R1-6 photoreceptors create the ring structure of the rhabdom. Only the R7 cell body is visible in this section. Fig. 1E shows a more distal section in which the R7 cell (labeled) projects a rhabdomere (marked by *) over the fused rhabdom. A cone cell (labeled CC) is also visible. The An. gambiae R7 rhabdomere is distinguished from that of Ae. aegypti because it is larger in volume and is consistently stained with less electron density than any of the other rhabdomeres in these two species. However, the major conclusion from the analysis is that An. gambiae and Ae. aegypti have similar photoreceptor organization as depicted in the diagram of Fig. 1C.
op8 rhodopsin and an op2 rhodopsin family member are specifically expressed in R7 photoreceptor cells in Ae. aegypti and An. gambiae
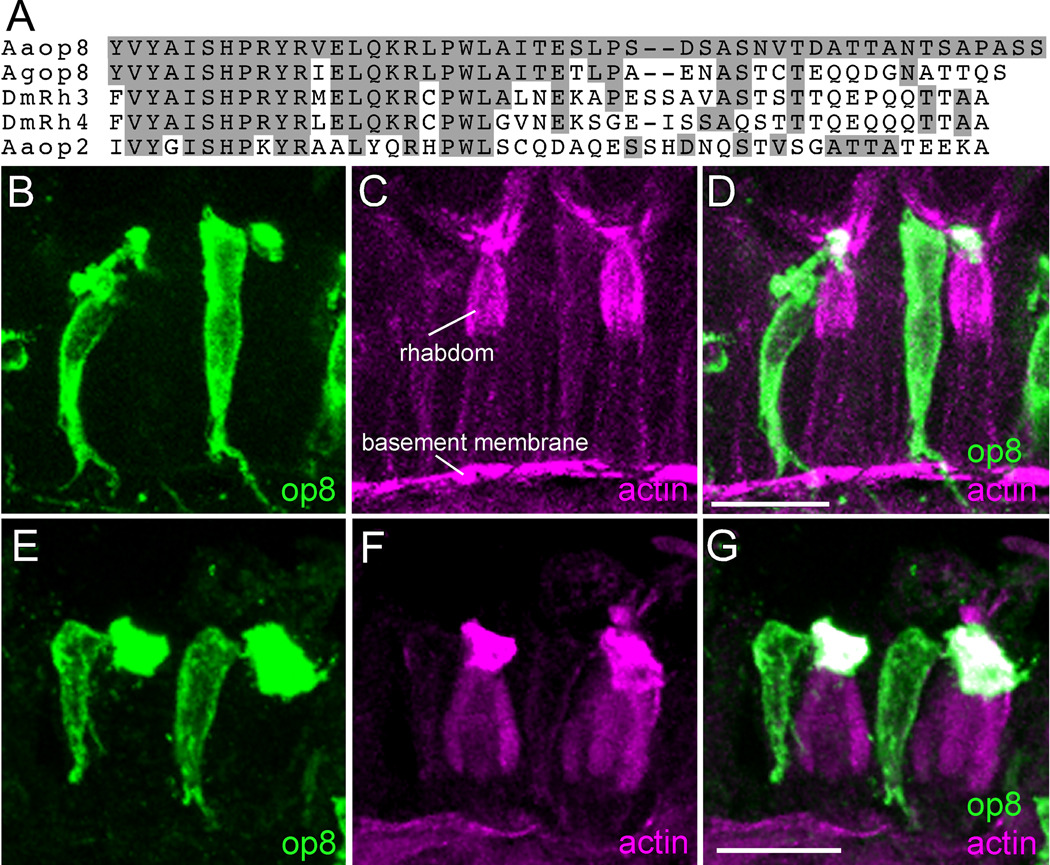
Ae. aegypti and An. gambiae each possess ten different rhodopsin genes. With the long range goal of understanding the cell-specific expression of rhodopsins in the mosquito eye, we made polyclonal antibodies against the carboxy tail sequence of different rhodopsins. In protein blots, the antisera raised against Ae. aegypti Aaop8 and Aaop2 stain a protein of 40 and 42 kDa molecular weight, respectively, as appropriate for each rhodopsin (supplemental Fig. S1). Further, the antisera prominently label retinal sections of transgenic Drosophila when these flies express the appropriate mosquito rhodopsin. These data provide evidence that these antisera detect the specified rhodopsins. The Aaop8 antiserum specifically recognized a single photoreceptor cell in many ommatidia of Ae. aegypti eye. To determine the identity of the labeled cell, the mosquito eye sections were counterstained with phalloidin to allow visualization of the actin-rich rhabdomeric membranes. Two neighboring ommatidial units are shown in Fig. 2B,C,D. The prominent actin staining in magenta identifies the fused rhabdomeres of all photoreceptor cells within the ommatidial unit. Actin staining is also detected in the membranes of the basal lamina. The Aaop8 protein is detected only at the most distal surface of the rhabdom structure. The only photoreceptor with this rhabdomeric projection is the R7 cell (Fig. 1), thus identifying R7 photoreceptor cell as the Aaop8-expressing cell. The Aaop8 antiserum also decorates the cell body of the R7 cell at a lower level, likely due to some localization of the Aaop8 rhodopsin within the plasma membrane of the R7 cell. This signal persists in the axonal projections from the R7 cell and extends into the optic lobe.
Figure 2.
Expression of op8 rhodopsin in Ae. aegypti and An. gambiae R7 photoreceptor cells. A: Multiple sequence alignment of the C-terminal regions of Ae. aegypti Aaop8 and Aaop2 with An. gambiae Agop8 and D. melanogaster Rh3 and Rh4 rhodopsins. Amino acids identical to the Aaop8 sequence are shaded gray. B, C, D: Ae. aegypti ommatidia labeled with Aaop8 antibody (green, B), phalloidin (magenta, C), and the resulting merged image (D). Phalloidin detects actin and heavily stains the rhabdom (labeled). E, F, G: An. gambiae ommatidia labeled with Aaop8 antibody (green, E), phalloidin (magenta, F), and the merged image (G). Scale bars in D and G are approximately 15 µm.
Given that An. gambiae and Ae. aegypti possess a similar photoreceptor organization and both genomes contain a rhodopsin, Agop8 and Aaop8, that share significant sequence homology, we investigated whether Agop8 is expressed in the An. gambiae R7 cell. The amino acid sequence of the Aaop8 carboxy tail region, used as antigen to generate antibody, is compared to the Agop8 carboxy tail region in Fig. 2A. Comparison to the Drosophila Rh3 and Rh4 UV rhodopsins (54% and 46% identical, respectively) and the Aaop2 rhodopsin (31% identical) is also shown. From testing the Aaop8 antibody on Drosophila eye sections, we found that the Aaop8 antibody labels Drosophila R7 cells and R8 cells of the dorsal rim area known to express Rh3 (Fortini and Rubin, 1990) indicating the cross reactivity of Aaop8 antibody with Drosophila Rh3. The fact that the C-terminal region of Aaop8 has more shared sequence identity with Agop8 (63%) than it does with Rh3 (54%) suggested the Aaop8 antiserum would also react with Agop8 rhodopsin. Fig. 2E,F,G shows that the Aaop8 antiserum labels a single photoreceptor in ommatidia of the An. gambiae retina. As in Ae. aegypti, this is the R7 cell, the only photoreceptor cell projecting a rhabdomere exclusively at the distal tip of the rhabdom (Fig. 1).
This analysis identified the R7 cell as the only photoreceptor cell type that expressed op8 rhodopsin in both the Ae. aegypti and An. gambiae retinas. However, not all R7 cells were stained by the op8 antibody in these two mosquitoes. This observation suggested that a similar situation exists in mosquitoes as previously seen in Drosophila in which the retina is a mosaic of different types of ommatidial units that are distinguished by the expression of different rhodopsins in the R7 cells. To identify the other rhodopsins expressed in the non-op8 expressing R7 cells in Ae. aegypti and An. gambiae, we tested antiserum raised against the Aaop2 carboxy tail sequence. Aaop2 is one member of a family of five closely related Ae. aegypti rhodopsins. Fig. 3A compares the carboxy terminus of Ae. aegypti Aaop2 family members as well as selected rhodopsins from An. gambiae. This comparison shows that Aaop2 retains the best sequence identity with Aaop1, and that contiguous sequence identity among the entire family is largely limited to the first 14 amino acids.
Figure 3.
Expression of op2 rhodopsin in Ae. aegypti and An. gambiae R7 photoreceptor cells. A: Multiple sequence alignment of the C-terminal regions of Ae. aegypti Aaop2, Aaop1, Aaop3, Aaop4, Aaop5 with An. gambiae Agop1, Agop3, Agop6 rhodopsins. Amino acids identical to the Aaop2 sequence are shaded gray. B, C, D: Ae. aegypti ommatidia labeled with Aaop2 antibody (green, B), phalloidin (magenta, C), and the merged image (D). E, F, G: An. gambiae ommatidia labeled with Aaop2 antibody (green, E), phalloidin (magenta, F), and the merged image (G). Scale bars in D and G are approximately 15 µm.
The immunostaining results show that Aaop2 antiserum also specifically recognized the R7 photoreceptor cell in some ommatidia of Ae. aegypti eye (Fig. 3B,C,D). As the C-terminal sequence of Aaop2 rhodopsin has good homology with An. gambiae Agop1, Agop3 and Agop6 rhodopsins, we also tested the Aaop2 antiserum on An. gambiae retinal sections. These experiments revealed that the Aaop2 antiserum specifically stained the rhabdomere of An. gambiae R7 photoreceptor cell in some ommatidia (Fig. 3E,F,G). The staining reaction was noticeably weaker in An. gambiae than Ae. aegypti, including the inability to decorate the An. gambiae R7 cell body.
op8 rhodopsin and an op2 rhodopsin family member are expressed in distinct domains of the Ae. aegypti and An. gambiae retinas
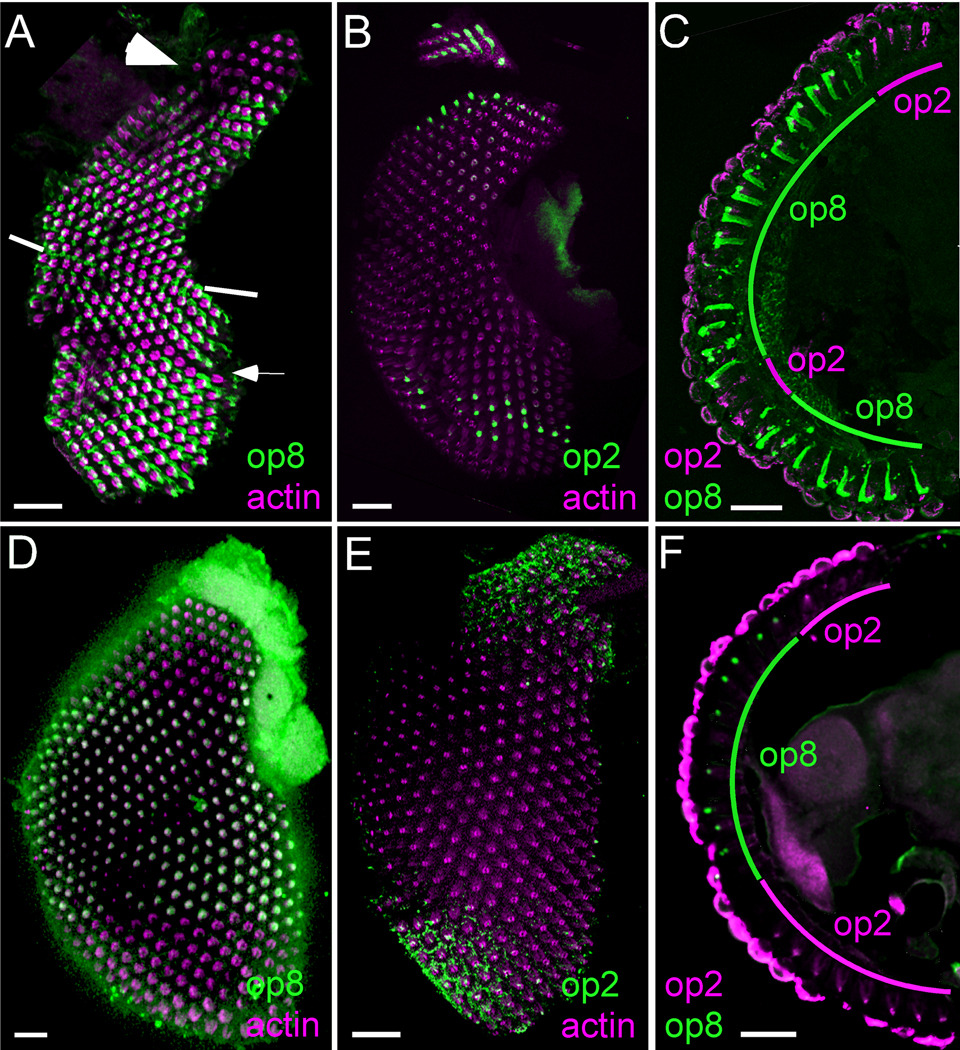
We used whole mount preparations, similar to those developed in Drosophila studies (Mazzoni et al., 2008), to understand the spatial patterning of the R7 cells in the Ae. aegypti and An. gambiae retinas. Fig. 4A shows Aaop8 antibody staining of the Ae. aegypti retina. While Aaop8 is broadly expressed within the retina, two retinal domains lack op8 staining. The first of these, marked by the large arrowhead in Fig. 4A, is found in the upper dorsal region. The second is a horizontal stripe, marked by the small white arrow, of one to three rows of ommatidia found within the ventral hemisphere. Mosquito eyes contain a dorsal/ventral equator, with each hemisphere having opposite orientations of the ommatidial units as previously observed in flies (Yang et al., 1999). The equator is marked in Fig. 4A by the white lines at the anterior and posterior margins. The R7 cell points upward in the ommatidia located above the equator and points downward in the ommatidia in the ventral domain. These observations placed the location of this stripe to be approximately four rows of ommatidia below the equator.
Figure 4.
op8 and op2 rhodopsins are expressed in nonoverlapping sets of R7 photoreceptor cells in Ae. aegypti and An. gambiae. A: Whole mount retina of Ae. aegypti stained with Aaop8 antibody (green) and co-labeled with phalloidin (magenta). The two regions not stained by Aaop8 antibody are the dorsal region (large white arrowhead) and the ventral stripe (small white arrow). The equator is marked by white lines at the margins of the eye. B: whole mount retina of Ae. aegypti stained with Aaop2 antibody (green) and co-labeled with phalloidin (magenta). C: Sectioned Ae. aegypti retina co-stained with Aaop8 antibody (green) and Aaop2 antibody (magenta). D: Whole mount retina of An. gambiae stained with Aaop8 antibody (green) and co-labeled with phalloidin (magenta). E: Whole mount retina of An. gambiae stained with Aaop2 antibody (green) and co-labeled with phalloidin (magenta). F: Sectioned An. gambiae retina co-stained with Aaop8 antibody (green) and Aaop2 antibody (magenta). Scale bars are approximately 50 µm.
The Aaop2 antiserum specifically recognized Ae. aegypti R7 cells in the ommatidia located in both the upper dorsal region and the stripe domain (Fig. 4B). We confirmed that Aaop8 and Aaop2 expression are found in nonoverlapping R7 cells in Ae. aegypti by examining retinal sections co-stained for Aaop2 (magenta) and Aaop8 (green). Fig. 4C shows that Aaop8 is broadly expressed through the central retina and ventral retina. Aaop2 specifically stains the dorsal domain and two rows of ommatidia located five ommatidia below the equator. Thus, this double labeling experiment confirmed the nonoverlapping expression of two different rhodopsins in the R7 photoreceptor cells of the Ae. aegypti retina. Both male and female retinas showed similar R7 photoreceptor patterning.
The expression pattern of rhodopsins in An. gambiae R7 photoreceptor cells was analyzed in a similar manner. Fig. 4D shows that Agop8 is expressed in all ommatidia in the equatorial region of the eye, but completely absent from both dorsal and ventral regions. Within the ventral region, Agop8 is expressed in the first four or five rows below the equator. Thus, the transition line from Agop8 expression occurs at the same location as the Aaop2 stripe of expression in Ae. aegypti. To determine the rhodopsin expressed in the dorsal and ventral regions lacking Agop8 expression, we stained the An. gambiae retina with the Aaop2 antibody. Weak R7 specific staining was observed in both the dorsal and ventral domains (Fig. 4E). Colabeling experiments using the Aaop2 and Aaop8 antibody in the same retinal sections (Fig. 4F) were consistent with the expectation that, like in Ae. aegypti, two nonoverlapping R7 cell types exist in the An. gambiae eye.
DISCUSSION
Similar photoreceptor organization is found in Ae. aegypti and An. gambiae
In a single ommatidium, the retinal photoreceptors of Ae. aegypti and An. gambiae assemble into conical rhabdom structure allowing photoreceptors to raise quantum capture efficiency without loss of spectral and polarization sensitivity (Land, 1997; Land et al., 1999; Snyder et al., 1973). The two species differ with respect to facet diameter and interommatidial angle. In a survey of mosquito species, these parameters correlate with photoperiod behavior, with An. gambiae and other nocturnal species having higher values suggesting structural modifications to increase sensitivity at the expense of spatial resolution (Kawada et al., 2006).
We extended the ultrastuctural analysis of Brammer (1970) to show that the ommatidia of both Ae. aegypti and An. gambiae possess eight photoreceptor cells in a stereotypical arrangement. The R8 cell body is located at the center of the ommatidium and projects a rhabdomere outward that contacts the R1 rhabdomere. The cell bodies of the other photoreceptors occupy peripheral positions and project rhabdomeres towards the center of the ommatidium. The rhabdomeric membranes of the R1-6 photoreceptors are fused with each other and form the ring structure of the rhabdom. The remaining photoreceptor cell is the R7 cell. The rhabdomere of the R7 cell is present only at the most distal rhabdom surface, lying on the top of the fused rhabdom structure. The R7 photoreceptor cell is also distinguished by the distal location of its nucleus, as the nuclei of all other photoreceptors are positioned at the proximal end of the cell body.
The Ae. aegypti and An. gambiae genome projects (Hill et al., 2002; Holt et al., 2002; Nene et al., 2007) revealed that these two mosquito species both possess ten different rhodopsin genes. From lineage relatedness to the Drosophila rhodopsins, the rhodopsin family in each mosquito species likely includes six or five genes encoding long wavelength (>500 nm) rhodopsins. Most of these rhodopsins are likely expressed in the adult retina. Stein et al. (1979) isolated a rhodopsin with a λmax 520 nm from adult Ae. aegypti, and a sensitivity peak at 523 nm is present in the Ae. aegypti retina (Muir et al., 1992). Two of the transcripts (Aaop1 and Aaop2) were detected by in situ hybridization to adult eyes (Graf et al., 1996), and all but three (Aaop7, Aaop10, Aaop12) were identified in the adult cDNAs characterized during execution of the Ae. aegypti genome project (according to GenBank's EST data).
Ae. aegypti and An. gambiae also contain one gene encoding a short wavelength rhodopsin (400–500 nm), and one gene encoding a UV rhodopsin (<400 nm). These two rhodopsins were also detected in adult Ae. aegypti cDNAs. Further, Muir et al. (1992) showed the adult eye possessed UV sensitivity peaks, at 333 nm in the dorsal eye region, and at 345 nm in the ventral eye region also indicating the presence of UV rhodopsin in Ae. aegypti. There are two or three additional rhodopsin genes for which there are no characterized close relatives in Drosophila and there is no information regarding their spectral properties (Nene et al., 2007).
Rhodopsin expression in mosquito R7 photoreceptor cells
By using antibodies raised against specific rhodopsins, we have shown that two different types of R7 photoreceptor cells, distinguished by the rhodopsin expressed, exist in Ae. aegypti and An. gambiae. One class of R7 photoreceptor cells expresses the op8 rhodopsin Aaop8 or Agop8. Aaop8 and Agop8 are the only rhodopsins found in the mosquito genomes with close relatedness to Drosophila UV-sensitive Rh3 and Rh4 rhodopsins, leading to the expectation that the mosquito R7 cells expressing op8 rhodopsin are the UV-sensitive photoreceptors in these two mosquitoes.
The second class of R7 photoreceptor cells expresses one or more members of the op2 family of rhodopsins. Whereas the mosquito op8 rhodopsins belong to the UV wavelength group, the op2 rhodopsin family members belong to the long wavelength group (Nene et al., 2007; Spaethe and Briscoe, 2004). The op2 rhodopsin family members are Aaop1, Aaop2, Aaop3, Aaop4 and Aaop5 in Ae. aegypti and Agop1, Agop3 and Agop6 in An. gambiae. The op2 rhodopsin family members retain high sequence identity throughout their length, with the N-terminal and C-terminal regions possessing the weakest identity. Our analysis relied on the antiserum raised against the C-terminus of the Aaop2 protein in an effort to create an Aaop2 specific reagent. However, the analysis of transgenic flies expressing other Ae. aegypti long wavelength rhodopsins shows the this antiserum will detect other family members. This observation precludes unambiguous identification of which long wavelength op2 family member is expressed in the R7 cell.
The retinal patterning of R7 photoreceptors in Ae. aegypti and An. gambiae
The nonoverlapping expression of two different rhodopsins, each with different spectral properties, in the R7 photoreceptor cells shows that both Ae. aegypti and An. gambiae are capable of gathering different qualities of visual information from this photoreceptor cell. Further, we have shown that these two classes of R7 cells are not randomly distributed, but rather divide the mosquito retina into four distinct domains. These four domains will be referred to as the upper dorsal region, the central region, the ventral stripe, and the lower ventral region.
Both Ae. aegypti and An. gambiae retinas possess a upper dorsal region that is populated by R7 cells expressing the op2 family of rhodopsins. Distinctive rhodopsin expression in the upper dorsal region is common in insect eyes. Drosophila has a row of ommatidia at the dorsal rim with precise orientation of the R7 and R8 cell rhabdomeres. Within this region the R7 and R8 cells express the same UV rhodopsin Rh3. These features enable the detection of polarized light (Labhart and Meyer, 2002). It is unlikely that mosquitoes use a similar mechanism for detection of polarized light because the R7 and R8 cells lack aligned rhabdomeres and do not express the same rhodopsin. A second set of observations involve sexual dimorphisms in the dorsal region of the housefly Musca domestica (Franceschini et al., 1981) and butterfly species (Briscoe and Chittka, 2001) that are likely key to mating behaviors in these species. However, we found both males and females of the two mosquito species have similar R7 photoreceptor patterning in this region. Thus, the earlier observations made in other insects do not account for the presence of a specialized upper dorsal region in the two mosquito species.
Below the upper dorsal region is the central region of the eye. In both Ae. aegypti and An. gambiae, the R7 cells in this region express the UV-sensitive op8 rhodopsin. The central region extends ventrally about four rows of ommatidia below the equator. This domain is the largest of the four regions thus representing the major visual field of the mosquito. The importance of UV vision has been documented in many animal behaviors including navigation, foraging, intraspecies communication, and the control of circadian rhythms (Hunt et al., 2001; Tovee, 1995). The exclusive presence of R7 cells expressing op8 is the basis of UV detection in the major visual field of these two mosquito species.
The ventral edge of the central region is bounded by a region that again expresses the op2 family member in the R7 cells. A unique feature of the Ae. aegypti eye is that these R7 cells are only found in a one to three ommatidia wide horizontal stripe. The function of this stripe is not known. One possibility is that this stripe allows direct comparison of the light intensity detected by the R7 cells of the upper dorsal region to the intensity detected by the R7 cells of the ventral stripe. Such a mechanism would allow the mosquito assess orientation and facilitate reorientation to a horizontal flight position. Although the stripe is only distinctive in Ae. aegypti, it should be noted that An. gambiae also has op2-type cells in the location of the Ae. aegypti stripe. Therefore An. gambiae may require and use the same types of visual inputs provided by the stripe domain in Ae. aegypti.
In the two mosquito species studied here, only the lower ventral region differs in R7 cell rhodopsin expression. Ae. aegypti expresses the UV-sensitive Aaop8 while An. gambiae extends the stripe domain of long wavelength op2-type R7 cells through the lower ventral region. These differences likely relate to adaptations based on the different lifestyles of the two species. An. gambiae is known to be nocturnal while Ae. aegypti shows diurnal rhythms (Day, 2005; Kawada et al., 2006), hence the two species are active in different levels and quality of environmental light. An important consideration is that this lower ventral region supplies the mosquito with a visual description of the surface directly underfoot upon and after flight landing. Therefore, the ventral region likely plays a large role in supplying visual input into feeding and egg laying behaviors.
In summary, the differential expression of rhodopsins within the R7 photoreceptors reveals that the eyes of the Ae. aegypti and An. gambiae mosquitoes are divided into multiple regions. The conserved expression of R7 cell rhodopsins within most of these regions suggest that these two mosquito species continue to share common visually-based behavioral strategies despite having diverged 150 million years ago. On the other hand, the R7 cell rhodopsin expressed within the lower ventral region is different in Ae. aegypti and An. gambiae. Because these two mosquitoes are active in different lighting environments, this adaptation likely allows better use of the available visual information and also suggests a specialized role of this ventral region in mosquito visual behaviors.
Supplementary Material
ACKNOWLEDGMENTS
Grant sponsor: National Institutes of Health; Grant number: EY006808 (to J.E.O.)
We thank David Severson for providing the Aaop8 and Aaop2 cDNA plasmids, the laboratories of Frank Collins, David Severson, and Malcolm Fraser for providing mosquito specimens, Yeona Chun, Bill Archer, and Jose Chaverri for assistance with electron microscopy, and Lisa Bartkowiak, Joe Real, and Zachary Lemmon for assistance with the fusion protein analysis.
LITERATURE CITED
- Ahmad ST, Natochin M, Artemyev NO, O'Tousa JE. The Drosophila rhodopsin cytoplasmic tail domain is required for maintenance of rhabdomere structure. Faseb J. 2007;21:449–455. doi: 10.1096/fj.06-6530com. [DOI] [PubMed] [Google Scholar]
- Brammer JD. The ultrastructure of the compound eye of a mosquito Aedes aegypti L. J Exp Zool. 1970;195:181–196. [Google Scholar]
- Briscoe AD, Chittka L. The evolution of color vision in insects. Annu Rev Entomol. 2001;46:471–510. doi: 10.1146/annurev.ento.46.1.471. [DOI] [PubMed] [Google Scholar]
- Clandinin TR, Zipursky SL. Making connections in the fly visual system. Neuron. 2002;35:827–841. doi: 10.1016/s0896-6273(02)00876-0. [DOI] [PubMed] [Google Scholar]
- Day JF. Host-seeking strategies of mosquito disease vectors. J Am Mosq Control Assoc. 2005;21:17–22. doi: 10.2987/8756-971X(2005)21[17:HSOMDV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Fortini ME, Rubin GM. Analysis of cis-acting requirements of the Rh3 and Rh4 genes reveals a bipartite organization to rhodopsin promoters in Drosophila melanogaster. Genes Dev. 1990;4:444–463. doi: 10.1101/gad.4.3.444. [DOI] [PubMed] [Google Scholar]
- Franceschini N, Hardie R, Ribi W, Kirschfled K. Sexual dimorphism in a photoreceptor. Nature. 1981;291:241–244. [Google Scholar]
- Graf R, Godknechi A, Nakano M, Li X, Ackermann U, Helbling P. Cloning and expression analysis of Aedes aegypti opsin: adaptation of an in situ hybridization protocol for mosquitoes. Insect Mol Biol. 1996;5:173–180. doi: 10.1111/j.1365-2583.1996.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Hill CA, Fox AN, Pitts RJ, Kent LB, Tan PL, Chrystal MA, Cravchik A, Collins FH, Robertson HM, Zwiebel LJ. G protein-coupled receptors in Anopheles gambiae. Science. 2002;298:176–178. doi: 10.1126/science.1076196. [DOI] [PubMed] [Google Scholar]
- Hill CA, Kafatos FC, Stansfield SK, Collins FH. Arthropod-borne diseases: vector control in the genomics era. Nat Rev Microbiol. 2005;3:262–268. doi: 10.1038/nrmicro1101. [DOI] [PubMed] [Google Scholar]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR, Wincker P, Clark AG, Ribeiro JM, Wides R, Salzberg SL, Loftus B, Yandell M, Majoros WH, Rusch DB, Lai Z, Kraft CL, Abril JF, Anthouard V, Arensburger P, Atkinson PW, Baden H, de Berardinis V, Baldwin D, Benes V, Biedler J, Blass C, Bolanos R, Boscus D, Barnstead M, Cai S, Center A, Chaturverdi K, Christophides GK, Chrystal MA, Clamp M, Cravchik A, Curwen V, Dana A, Delcher A, Dew I, Evans CA, Flanigan M, Grundschober-Freimoser A, Friedli L, Gu Z, Guan P, Guigo R, Hillenmeyer ME, Hladun SL, Hogan JR, Hong YS, Hoover J, Jaillon O, Ke Z, Kodira C, Kokoza E, Koutsos A, Letunic I, Levitsky A, Liang Y, Lin JJ, Lobo NF, Lopez JR, Malek JA, McIntosh TC, Meister S, Miller J, Mobarry C, Mongin E, Murphy SD, O'Brochta DA, Pfannkoch C, Qi R, Regier MA, Remington K, Shao H, Sharakhova MV, Sitter CD, Shetty J, Smith TJ, Strong R, Sun J, Thomasova D, Ton LQ, Topalis P, Tu Z, Unger MF, Walenz B, Wang A, Wang J, Wang M, Wang X, Woodford KJ, Wortman JR, Wu M, Yao A, Zdobnov EM, Zhang H, Zhao Q, Zhao S, Zhu SC, Zhimulev I, Coluzzi M, della Torre A, Roth CW, Louis C, Kalush F, Mural RJ, Myers EW, Adams MD, Smith HO, Broder S, Gardner MJ, Fraser CM, Birney E, Bork P, Brey PT, Venter JC, Weissenbach J, Kafatos FC, Collins FH, Hoffman SL. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- Hunt DM, Wilkie SE, Bowmaker JK, Poopalasundaram S. Vision in the ultraviolet. Cell Mol Life Sci. 2001;58:1583–1598. doi: 10.1007/PL00000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H, Honda S, Takagi M. Comparative laboratory study on the reaction of Aedes aegypti and Aedes albopictus to different attractive cues in a mosquito trap. J Med Entomol. 2007;44:427–432. doi: 10.1603/0022-2585(2007)44[427:clsotr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kawada H, Takemura SY, Arikawa K, Takagi M. Comparative study on nocturnal behavior of Aedes aegypti and Aedes albopictus. J Med Entomol. 2005;42:312–318. doi: 10.1093/jmedent/42.3.312. [DOI] [PubMed] [Google Scholar]
- Kawada H, Tatsuta H, Arikawa K, Takagi M. Comparative study on the relationship between photoperiodic host-seeking behavioral patterns and the eye parameters of mosquitoes. J Insect Physiol. 2006;52:67–75. doi: 10.1016/j.jinsphys.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Kirschfeld K. The projection of the optical environment on the screen of the rhabdomere in the compound eye of the Musca. Exp Brain Res. 1967;3:248–270. doi: 10.1007/BF00235588. [DOI] [PubMed] [Google Scholar]
- Labhart T, Meyer EP. Neural mechanisms in insect navigation: polarization compass and odometer. Curr Opin Neurobiol. 2002;12:707–714. doi: 10.1016/s0959-4388(02)00384-7. [DOI] [PubMed] [Google Scholar]
- Land MF. Visual acuity in insects. Annu Rev Entomol. 1997;42:147–177. doi: 10.1146/annurev.ento.42.1.147. [DOI] [PubMed] [Google Scholar]
- Land MF, Gibson G, Horwood J, Zeil J. Fundamental differences in the optical structures of the eyes of nocturnal and diurnal mosquitoes. J Comp Physiol A. 1999;185:91–103. [Google Scholar]
- Mazzoni EO, Celik A, Wernet MF, Vasiliauskas D, Johnston RJ, Cook TA, Pichaud F, Desplan C. Iroquois complex genes induce co-expression of rhodopsins in Drosophila. PLoS Biol. 2008;6:e97. doi: 10.1371/journal.pbio.0060097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir LE, Thorne MJ, Kay BH. Aedes aegypti (Diptera: Culicidae) vision: spectral sensitivity and other perceptual parameters of the female eye. J Med Entomol. 1992;29:278–281. doi: 10.1093/jmedent/29.2.278. [DOI] [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, Ren Q, Zdobnov EM, Lobo NF, Campbell KS, Brown SE, Bonaldo MF, Zhu J, Sinkins SP, Hogenkamp DG, Amedeo P, Arensburger P, Atkinson PW, Bidwell S, Biedler J, Birney E, Bruggner RV, Costas J, Coy MR, Crabtree J, Crawford M, Debruyn B, Decaprio D, Eiglmeier K, Eisenstadt E, El-Dorry H, Gelbart WM, Gomes SL, Hammond M, Hannick LI, Hogan JR, Holmes MH, Jaffe D, Johnston JS, Kennedy RC, Koo H, Kravitz S, Kriventseva EV, Kulp D, Labutti K, Lee E, Li S, Lovin DD, Mao C, Mauceli E, Menck CF, Miller JR, Montgomery P, Mori A, Nascimento AL, Naveira HF, Nusbaum C, O'Leary S, Orvis J, Pertea M, Quesneville H, Reidenbach KR, Rogers YH, Roth CW, Schneider JR, Schatz M, Shumway M, Stanke M, Stinson EO, Tubio JM, Vanzee JP, Verjovski-Almeida S, Werner D, White O, Wyder S, Zeng Q, Zhao Q, Zhao Y, Hill CA, Raikhel AS, Soares MB, Knudson DL, Lee NH, Galagan J, Salzberg SL, Paulsen IT, Dimopoulos G, Collins FH, Birren B, Fraser-Liggett CM, Severson DW. Genome sequence of Aedes aegypti, a major arbovirus vector. Science. 2007;316:1718–1723. doi: 10.1126/science.1138878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sison-Mangus MP, Bernard GD, Lampel J, Briscoe AD. Beauty in the eye of the beholder: the two blue opsins of lycaenid butterflies and the opsin gene-driven evolution of sexually dimorphic eyes. J Exp Biol. 2006;209:3079–3090. doi: 10.1242/jeb.02360. [DOI] [PubMed] [Google Scholar]
- Snyder A, Menzel R, Laughlin S. Structure and Function of the Fused Rhabdom. J Comp Physiol. 1973;87:99–135. [Google Scholar]
- Spaethe J, Briscoe AD. Early duplication and functional diversification of the opsin gene family in insects. Mol Biol Evol. 2004;21:1583–1594. doi: 10.1093/molbev/msh162. [DOI] [PubMed] [Google Scholar]
- Stein PJ, Brammer JD, Ostroy SE. Renewal of opsin in the photoreceptor cells of the mosquito. J Gen Physiol. 1979;74:565–582. doi: 10.1085/jgp.74.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovee MJ. Ultra-violet photoreceptors in the animal kingdom-their distribution and function. Trends Ecol Evol. 1995;5:455–460. doi: 10.1016/s0169-5347(00)89179-x. [DOI] [PubMed] [Google Scholar]
- Yang CH, Simon MA, McNeill H. mirror controls planar polarity and equator formation through repression of fringe expression and through control of cell affinities. Development. 1999;126:5857–5866. doi: 10.1242/dev.126.24.5857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.