It is known from animal studies 1 that malignant tumors may develop in certain conditions characterized by disturbances of the host’s immune defenses. The same is apparently true in man. For example, patients suffering from agammaglobulinemia, hypogammaglobulinemia and deficiencies of cell mediated immunity have a high incidence of malignancy involving either lymphoid or other tissues.2,4 An increased frequency of neoplasms has also been observed in a variety of “autoimmune” disorders.3
A prerequisite for success in clinical organ transplantation is the iatrogenic alteration of the host immune apparatus. A coincidental effect might be predicted to be an increased incidence of neoplasia. This possibility is supported in the present communication, which describes the development of malignant lymphoid tumors in 5 recipients of renal homografts, treated with differing immunosuppressive regimens at 3 widely separated transplantation centers (Table 1). A brief summary of the cases has already been reported.5 The occurrence of these tumors indicates that malignant disease may be a significant hazard of organ transplantation and long continued immunosuppressive therapy.
Table 1.
Clinical Features of Lymphomas in Renal Transplant Recipients
| Number | Patient | Transplant Center |
Age | Sex | Date of Transplant |
Donor | Date Malignancy Diagnosed |
Organs Involved |
Splenec- tomy |
Thymec- tomy |
Imuran | Predni- sone |
ALG | Type of Tumor |
Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | T.C. | Denver | 14 | M | 5/29/67 | Mother | 11/16/67 | Brain | Yes | No | Yes | Yes | Yes | Reticulum Cell Sarcoma |
Died 12/4/67 |
| 2 | S.D. | Denver | 23 | M | 6/15/65 | Father | 12/6/67 | Thyroid Liver Lung Stomach Prostate Pituitary Skin Psoas Muscle |
Yes | Yes | Yes | Yes | No | Reticulum Cell Sarcoma |
Died 12/6/67 |
| 3 | E.C. | Denver | 20 | F | 9/15/67 | Father | 4/11/68 | Brain | Yes | No | Yes | Yes | Yes | Possible Plasma- cytoma |
Alive 8/20/68 |
| 4 | W.A. | Minneapolis ☼ |
27 | M | Sept 1964 | Brother | June 1965 | Liver Brain Bone Marrow |
Yes | No | Yes | Yes | No | Lympho- sarcoma |
Died 11/6/65 |
| 5 | M.M. | Edinburgh Scotland ☼☼ |
26 | F | 1/17/66 | Mother | 2/1/68 | Mediastinal Lymph nodes Pleura |
No | No | Yes | Yes | Yes | Reticulum Cell Sarcoma |
Died 2/16/68 |
C. R. Hitchcock—Personal communication
M. F. A. Woodruff—Personal communication
CLINICAL MATERIAL
Case 1 (Denver)
Prior to transplantation in May 1967, the patient complained of headaches, dizziness, blurred vision, nausea and vomiting. He also suffered 2 generalized epileptiform seizures. Neurologic examination was negative and his symptoms were attributed to severe hypertension.
Renal homotransplantation was performed in May 1967. Immunosuppression was with azathioprine, prednisone, and horse antilymphocyte globulin (ALG). Two months after operation, he had a generalized seizure and remained confused and incoherent for several days. At that time he was being treated for threatened rejection of the homograft, was hypertensive, and was observed to be exceeding his restricted fluid allowance. Serum chemistries showed evidence of marked hemodilution. He was considered to be suffering from water intoxication. His condition promptly improved on a regimen of fluid restriction and administration of sodium chloride and potassium supplements. There were no further neurologic symptoms until 9 days before the diagnosis of reticulum cell sarcoma was made by craniotomy. The very extensive tumor of the brain (Figure 1) was the cause of the patient’s death. Renal function was normal in the last months of life.
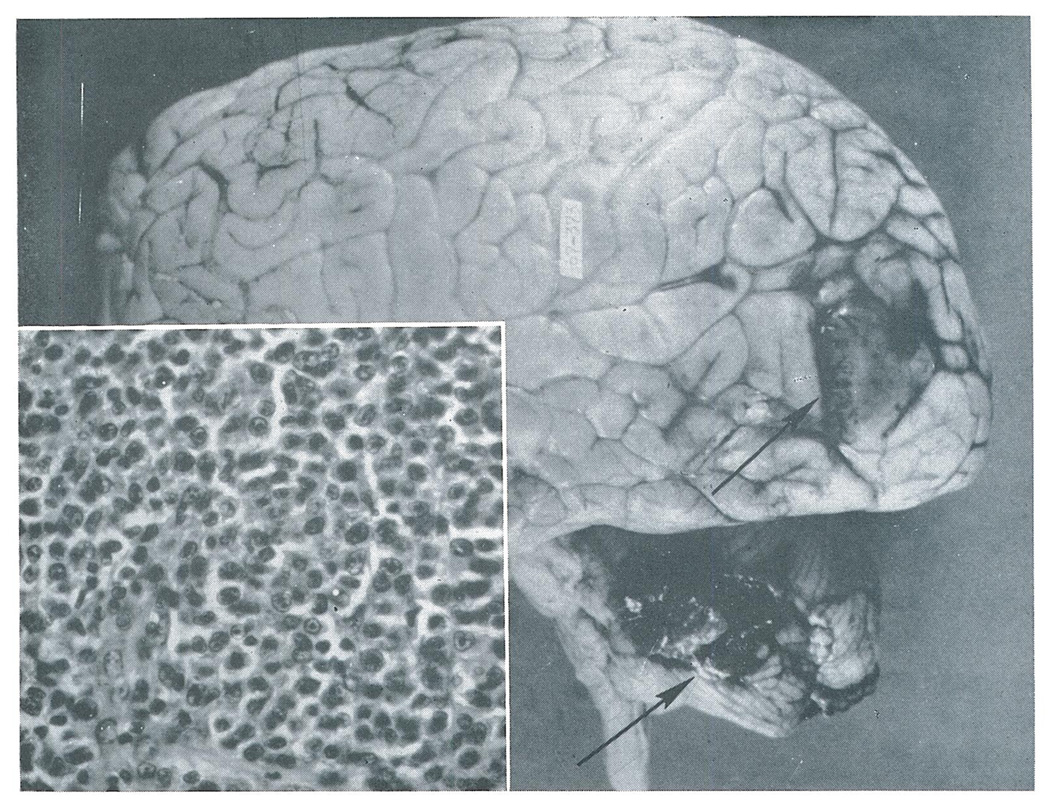
Fig. 1.
Case 1. Tumor nodules (arrows) in the left occipital lobe and cerebellum. The flattened gyri reflect increased intracranial pressure caused by the tumor. Insert–The large, uniform cells with indistinct cytoplasm and round to oval nuclei are characteristic of reticulum cell sarcoma (×350).
In this case, the possibility could not be excluded that the tumor was present at the time of transplantation and was the cause of his earlier neurologic symptoms.
Case 2 (Denver)
The patient was treated with continuous azathioprine and prednisone, and with actinomycin C and local homograft irradiation for threatened rejection. He had persistent hypercalcemia following his renal transplant operation. A parathyroidectomy performed on March 29, 1966. The thyroid gland was found to be normal at this operation, but at autopsy on December 6, 1967 was found to be enlarged and almost completely replaced by tumor. Renal function was subnormal, but adequate, throughout life.
Death occurred 6 days after emergency vagotomy and gastrectomy performed to control massive upper gastrointestinal bleeding. The resected portion of stomach contained several ulcers, in the bases of which were small collections of tumor cells that were strikingly similar to those found in the brain of the first patient. At autopsy widespread tumor was found (Figure 2).
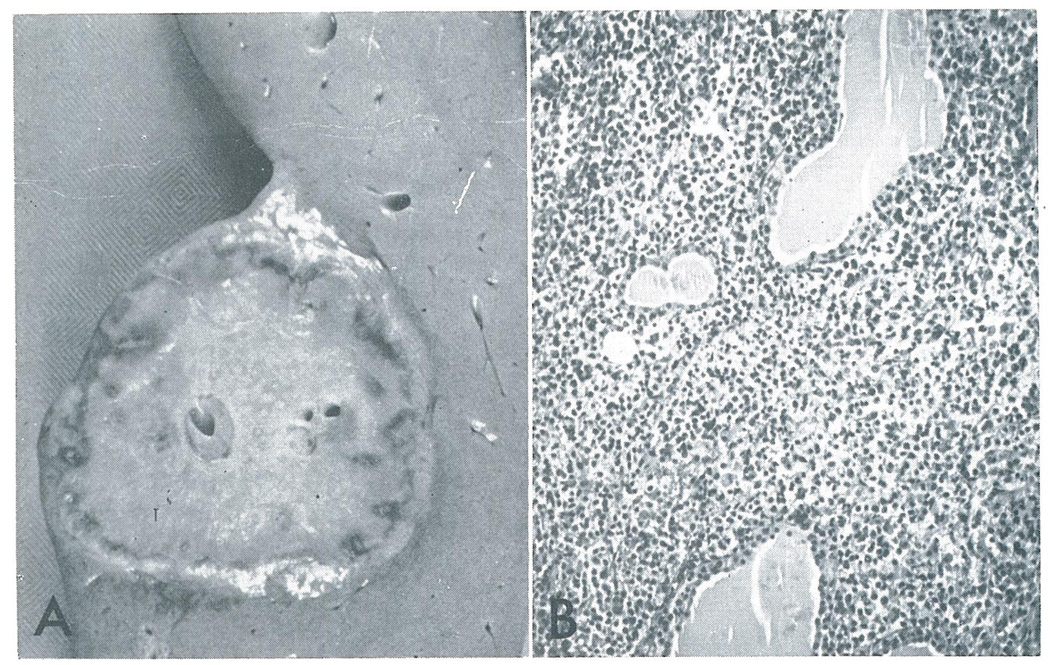
Fig. 2.
Case 2. A.–This 5 cm nodule was one of several found in the liver. B.–Malignant reticulum cells have massively infiltrated the thyroid, separating the follicles widely (×80).
Case 3 (Denver)
Immunosuppression was with azathioprine, prednisone and a 7 week course of heterologous ALG. Seven months after renal homotransplantation the patient developed a rapidly progressing left hemiparesis. A lesion in the right anterior and mid-thalamic areas was identified with pneumoencephalography and biopsied with a stereotaxic technique. The histologic findings were consistent with a tumor of lymphoid origin, possibly a plasmacytoma. Extensive special diagnostic studies revealed no evidence of multiple myeloma or any other site of tumor.
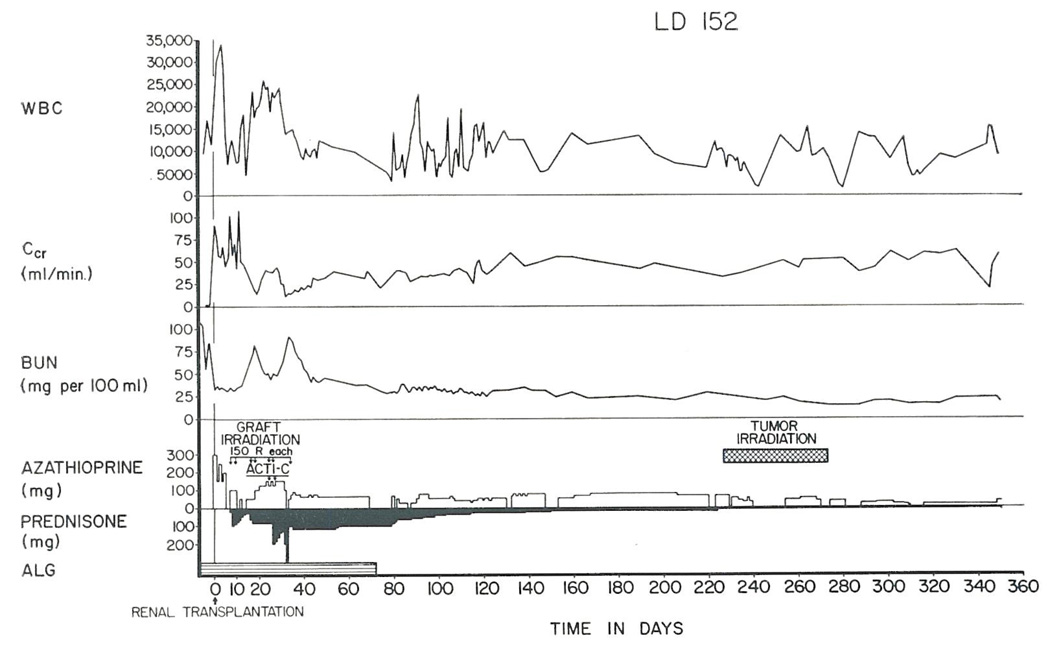
Radiotherapy to the brain (5650 rads) combined with reduction in the dosage of prednisone and azathioprine (Figure 3) resulted in striking improvement in the patient’s neurologic condition. She is well with moderate but stable residual hemiparesis at the present time.
Fig. 3.
The course of a patient (Case 3) who developed a plasmacytoma in the diencephalon more than 7 months after renal homotransplantation from her father. The tumor was irradiated and the doses of both azathioprine and prednisone were drastically reduced. The neurologic deficit was partially reversed and has not progressed subsequently. Renal function has remained good.
Case 4 (Minneapolis, Dr. Claude Hitchcock)
Renal homotransplantation was performed under treatment with azathioprine and prednisone. Ten months later, the liver became enlarged and was proved by biopsy to contain a lymphosarcoma. Two courses of radioactive cobalt therapy, each of 2,500 rads, were given to the liver area. Thirteen months after transplantation the patient became jaundiced and developed ascites. Death in hepatic coma occurred one month later. Autopsy examination revealed tumor nodules in the liver, varying from 2 to 10 cm in diameter. Similar tumor involvement was found in the left cerebellar cortex. The bone marrow was filled with lymphosarcoma.
Case 5 (Edinburgh, Mr. M.F.A. Woodruff)
A patient being treated with azathioprine and prednisone developed right-sided pleurisy 518 days after renal homotransplantation. About 1½ months earlier, a 10 day course of horse ALG had been given for the indication of late rejection. A loculated effusion between the right upper lobe and mediastinum was seen in the chest x-ray. This slowly diminished in size when antibiotics were given and could no longer be easily seen 2½ months later. A chest x-ray 725 days post-transplantation showed a recurrence and extension of the aforementioned radiographic abnormalities. Operation revealed a reticulum cell sarcoma affecting the mediastinal lymph nodes and ulcerating through the pleura. The patient died 760 days after transplantation. The kidney functioned throughout life.
OTHER PATHOLOGIC STUDIES
The lymphoid tissues from autopsy examination were reviewed in 24 recipients of kidney or liver homografts who died without previous evidence of malignancy. In 13 of these cases, immunosuppressive therapy had been with azathioprine and prednisone and in the other 11, ALG had also been administered. In no case was there any abnormal proliferation of lymphoid tissue. In fact, the lymphoid tissues were grossly depleted, more so in the thymus and gastrointestinal tract than in the lymph nodes. In the lymph nodes, lymphoid follicles were small and poorly formed and germinal centers were extremely rare. There was a proportionately greater reduction in small lymphocytes than in plasma cells.
DISCUSSION
In these patients, the probability is strong that the neoplasms began de novo at some time after the transplantation. Only in Case 1 was there any suggestion that the tumor might have antedated the original operation. Moreover, there was no evidence that the tumors were transplanted from the donors. All 5 kidneys were obtained from living related volunteers who have remained in good health from one to 4 years after their nephrectomies.
A number of the conditions present in the human recipients of renal homografts herein reported have been shown in experimental animals to be capable of inducing or influencing oncogenesis under the appropriate circumstances. These include the depression of immunologic reactivity by thymectomy,6,8 antilymphocyte serum, 7,8 and azathioprine9 or by the provision of chronic antigenic stimulation.1,10,11
In the clinical situation, the relative contribution of any single factor is impossible to assess. All 5 of the patients received immunosuppressive therapy with azathioprine and prednisone, 4 underwent splenectomy, one had a thymectomy and 3 received heterologous ALG. Beside therapy with azathioprine and prednisone, the common feature in all cases was the continuous presence of antigen in the form of a homograft. Four of the 5 patients had severe problems with rejection and received large doses of immunosuppressive agents to control this reaction.
It has been suggested that the increased incidence of malignancy which follows immunologic depression is due to a loss of the surveillance mechanism by which mutant cells are repudiated as “non-self.”3,12 This point of view has been strongly supported by the ease with which neoplasms have been accidentally transplanted from cadaveric renal donors who died of cancer,13 and by the subsequent disappearance of a transplanted malignant growth in at least one case after immunosuppression had been discontinued.14
While probably germane to the presently reported cases, the foregoing sequence of events does not explain the peculiar predisposition to the development of lympho-reticular tumors. It is conceivable that chronic antigenic stimulation was an added pathogenetic determinant, even though the lymphoid tissues in our 3 patients (Cases 1–3), as well as in 24 others who died without neoplasia, had variable involution.
It remains to be determined to what extent the appearance of neoplastic growth will be a complication of clinical organ transplantation. At the moment, it appears that the incidence will be low enough so that the usefulness of such procedures will not be vitiated. In the meanwhile, it is important to be alert to this diagnostic possibility since only in this way can effective therapy be instituted as was accomplished in Case 3.
So far the predominant risk after transplantation has seemed to be the appearance of mesenchymal tumors, although there have also been 2 reported epithelial malignancies which developed anew in recipients of renal homografts. One was a fatal dysgerminoma of the ovary in Good’s patient.3 The other was a squamous cell carcinoma of the ear in one of our patients who was included in Hume’s inter-institutional compilation.13 In the latter case the lesion of the ear appeared 2 years after renal homotransplantation and was cured by a radical excision. The patient died of a perforated sigmoid diverticulitis 2 years later.
Further knowledge about the general effect of transplantation and immunosuppression on tumor growth will have an important bearing on the advisability of organ replacement for the treatment of primary malignancies of vital structures such as the kidney, liver, and lung. In Hume’s report13 there were several examples of renal homotransplantation in patients with Wilm’s tumor or hypernephroma. There was only one example of a recurrence. In our series of orthotopic liver transplantations for hepatoma, there have been 3 recipients with survival of 5 months or longer. One of these patients died of widespread metastases after 13½ months, another has radiographic evidence of early pulmonary metastases at 5 months, and the third is free of detectable tumor after 6 months.15
SUMMARY
Malignant lymphomas developed in 5 renal homograft recipients treated at 3 widely separated transplantation centers. The development of these tumors appears to be an indirect complication of organ transplantation and/or the measures taken to prevent rejection.
Acknowledgments
Supported by United States Public Health Service grants AM-06344, HE-07735, AM-07772, AI-04152, FR-00051, FR-00069, AM-12148 and AI-AM-08898.
REFERENCES
- 1.Schwartz R, Andre-Schwartz J, Armstrong MYK, Beldotti L. Neoplastic sequelae of allogenic disease. I. Theoretical considerations and experimental design. Ann. N.Y. Acad. Sci. 1966;129:804. [Google Scholar]
- 2.Fialkow PJ. “Immunologic” oncogenesis. Blood. 1967;30:388. [PubMed] [Google Scholar]
- 3.Good FA. Experimental and clinical experiences with chemical suppression of immunity. A personal review. In: Miescher PA, Graber P, editors. Immunopathology Fifth International Symposium; New York: Grune and Stratton, Inc.; 1967. pp. 366–416. [Google Scholar]
- 4.Schwartz R. Immunologic disorders in malignant lymphomas; Proceedings of the Fifth National Cancer Conference; Philadelphia: J. B Lippincott Company; 1964. p. 645. [PubMed] [Google Scholar]
- 5.Starzl TE, Groth CG, Brettschneider L, Smith GV, Penn I, Kashiwagi N. Perspectives in organ transplantation; Proceedings of the Swiss Society of Immunology; Excerpta Medica; in press. [DOI] [PubMed] [Google Scholar]
- 6.Martinez C, Dalmasso AP, Good RA. Homotransplantation of normal and neoplastic tissue in thymectomized mice. In: Good RA, Gabrielsen AE, editors. The Thymus in Immunobiology. New York: Hoeber Medical Division, Harper and Row; 1964. p. 465. [Google Scholar]
- 7.Allison AC, Law LW. Effects of antilymphocyte serum on virus oncogenesis. Proc. Soc. Exp. Biol. Med. 1968;127:207. doi: 10.3181/00379727-127-32657. [DOI] [PubMed] [Google Scholar]
- 8.Davis RC, Lewis J., Jr Effect of thymectomy on an antilymphocyte serum treated human tumor xenograft. Surg. Forum. 1967;18:229. [Google Scholar]
- 9.Casey TP. Azathioprine (Imuran) administration and the development of malignant lymphomas in NZB mice. Clin. Exp. Immunol. 1968;3:305. [PMC free article] [PubMed] [Google Scholar]
- 10.Walford RL. Increased incidence of lymphoma after injections of mice with cells differing at weak histocompatibility loci. Science. 1966;152:78. doi: 10.1126/science.152.3718.78. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong MYK, Schwartz RS, Beldotti L. Neoplastic sequelae of allogeneic disease. III. Histological events following transplantation of allogeneic spleen cells. Transplantation. 1967;6:1380. [Google Scholar]
- 12.Prehn RT. Tumor antigens. In: Michich E, editor. Immunity, Cancer and Chemotherapy. New York: Academic Press; 1967. p. 265. [Google Scholar]
- 13.Hume DM. Progress in clinical renal homotransplantation. In: Welch CE, editor. Advances in Surgery. Vol. 2. Chicago: Year Book Medical Publishers, Inc.; 1966. p. 419. [PubMed] [Google Scholar]
- 14.Wilson RE, Hager EB, Hampers CL, Corson JM, Merrill JP, Murray JE. Immunologic rejection of human cancer transplanted with a renal allograft. New Eng. J. Med. 1968;278:479. doi: 10.1056/NEJM196802292780904. [DOI] [PubMed] [Google Scholar]
- 15.Starzl TE, Brettschneider L, Groth CG, Penn I, Blanchard H. Orthotopic liver transplantation in man; Proceedings of the Second International Congress of the Transplantation Society; New York: 1968. in press. [PMC free article] [PubMed] [Google Scholar]