Abstract
Binge alcohol use is common among teenagers with 28% of 12th graders reporting getting drunk in the past month. Chronic heavy drinking has been associated with verbal learning and memory deficits in adolescents and adults, yet verbal encoding in less frequently drinking teens has not yet been studied. Here, we examined functional magnetic resonance imaging (fMRI) response during verbal encoding among adolescent binge drinkers. Participants recruited from local high schools were of ages 16–18 and consisted of 12 binge drinkers and 12 demographically similar nondrinkers. Participants were all nonsmokers, and drinkers were abstinent from alcohol for an average of 33 days at the time of scanning. Participants performed a verbal paired associates learning task during fMRI acquisition. Drinkers recalled marginally fewer words than nondrinkers (P = .07). Compared with nondrinkers, bingers showed more response in right superior frontal and bilateral posterior parietal cortices but less response in occipital cortex during novel encoding (Ps < .05, clusters > 1,512 µL). In addition, controls showed significant activation in the left hippocampus during novel encoding, whereas binge drinkers did not. Adolescent binge drinkers demonstrated (1) more response than nondrinkers in frontal and parietal regions, which could suggest greater engagement of working memory systems during encoding; (2) no hippocampal activation to novel word pairs; and (3) slightly poorer word pair recall, which could indicate disadvantaged processing of novel verbal information and a slower learning slope. Longitudinal studies will be needed to ascertain the degree to which emergence of binge drinking is linked temporally to these brain response patterns.
Keywords: Alcohol, Functional MRI, Adolescence, Memory
Introduction
Heavy episodic or binge alcohol use is common among adolescents and is commonly defined as consuming at least four or five drinks on an occasion for females and males, respectively. Although 6–10% of adolescents meet diagnostic criteria for alcohol use disorders (Clark et al., 2002; Rohde et al., 1996), binge alcohol use is much more prevalent and often viewed by teens as innocuous. Recent surveys of 12th graders reveal that 28% report getting drunk in the past month and only 49% perceived harm in binge drinking once or twice each weekend (Johnston et al., 2009). Despite the perceived harmlessness of binge alcohol use in adolescence, the influence of binge drinking on adolescent neurodevelopment and brain functioning is unclear. Of significant concern, learning and memory abilities are key to academic success but may be particularly sensitive to the influence of alcohol use. Here, we examined brain response during verbal learning among adolescent binge drinkers using functional magnetic resonance imaging (fMRI).
Chronic heavy drinking has been associated with poorer verbal learning among both adults (for review, see Grant, 1987) and adolescents (Brown et al., 2000). More recently, structural neuroimaging studies have begun to explore the neurobiological substrates of altered memory functioning. Teens with alcohol use disorders have demonstrated reduced volumes of the hippocampus (De Bellis et al., 2000; Medina et al., 2007; Nagel et al., 2005) and prefrontal cortex (De Bellis et al., 2005; Medina et al., 2008), areas crucial to intact learning and memory (Budson, 2009; Squire and Schacter, 2002). We have previously shown altered frontal lobe fMRI response during working memory among adolescents with alcohol use disorders (Tapert et al., 2004), which may contribute to modestly reduced learning performance among the broader population of adolescent drinkers. To our knowledge, no study has yet examined fMRI response during verbal learning among adolescent binge drinkers.
To characterize the neural substrates of learning and memory in binge drinking adolescents, we collected blood oxygen level–dependent (BOLD) fMRI during a verbal paired associates encoding task. Previous research using this verbal learning task has suggested different BOLD response patterns during encoding of novel as compared with familiar information (Han et al., 2007). Because alcohol use has been associated with impaired initial learning (Brown et al., 2000), we predicted that binge drinking teens would exhibit poorer learning and reduced prefrontal and hippocampal fMRI response during novel verbal encoding.
Materials and methods
Participants
Adolescents aged 16–18 years were recruited from local high schools and colleges as part of an ongoing study (Tapert et al., 2007). After obtaining written informed assent and consent, approved by the University of California San Diego Human Research Protection Program, eligibility was determined with telephone interviews. Exclusion criteria included history of medical or neurological disorders, psychiatric disorders, more than 10 lifetime uses of drugs, significant head injury, learning disabilities, significant maternal drinking or drug use during pregnancy, immediate family history of psychotic disorders or bipolar I, left-handedness, and MRI contraindications.
The final sample included 12 binge drinkers and 12 demographically similar nondrinkers. As previously defined by our group (McQueeny et al., 2009) and others (for review, see Courtney and Polich, 2009), binge drinkers were those who typically drank ≥4 drinks per occasion for females and ≥5 drinks per occasion for males. Groups were similar on demographics, mood, and estimated premorbid IQ based on vocabulary score (Table 1). Binge drinkers reported 10.4 ± 4.8 drinks per occasion but drank an average of just 21.2 ± 14 drinks per month during the 3 months before scanning and had been drinking for an average of 3.1±1.8 years. At the time of scanning, drinkers had been abstinent for an average of 32.5 ± 10.5 days. Binge drinkers had more lifetime experiences with marijuana and other drug use than controls, but this use was limited to less than 10 total lifetime uses for an average of 2.9 ± 3.6 uses. Controls and binge drinkers were all nonsmokers.
Table 1.
Participant demographic and substance use characteristics
| Binge drinkers (n = 12), M (S.D.) or % |
Controls (n = 12), M (S.D.) or % |
|
|---|---|---|
| Age (range 16–18 years) | 18.2 (0.8) | 17.8 (0.9) |
| % Female | 16.7 | 33.3 |
| % Caucasian | 72.7 | 66.7 |
| % Family history negativea | 91.9 | 91.9 |
| Parent annual salary (thousands) | 118.2 (72.2) | 102.9 (82.6) |
| Vocabulary T-scoreb | 55.8 (10) | 56.3 (8) |
| Spielberger State Anxiety T-score | 36.2 (4.7) | 35.0 (2.9) |
| Beck Depression Inventory total | 2.5 (2.4) | 1.5 (2.1) |
| Child Behavior Checklist, externalizing T-score |
40.8 (10.2) | 47.5 (10.1) |
| Child Behavior Checklist, internalizing T-score |
45.6 (9.3) | 46.3 (7.1) |
| Lifetime drinking episodes** | 55.3 (48.0) | 1.3 (2.7) |
| Drinks per month, past 3 months** | 21.2 (13.7) | 0.0 (0.0) |
| Drinks per occasion, past 3 months** | 10.4 (4.8) | 0.0 (0.0) |
| Drinking days, past month | 1.5 (1.1) | 0.0 (0.0) |
| Lifetime alcohol abuse and dependence criteria* |
1.1 (1.3) | 0.0 (0.0) |
| Lifetime marijuana use episodes* | 2.1 (3.1) | 0.0 (0.0) |
| Lifetime other drug use episodes | 0.8 (2.3) | 0.0 (0.0) |
| Fagerstrom Test for Nicotine Dependence total (max = 10) |
0.0 (0.0) | 0.0 (0.0) |
P < 05.
P < 001.
No first-degree biological relative with alcohol or drug abuse or dependence.
Based on Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), age-corrected T-scores (mean = 50, S.D. = 10).
Measures
Substance involvement
Detailed information on alcohol and other drug involvement was collected with the Customary Drinking and Drug Use Record (CDDR, Brown et al., 1998). The CDDR is a semistructured interview that ascertains lifetime and past 3-month use of alcohol, nicotine, and other drugs; Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) substance use disorder (APA, 1994) symptoms; withdrawal experiences; and peak and typical consumption amount and duration. Blood alcohol concentrations were estimated by taking into consideration drinking amount and duration, height, weight, and gender (Fitzgerald, 1995). The CDDR also incorporates the Fagerstrom Test for Nicotine Dependence (Heatherton et al., 1991). In addition to the CDDR, participants completed one 28-day Timeline Followback (Sobell and Sobell, 1992) 28 days before scanning and another 28-day Timeline Followback on the day of scanning. Together, these provided detailed substance use patterns for the 2 months before scanning.
Behavior and mood
Participants completed several behavioral and mood assessments at the time of fMRI scanning. The Child Behavior Checklist (Achenbach and Rescorla, 2001) ascertained internalizing and externalizing behaviors. The Beck Depression Inventory (Beck, 1978) and Spielberger State Trait Anxiety Inventory (Spielberger et al., 1970) assessed mood at the time of scanning.
Verbal paired associates task
The verbal encoding task (Eyler et al., 2008; Fleisher et al., 2005; Han et al., 2007) required participants to memorize word pairs both before and during fMRI scanning. Participants were asked to memorize a list of 16 highly associated pairs of monosyllabic nouns that were presented for 5 s each. After the word pairs were presented, recall was tested by showing the first member of a pair and asking subjects to verbalize the second member of that pair. Learning and recall trials were repeated until the subject was able to correctly recall 10 of the 16 pairs. This learning and recall session took place while participants were lying in the scanner, immediately before fMRI acquisition. After learning and recall were performed to criterion, the scanning portion of the task began. During scanning, participants viewed 32 pairs of associated words (for 5 s each). During the repeated pairs condition, subjects were presented with the pairs of words on which they were trained before scanning. For the novel encoding condition, subjects were presented with novel word pairs. For both of these conditions, subjects were instructed to learn the word pairs. The task also consisted of “fixation” trials with variable durations of 8, 12, and 16 s. Total scanning time was 4 min 36 s. Immediately after scanning, recall for both repeated and novel word pairs was assessed.
Procedures
Image acquisition
During scanning, participants viewed a screen at the foot of the scanner bed via a mirror attached to the head coil, and the verbal encoding task was projected onto the screen from a laptop computer. After situation in the scanner and collection of structural imaging, participants were asked to learn the first set of word pairs, and recall was subsequently tested as previously described. Functional imaging was acquired during the second phase of paired associates learning, after which recall was again tested.
Images were acquired on a 3-tesla General Electric scanner. Scanning included a structural image collected in the sagittal plane with an inversion recovery–prepared T1-weighted spoiled gradient recalled acquisition in the steady state (SPGR) sequence (repetition time = 8 ms, echo time = 3 ms, field of view = 240 mm, resolution = 1 mm3, 176 continuous slices, and acquisition time = 7 min 19 s). During the verbal encoding task, functional imaging was acquired axially, using T2*-weighted echo planar imaging (repetition time = 4,000 ms, echo time = 30 ms, flip angle = 90°, field of view = 240 mm, 32 slices covering the whole brain, slice thickness = 3.8 mm, in-plane resolution = 3.75 × 3.75 mm, 69 repetitions, and acquisition time = 4 min 36 s).
Data analysis
Imaging data were processed and analyzed using Analysis of Functional NeuroImages (AFNI) (Cox, 1996). First, the time series data sets were corrected for motion with an automated algorithm. Two independent raters then identified and removed repetitions containing visible head motions that were not corrected by the algorithm; all participants retained at least 90% of repetitions. Time series data were then deconvolved with a reference function that represented the time course of the task while accounting for hemodynamic responses (Bandettini et al., 1993) and covarying for the estimated degree of motion and linear trends. This yielded fit coefficients representing the BOLD response contrast during encoding for each voxel in the brain for each subject. As previous work using a similar version of this task revealed that the most robust contrast was encoding of novel word pairs versus fixation (Eyler et al., 2008), this was the contrast of focus for the present analyses. Data were transformed into standard Talairach coordinates (Lancaster et al., 2000; Talairach and Tournoux, 1988). Finally, functional data were resampled into 3-mm isotropic voxels and spatially smoothed with a Gaussian filter (5 mm full width half maximum) to account for anatomic variability between participants.
Whole-brain BOLD response data were analyzed between groups with t-tests. To control for Type I error, we used a Monte Carlo simulation to determine the minimum cluster volume and statistical threshold of significant clusters (Forman et al., 1995). Clusters were considered significant if they comprised contiguous activated (α < 0.05) voxels that exceeded 1,512 µL in volume (cluster-wise α < 0.05).
In addition to the whole-brain analysis, we also examined BOLD response within a hippocampal region of interest (ROI) defined by Talairach space, because of the critical role of the hippocampus in encoding. This hippocampal ROI was defined a priori using the ROI feature of AFNI, which is based on the Talairach atlas. Thus, ROI boundaries are defined independently from activation patterns in these data. The hippocampal ROI was resampled to the same resolution as functional data sets to include in analyses all functional voxels that were partially located within the hippocampal ROI. The resulting left and right hippocampal ROIs were 3.3 cm3 each. BOLD response during novel encoding was averaged across the hippocampal ROIs for each subject and then examined within and between groups.
Results
Behavioral results
During the scanning portion of the verbal paired associates task, controls recalled 85 ± 9% of words, whereas binge drinkers recalled 78 ± 11% of word pairs (t[22] = 1.87, P = .07). Although 8% of controls did not correctly recall 10 out of 16 pairs on the initial learning trial and therefore required a second training session, 42% of binge drinkers did not meet criterion on the first trial (χ2[1] = 3.56, P = .06).
fMRI results
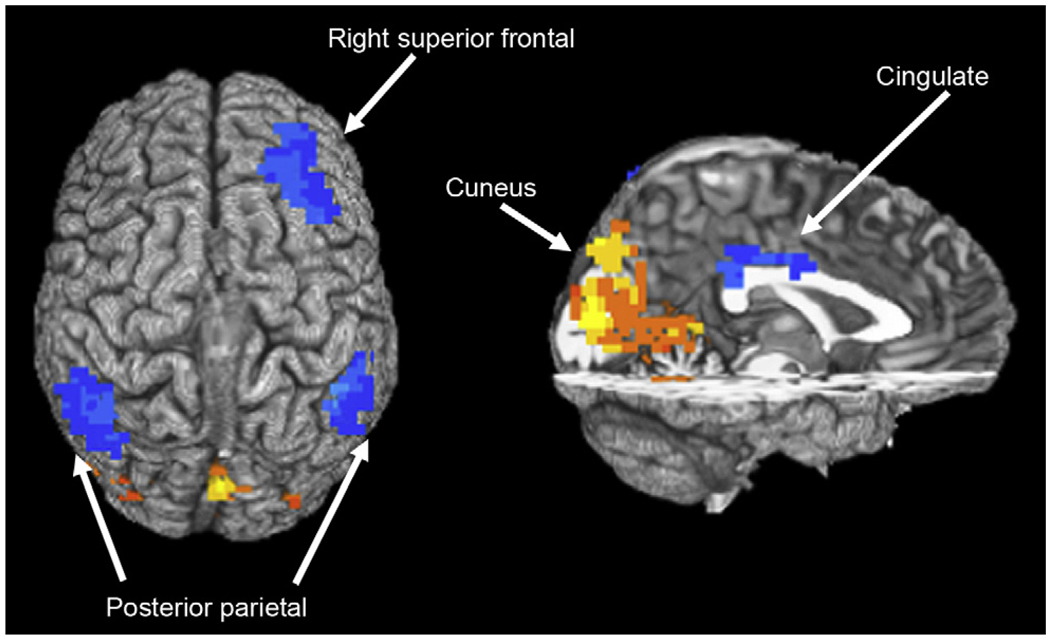
Binge drinkers exhibited significantly less BOLD response than controls while encoding new word pairs in a large posterior cluster spanning bilateral cuneus, lingual gyrus, parahippocampal gyrus, and right medial precuneus (Brodmann’s areas [BA] 18, 19, 31; see Table 2 and Fig. 1). Binge drinkers demonstrated significantly more BOLD response than controls during learning in several brain regions, including the right inferior parietal lobule (BA 40), left superior/inferior parietal lobule (BA 7, 40), right middle/superior frontal gyrus (BA 8), and cingulate (BA 24, 30).
Table 2.
Regions showing significant blood oxygen level–dependent response difference between controls and binge drinkers during learning of new word pairs versus fixation
| Talairach coordinates |
||||||
|---|---|---|---|---|---|---|
| Anatomic region | Brodmann’s area | Volume (µL) | x | y | z | t Value |
| Binge drinkers > controls | ||||||
| R superior frontal gyrus | 8 | 3,915 | −23 | −38 | 48 | 4.28 |
| L superior/inferior parietal lobule | 7, 40 | 4,185 | 41 | 56 | 51 | 3.46 |
| R inferior parietal lobule | 40 | 4,536 | −50 | 47 | 51 | 3.49 |
| B cingulate gyrus | 24, 31 | 1,512 | 2 | 35 | 24 | 3.96 |
| Binge drinkers < controls | ||||||
| B cuneus, lingual gyrus, parahippocampal gyrus, R precuneus | 18, 19, 30 | 29,376 | 2 | 86 | 12 | 5.72 |
R = right; L = left; B = bilateral. Talairach coordinates refer to maximum signal intensity group difference within cluster; t value represents t statistic for group difference in average brain response within cluster.
Fig. 1.
Group differences in blood oxygen level–dependent response during novel word pair encoding among adolescent nondrinkers and binge drinkers. White regions indicate areas where nondrinkers showed greater response than binge drinkers, and black clusters represent regions where binge drinkers showed more response than nondrinkers (clusters > 1,512 µL, P < .05).
Within the hippocampal ROIs, controls demonstrated a significant BOLD response increase during novel encoding (i.e., significant change from rest) in the left hippocampus (t[11] = 2.66, P = .02) and a trend for increased activation in the right hippocampus (t[11] = 1.91, P = .08). Among binge drinkers, there was a trend for greater BOLD response in the left hippocampus (t[11] = 1.94, P = .08) but no difference in the right hippocampus (P > .10) during novel encoding relative to fixation. There was no significant between-subjects effect in the right or left hippocampal ROI (Ps > .10).
Discussion
Despite relatively short drinking histories, adolescents with a history of binge drinking demonstrated different patterns of brain functioning and somewhat poorer performance during verbal encoding compared with nondrinkers. Binge drinkers recalled 7% fewer word pairs correctly, and nearly half of binge drinkers did not adequately recall the word pair list (10 out of 16 or 63% accuracy) on the first training session, whereas only one nondrinker did not reach this benchmark on the first trial. Although performance differences were only trends, likely because of limited power, they may point to important implications for academic achievement. Binge drinkers in this study reported relatively few problems related to alcohol use, yet their somewhat poorer verbal learning performance is consistent with previous work showing poorer verbal recall among adolescents with alcohol use disorders (Brown et al., 2000).
In addition to subtle performance decrements, binge drinkers exhibited reduced BOLD response during novel verbal encoding in a large region spanning occipital cortex and extending into the right parahippocampal gyrus and medial right precuneus. We observed similar patterns of diminished occipital response during spatial working memory among adolescents with alcohol use disorders (Tapert et al., 2004) as well as adolescents using marijuana heavily (Schweinsburg et al., 2008). The reduced occipital cortex activation among binge drinkers could indicate less involvement of visual and linguistic processing while learning verbal material.
Hippocampal ROI analyses revealed no significant group difference. Within-group analyses demonstrated that controls evinced significant activation during novel encoding in the left hippocampus and binge drinkers exhibited such activation at the trend level. In addition, binge drinkers demonstrated significantly diminished fMRI response that extended into the parahippocampal gyrus. The hippocampus and surrounding medial temporal lobe structures, including the parahippocampal gyrus, are known for their role in memory (e.g., Squire and Schacter, 2002) and may be particularly susceptible to alcohol-related damage. Smaller hippocampal volumes have been observed in adolescents with alcohol use disorders (De Bellis et al., 2000; Medina et al., 2007; Nagel et al., 2005). Furthermore, compromised white matter integrity of fibers originating in the left hippocampus has been shown in an overlapping sample of binge-drinking youths (McQueeny et al., 2009). Given that rodent studies have indicated that adolescents are more sensitive than adults to alcohol-induced hippocampal neurotoxicity (Pyapali et al., 1999; Slawecki et al., 2001; White and Swartzwelder, 2004), intense levels of alcohol exposure during this developmental stage may be particularly detrimental to brain functioning (Pascual et al., 2007; Tokunaga et al., 2006). Although subtle, our results are consistent with these findings of hippocampal differences in adolescent alcohol users.
Binge drinkers exhibited increased BOLD response during learning of new word pairs in frontal, parietal, and cingulate regions. Frontal and parietal cortices have been consistently implicated in working memory, and the cingulate may have a role in verbal storage (for review, see Wager and Smith, 2003). Our previous work has characterized increased bilateral parietal (BA 7) BOLD response during spatial working memory among adolescents with alcohol use disorders, which may indicate greater working memory–related neural effort among drinkers (Tapert et al., 2004). A similar pattern of increased response in the same bilateral parietal regions (BA 7, 40) was observed among binge drinkers in the present study. Given our previous findings of diminished prefrontal activation in alcohol-dependent young women (Tapert et al., 2001) and adolescent marijuana users (Schweinsburg et al., 2008), we initially hypothesized that binge drinkers in the present study would show a similar reduction in prefrontal activation. However, we instead observed an increase in right superior prefrontal fMRI response during verbal learning. An fMRI study of paired associates learning among youths likewise revealed increased right dorsolateral prefrontal activation among those prenatally exposed to alcohol compared with control youths (Sowell et al., 2007). The authors concluded that youths with prenatal alcohol exposure may compensate for deficient medial temporal lobe function by using frontal lobe memory networks. Coupled with the somewhat reduced hippocampal and parahippocampal response among binge drinkers, the observed over-activation of frontoparietal systems by binge drinkers in the present study may similarly suggest greater reliance on alternate memory systems during verbal learning.
In addition, left hemispheric activation is typically dominant during verbal learning and working memory tasks (e.g., Wager and Smith, 2003), yet the prefrontal group difference in the present study was localized in the right hemisphere. Using this same task, we have observed increased activation throughout the right hemisphere, including prefrontal cortex, among older adults with genetic risk for developing Alzheimer’s disease (Han et al., 2007). We suggested that this right hemispheric activation among those at risk for Alzheimer’s may reflect recruitment of bilateral systems to maintain performance. Furthermore, a recent transcranial magnetic stimulation study noted that intact right prefrontal systems are crucial for suppressing task-irrelevant information during high-load verbal working memory (Sandrini et al., 2008). Thus, increased right prefrontal activation among binge drinkers in the present study could, in part, reflect increased effort to suppress irrelevant information.
White matter abnormalities may, in part, underlie these brain function differences. Our recent study of an overlapping sample identified reduced white matter integrity among binge drinkers in frontal and parietal regions, as well as in projections to the left hippocampus (McQueeny et al., 2009). Microstructural white matter disruption has been related to cognitive decrements among adult alcoholics (Pfefferbaum and Sullivan, 2002; Pfefferbaum et al., 2009). In addition, we observed abnormal prefrontal white matter volumes among adolescents with alcohol use disorders (Medina et al., 2008). Thus, altered white matter integrity could partially underlie functional aberrations observed in these regions. Multimodal functional connectivity analyses will refine the nature of these relationships.
The results of this study should be considered in light of possible limitations and considerations for future work. Although a sample size of 12 can be sufficient to characterize differences in whole-brain functioning (Desmond and Glover, 2002), we may have had limited power to detect subtle differences in hippocampal response. Longitudinal investigations will begin to determine whether binge drinkers exhibit preexisting differences that may contribute to these findings, as well as the neurocognitive implications of continued drinking or cessation. Finally, the paired associates task used in this study is relatively easy, with an average accuracy of 78% in binge drinkers. A more difficult task, such as a verbal list learning task, might have greater ecological validity to late-adolescent academic settings that require more challenging memorization.
In sum, adolescents with histories of heavy episodic drinking demonstrated functional differences and marginally poorer performance during verbal learning as compared with demographically similar nondrinkers. The pattern of increased frontoparietal response and somewhat reduced hippocampal activation could indicate greater reliance on working memory and subtle deficits in consolidation among drinkers. These preliminary results suggest the possibility of altered neural processing of novel verbal information that could underlie verbal learning decrements, despite relatively short and problem-free drinking histories.
Acknowledgments
This research was made possible by grant support from the National Institute on Alcohol Abuse and Alcoholism (R01 AA13419, PI: S.F.T.) and the National Institute on Drug Abuse (R01 DA021182, PI: S.F.T.). Portions of this work were presented at the International Conference on Applications of Neuroimaging in Alcoholism, January 2008. The authors thank Dr. Brian Schweinsburg, Dr. Krista Medina, Dr. M.J. Meloy, Jennifer Winward, the staff of the Adolescent Brain Imaging Project, and the participating adolescents and parents.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, &Families; 2001. [Google Scholar]
- APA. American Psychiatric Association (4th ed.) Washington, DC: American Psychiatric Association; 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn. Reson. Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Beck AT. Beck Depression Inventory (BDI) San Antonio, TX: Psychological Corp.; 1978. [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J. Stud. Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neuro-cognitive functioning of adolescents: effects of protracted alcohol use. Alcohol. Clin. Exp. Res. 2000;24:164–171. [PubMed] [Google Scholar]
- Budson AE. Understanding memory dysfunction. Neurologist. 2009;15:71–79. doi: 10.1097/NRL.0b013e318188040d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Bukstein O, Cornelius J. Alcohol use disorders in adolescents: epidemiology, diagnosis, psychosocial interventions, and pharmacological treatment. Paediatr. Drugs. 2002;4:493–502. doi: 10.2165/00128072-200204080-00002. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking in young adults: data, definitions, and determinants. Psychol. Bull. 2009;135:142–156. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, et al. Hippocampal volume in adolescent-onset alcohol use disorders. Am. J. Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescentonset alcohol use disorders and comorbid mental disorders. Alcohol. Clin. Exp. Res. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Glover GH. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J. Neurosci. Methods. 2002;118:115–128. doi: 10.1016/s0165-0270(02)00121-8. [DOI] [PubMed] [Google Scholar]
- Eyler LT, Jeste DV, Brown GG. Brain response abnormalities during verbal learning among patients with schizophrenia. Psychiatry Res. 2008;162:11–25. doi: 10.1016/j.pscychresns.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EF. Intoxication Test Evidence. 2nd ed. Deerfield, IL: Clark Boardman Callaghan; 1995. [Google Scholar]
- Fleisher AS, Houston WS, Eyler LT, Frye S, Jenkins C, Thal LJ, et al. Identification of Alzheimer disease risk by functional magnetic resonance imaging. Arch. Neurol. 2005;62:1881–1888. doi: 10.1001/archneur.62.12.1881. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Grant I. Alcohol and the brain: neuropsychological correlates. J. Consult. Clin. Psychol. 1987;55:310–324. doi: 10.1037//0022-006x.55.3.310. [DOI] [PubMed] [Google Scholar]
- Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, Fleisher AS, et al. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiol. Aging. 2007;28:238–247. doi: 10.1016/j.neurobiolaging.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. The Monitoring the Future National Survey Results on Adolescent Drug Use: Overview of Key Findings, 2008. ethesda, MD: National Institute on Drug Abuse; 2009. [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, et al. Altered white matter integrity in adolescent binge drinkers. Alcohol. Clin. Exp. Res. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol. Clin. Exp. Res. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol. Teratol. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur. J. Neurosci. 2007;25:541–550. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol. Psychiatry. 2009;65:680–690. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Microstructural but not macro-structural disruption of white matter in women with chronic alcoholism. Neuroimage. 2002;15:708–718. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19:107–111. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Seeley JR. Psychiatric comorbidity with problematic alcohol use in high school students. J. Am. Acad. Child Adolesc. Psychiatry. 1996;35:101–109. doi: 10.1097/00004583-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Sandrini M, Rossini PM, Miniussi C. Lateralized contribution of prefrontal cortex in controlling task-irrelevant information during verbal and spatial working memory tasks: rTMS evidence. Neuropsychologia. 2008;46:2056–2063. doi: 10.1016/j.neuropsychologia.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res. 2008;163:40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Res. Dev. Brain Res. 2001;128:63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press, Inc.; 1992. pp. 41–72. [Google Scholar]
- Sowell ER, Lu LH, O’Hare ED, McCourt ST, Mattson SN, O’Connor MJ, et al. Functional magnetic resonance imaging of verbal learning in children with heavy prenatal alcohol exposure. Neuroreport. 2007;18:635–639. doi: 10.1097/WNR.0b013e3280bad8dc. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Squire LR, Schacter DL. The Neuropsychology of Memory. 3rd ed. New York, NY: Guilford; 2002. [Google Scholar]
- Talairach J, Tournoux P. Coplanar Stereotaxic Atlas of the Human Brain. Three-Dimensional Proportional System: An Approach to Cerebral Imaging. New York, NY: Thieme; 1988. [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol. Clin. Exp. Res. 2001;25:236–245. [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown GG, Brown SA, Frank LR, et al. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol. Clin. Exp. Res. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl.) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga S, Silvers JM, Matthews DB. Chronic intermittent ethanol exposure during adolescence blocks ethanol-induced inhibition of spontaneously active hippocampal pyramidal neurons. Alcohol. Clin. Exp. Res. 2006;30:1–6. doi: 10.1111/j.1530-0277.2006.00020.x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn. Affect. Behav. Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- White AM, Swartzwelder HS. Hippocampal function during adolescence: a unique target of ethanol effects. Ann. N. Y. Acad. Sci. 2004;1021:206–220. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]