Abstract
The purpose of this study was to evaluate the immune status of women with stage I–III breast cancer after receiving external beam radiotherapy (RT). Fourteen stage I–III, estrogen or progesterone receptor–positive or–negative (FER/PR +\−), postsurgical breast cancer patients undergoing a standard course of chemotherapy and radiation were studied. Complete blood counts (CBC) with differential, phagocytic activity, natural killer (NK) cell functional activity, and tumor necrosis factor-α (TNF-α) and interferon-γ cytokine activity were measured immediately before and for the six weeks following the completion of radiation therapy. Fatigue levels after completion of RT were measured using the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale. Nonparametric statistical methods (Wilcoxon rank and Spearman correlations) were used to analyze the data. Compared with postchemotherapy, following the completion of RT, these breast cancer patients showed lymphopenia, low functional activity of natural killer lymphocytes, decreased monocyte phagocytic activity, and decreased TNF-α production but no neutropenia, no anemia, and no change in interferon-γ production. Lymphocyte count did not return to normal by the end of the 6-week post-RT observation period. The severity of lymphopenia and low natural killer cell activity was related to RT area but not radiation dose. Patients did not report significant fatigue levels for the 6 weeks after completing RT.
Significant decreases in the numbers and functions of cells from both the innate and adaptive immune system were detected following a standard course of radiation therapy for the treatment of breast cancer. Immune deficits in lymphocyte populations and TNF-α production, should they persist, may have consequences for immune response to residual or recurrent malignancy following completion of conventional treatment. The use of adjunctive immune therapies which target these specific defects may be warranted in the post-treatment period.
Keywords: breast cancer, fatigue, immune defects, natural killer cell activity, phagocytic activity, radiotherapy, tumor necrosis factor
This study evaluated the immune status of women after receiving primary treatment for stage I, II, or III breast cancer. Although limited information exists, current data suggest that breast cancer patients who have completed surgery, chemotherapy, and radiotherapy (RT) have immunologic deficits,1–4 and in some studies, this has been associated with a poor prognosis.5,6 For more than 20 years, it has been known that local RT for breast cancer causes long-term effects on both the adaptive and the innate immune system. External beam radiation results in reduced secretion of immunoglobulins IgM, IgA, and IgG7; lymphopenia for as long as a decade in some patients8,9; and a decrease in absolute numbers of T cells.10,11 Low natural killer (NK) cell activity and low apoptotic cytokine levels have been associated with poor cancer prognosis.1,4,5,7,10,12–15 However, little is known about the functional activity of NK cells and monocytes or cytokine levels in breast cancer patients following completion of RT.
The immune cells of breast cancer patients have been shown to be impaired in their ability to produce tumor necrosis factor α (TNF-α).12 The ability to produce TNF-α and interferon-γ (IFN-γ) is associated with tumor regression and increased survival for cancer patients.13–15 Longer disease-free survival among breast cancer patients is associated with relatively higher circulating levels of TNF-α.16 Altered secretion of TNF-α and other inflammatory mediators has been associated with persistent posttreatment fatigue in breast cancer survivors.17–20 Although inflammatory alterations appear to underlie persistent fatigue in breast cancer survivors, the specific relationship between TNF-α levels and fatigue has yet to be elucidated.
The data reported here fill a gap in the knowledge regarding the immune status of breast cancer patients in the first weeks after completion of surgery, chemotherapy, and RT. Knowing more about the status of breast cancer patients’ innate and adaptive immune status after completing primary breast cancer treatment will assist in the development and evaluation of novel adjunctive immune therapies. Our long-term goal is the development of immunomodulatory therapies to be used in both the neoadjuvant and the adjuvant setting for the immune defects reported here in women who have completed RT for early-stage breast cancer.
Methods
Subjects
Complete blood counts with differential, phagocytosis activity, NK cell activity, and cytokine activity measurements were made in breast cancer patients before and after a standard course of external beam radiotherapy. Women aged 21 to 75 years with stage I, II, or III breast cancer, who had undergone surgery and chemotherapy and were scheduled to begin a standard course of RT, were invited to participate in this observational study at the University of Minnesota and Bastyr University under Institutional Review Board–approved protocols. Patients recruited into the study agreed to avoid taking any products containing known immunomodulating compounds during the 2-week period prior to RT, during RT, and during the 6-week study after RT. Fourteen subjects with newly diagnosed breast cancer were recruited between September 2005 and March 2007. Because the results were consistent among subjects, recruitment was discontinued after the enrolment of 14 participants. A sample size of 12 to 15 is considered adequate to achieve significance in describing intersubject variability.21 See Table 1 for patient characteristics and treatments.
Table 1.
Breast Cancer Patient Study Participants
| Patient | Age (yrs) |
Stage | ER | PR | HER 2/neu |
Chemotherapy Protocol |
Hematopoietic Drugs |
Hormonal Therapy and Initiation Timeline |
Dose (cGy) and Location |
Total Dose (cGy) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | 2 | Y | Y | N | Doxorubicin | EPO | Anastrozole, letrozole | Breast | 6,000 |
| Cyclophosphamide | Filgrastim | initiated pre-RT | 5,000 to L chest wall | |||||||
| Paclitaxel | 1,000 boost to surgical | |||||||||
| scar | ||||||||||
| 2 | 37 | 1 | N | N | N | Doxorubicin | Pegfilgrastim | None | Breast | 6,400 |
| Cyclosphosphamide | 5,000 to R | |||||||||
| Paclitaxel | tangent breast | |||||||||
| 1,400 boost to R tangent breast | ||||||||||
| 3 | 42 | 3 | N | N | P | Doxorubicin | Pegfilgrastim | None | Locoregional | 6,040 |
| Cyclophosphamide | 5,040 to whole R breast | |||||||||
| Paclitaxel | 1,000 intramammary boost | |||||||||
| Trastuzumab | 5,040 to IMC | |||||||||
| 5,040 to R SCF | ||||||||||
| 4 | 38 | 3 | Y | Y | N | Doxorubicin | None | Letrozole initiated | Locoregional | 6,040 |
| Cyclophosphamide | post-RT | 1,000 to mastectomy scar | ||||||||
| Paclitaxel | 5,040 to R SCF | |||||||||
| 4,500 to PAB | ||||||||||
| 5,040 to R CW tangents | ||||||||||
| 5,040 R IM electron field | ||||||||||
| 5 | 32 | 1 | Y | N | I | Doxorubicin | Filgrastim | Tamoxifen initiated | Breast | 5,400 |
| Cyclophosphamide | Epoetin alfa | post-RT | 5,400 to LCW | |||||||
| Paclitaxel | 4,680 to L SCF | |||||||||
| Trastuzumab | ||||||||||
| 6 | 52 | 1 | Y | Y | N | Doxorubicin | GM-CSF | Tamoxifen initiated | Breast | 6,640 |
| Cyclophosphamide | during RT | 1,600 scar boost | ||||||||
| 5,040 to UOQ of L breast | ||||||||||
| 7 | 35 | 2 | Y | Y | N | Doxorubicin | EPO | Tamoxifen initiated | Breast | 6,000 |
| Cyclophosphamide | Pegfilgrastim | post-RT | 5,000 to R breast | |||||||
| Paclitaxel | 1,000 to tumor bed and excisional | |||||||||
| bx scar | ||||||||||
| 8 | 39 | 2 | N | N | N | Doxorubicin | Pegfilgrastim | None | Locoregional | 6,000 |
| Cyclophosphamide | 4,600 to L breast IM | |||||||||
| Paclitaxel | 4,600 to L SCF/axilla | |||||||||
| 690 to PAB | ||||||||||
| 1,400 boost to tumor bed | ||||||||||
| 9 | 49 | 3 | Y | Y | N | Doxorubicin | Pegfilgrastim | Letrozole initiated | Locoregional | 6,640 |
| Cyclophosphamide | post-RT | 6,640 to L chest wall, axilla, and | ||||||||
| Docetaxel | paraclavicular region and scar | |||||||||
| margins | ||||||||||
| 10 | 47 | 3 | Y | Y | N | Doxorubicin | Filgrastim | Tamoxifen initiated | Locoregional | 6,040 |
| Cyclophosphamide | Epoetin alfa | post-RT | 6,040 to R chest wall | |||||||
| Paclitaxel | 5,040 to paraclavicular region | |||||||||
| 11 | 60 | 2 | N | N | P | Doxorubicin | Pegfilgrastim | None | Breast | 6,300 |
| Cyclophosphamide | 1,260 boost to lumpectomy site | |||||||||
| Paclitaxel | 5,040 to R breast | |||||||||
| 12 | 59 | 3 | Y | Y | N | Doxorubicin | None | Anastrozole initiated | Breast | 6,440 |
| Cyclophosphamide | pre-RT | 1,440 boost to L chest wall scar | ||||||||
| Paclitaxel | 5,080 IM | |||||||||
| Gemcitabine | 5,040 to L breast | |||||||||
| 5,040 to L supraclavicular and | ||||||||||
| axillary area | ||||||||||
| 13 | 42 | 2 | Y | Y | P | Doxorubicin | Pegfilgrastim | Tamoxifen initiated | Breast | 6,440 |
| Cyclophosphamide | post-RT | 1,400 boost to lumpectomy scar | ||||||||
| Docetaxel | 5,040 to R breast | |||||||||
| 14 | 61 | 3 | Y | Y | I | Doxorubicin | Pegfilgrastim | Anastrozole initiated | Locoregional | 6,520 |
| Cyclophosphamide | during RT | 5,080 IM | ||||||||
| Docetaxel | 5,040 to L chest wall | |||||||||
| 1,400 boost to L chest wall | ||||||||||
| 5,040 to L supraclavicular and | ||||||||||
| axillary area |
bx = biopsy; CW = chest wall; EPO = erythropoietin; ER = estrogen receptor; GM-CSF = granulocyte-macrophage colony-stimulating factor; I = indeteminant; IM = internal mammary; IMC = internal mammary chain; LCW = left chest wall; N = negative; P = positive; PAB = posterior axillary boost; PR = progesterone receptor; RT = radiotherapy; SCF = supraclavicular field; UOQ = upper outer quadrant.
Protocol
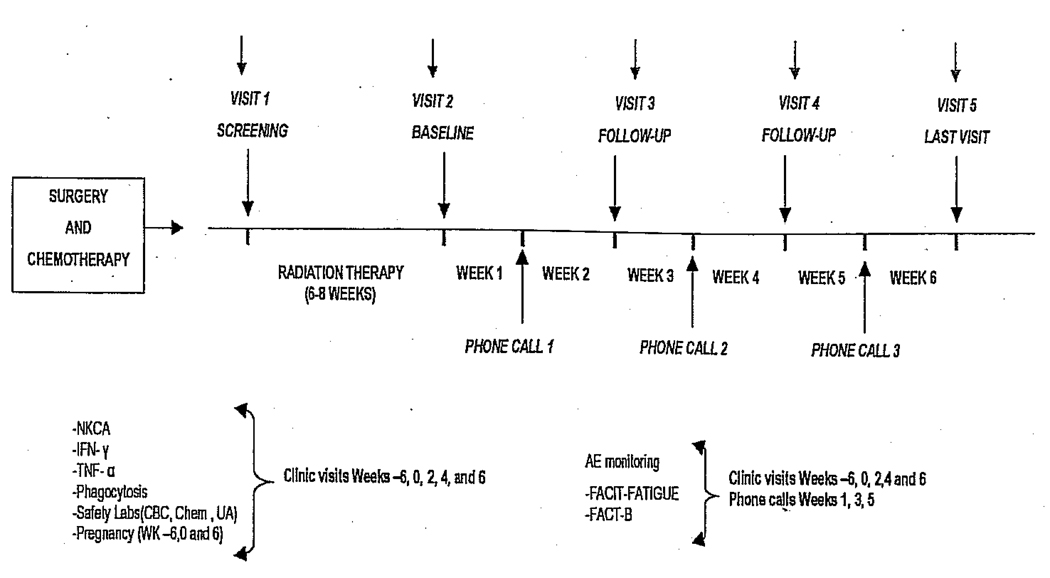
Figure 1 shows the timeline of the observational study.
Figure 1.
Breast cancer participant study timeline. AE = adverse event; CBC = complete blood count; Chem = chemistry; FACIT = Functional Assessment of Chronic Illness Therapy; FACT-B = Functional Assessment of Cancer Therapy-Breast; IFN = interferon; NKCA = natural killer cell activity; TNF-α = tumor necrosis factor α; UA = urinalysis.
Complete Blood Count with Differential
The clinical laboratory tests (complete blood count, chemistry, serum pregnancy tests, and urinalysis) were performed at the Department of Laboratory Medicine at University of Washington for Seattle participants and at the University of Minnesota Fairview laboratory for participants recruited in Minneapolis. All samples were hand delivered within 4 hours of blood draw.
NK Cell Functional Activity
To ensure uniform assessment of immune response, immunologic assays were carried out at Bastyr University for subjects recruited from both sites. Blood from patients at the University of Minnesota was collected at the University of Minnesota General Clinical Research Center and shipped overnight to Bastyr University.
Owing to the possibility of blood sample transport conditions from the University of Minnesota to Bastyr University affecting the accuracy of measuring NK cell activity, a blood sample quality control assessment was included. If specimens were more than 48 hours old when they arrived at the Bastyr Tierney laboratory, the lymphocytes were isolated from the blood specimens and cell viability was determined. If the viability was greater than 80%, the assay was performed. If the viability was less than 80%, the specimens were rejected.
Peripheral blood mononuclear cells (PBMCs) were isolated by ficoll–hypaque gradient separation, washed twice in phosphate-buffered saline, and maintained in RPMI 1640, 10% fetal bovine serum (FBS) with 2 mM l-glutamine (l-Gln) and penicillin-streptomycin (1,000 U/mL/1 mg/mL). Monocytes were depleted from PBMC samples by adherence to cell culture flasks for 60 minutes, and nonadherent peripheral blood lymphocytes (PBLs) were collected. NK cell activity of these PBL samples, as measured by the ability to kill K562 tumor target cells (an NK cell–sensitive human tumor cell line), was assessed in triplicate at the effector to target (E:T) ratios of 50:1, 25:1, and 12.5:1 following published methods.22 Target cells were labeled with DiOC183 and cocultured with PBL effector cell samples for 4 hours.
A control sample with K562 cells only was included to determine spontaneous target cell killing. Following incubation, propidium iodide was added to detect dead cells. The percentage of killed target cells was determined using flow cytometry, and percent specific lysis (PSL) was calculated at each E:T ratio. To standardize NK cell activity so that accurate comparisons were possible between PBL samples from different study participants, lytic units (LUs) were calculated by a previously published and validated software program (Whiteside TL, personal communication, 2007). LU20 values, defined as the E:T ratio at which 20% of target cell death occurs, were extrapolated from dose-response curves of PSL versus log E:T ratio for each blood sample assayed. LUs of NK cell activity, defined as the number of cells required to cause 20% target cell lysis relative to 107 effector cells, were determined by the equation 107/LU20 and thus increase with increasing lyric activity.
Phagocytic Activity
Phagocytic activity was determined as a functional assessment of polymorphonuclear (PMN) leukocytes and monocytes in whole blood samples collected from study volunteers. Whole blood samples (100 µL) were incubated in triplicate with fluorescently labeled Escherichia coli (K-12 strain conjugated to fluorescein isothiocyanate [FITC] and opsonized with antibodies and complement; Invitrogen, Carlsbad, CA) at 37°C for 30 minutes (maximum uptake samples) or 8 minutes (test samples) or left on ice (control). Internalization of FITC-conjugated E. coli by the two subpopulations was measured using flow cytometry, gating on monocyte and PMN leukocyte populations. Phagocytic activity (percentage of maximal FITC–E. coli uptake) was determined by the following formula:
Ex Vivo Cytokine Measurement
Blood was collected from participants at each of five time points: within 72 hours prior to the start of RT, within 72 hours following completion of RT, and at 2, 4, and 6 weeks following completion of RT. Isolated PBMCs (1 × 106 cells/mL) were incubated for 24 hours in the presence or absence of 1 µg/mL phytohemagglutinin (PHA). Each of six wells in a 24-well tissue culture plate were inoculated with 1 × 106 cells in 1 mL complete media (RPMI 1640, 2% FBS, l-glutamine, 1× Pen/Strep); three wells included 1 µg/mL PHA and three did not. Following 24-hour incubation, the supernatant was collected and centrifuged to remove cell solids. Following 24-hour incubation, the supernatant was collected and nonadherent cells were removed by centrifuge in an Eppendorf 5415c benchtop microcentrifuge (room temperature, 2 minutes, 3,500g). Samples were stored at −80°C until assay. IFN-γ and TNF-α concentrations were determined using enzyme-linked immunosorbent assay according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). Plates were read on a SPECTRAmax 384 PLUS plate reader (Molecular Devices, Sunnyvale, CA). Data results were analyzed using SoftMax Pro (2006 Molecular Devices Corp.) and SPSS (version 15.0; SPSS, Chicago, IL) software.
Statistical Methods
Owing to the small sample size and the nongaussian distribution of the data, nonparametric statistics were used to analyze paired data (Wilcoxon signed rank sum test or Mann-Whitney test). These tests are used to analyze paired data points that are equally likely to go in either direction (positive or negative) and do not assume gaussian distribution or address average absolute difference. Spearman correlations were calculated to detect associations between total RT dose and immune response.
Results
Subjects
Of 27 women screened for this study, 17 met our diagnostic and temporal inclusion criteria. Of these 17 study subjects enrolled, 14 completed the preRT baseline and the 6-week postRT observational study and are included in this analysis. Four participants were seen at the University of Minnesota, and 10 were recruited from the Seattle area and seen at Bastyr University or the University Health Clinic Specialty Care and Research Center. They ranged in age from 32 to 61 years. Table 1 shows that all study subjects received both chemotherapy and RT; 21% were stage I, 36% stage II, and 43% stage III. Most (71%) had estrogen or progesterone receptor–positive breast tumors, 100% had invasive ductal carcinoma, and 15% also had ductal carcinoma in situ. HER2/neu overexpression was present in 23% of the participants’ biopsied tissue samples. Hematopoietic growth factor therapy (erythropoietin, granulocyte colony stimulating factor [GCSF], peg-GCSF, and granulocyte-macrophage colony-stimulating factor) was used in 92% of patients. Doxorubicin, cyclophosphamide, and taxanes were the most commonly used chemotherapeutic agents. Total RT doses ranged from 60 to 65 Gy delivered over a range of 30 to 36 days of treatments. Most patients (79%) received a radiation “boost” to surgical sites and the tumor margin area. Of the 10 estrogen receptor–positive patients, 6 initiated antiestrogen hormone therapy after completion of RT, two during and two before RT.
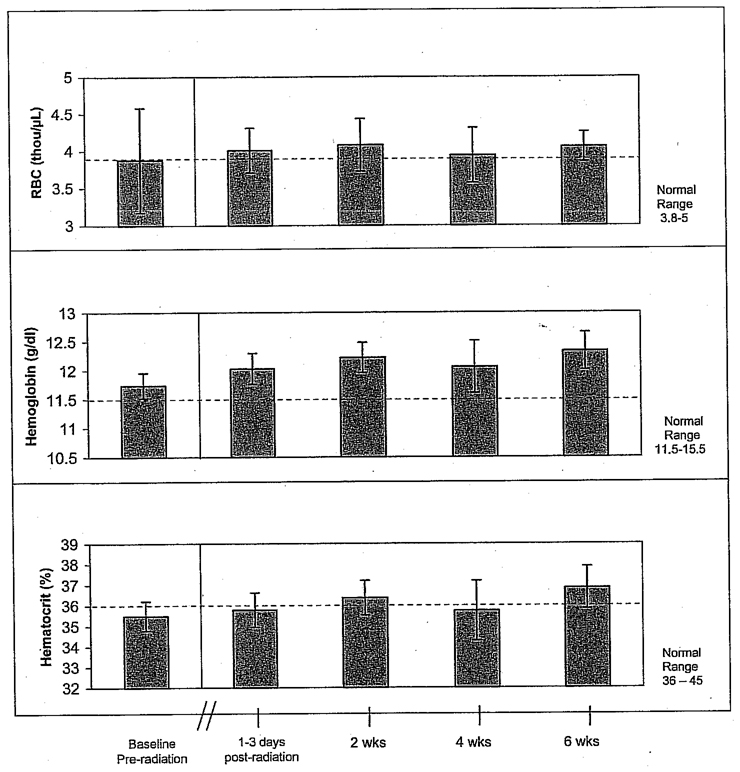
Changes in Red Blood Cell Measures
As shown in Figure 2, the red blood cell (RBC) parameters were not affected by RT. Both total RBCs and hemoglobin were normal before or after RT. Although hematocrit at the preradiation baseline was slightly below normal (35.5%), it returned to normal by 2 weeks after completion of RT. However, none of these differences were statistically significant using a two-tailed, nonparametric Wilcoxon signed rank test.
Figure 2.
Red blood cell (RBC) parameters are unaffected by radiotherapy in stage I–III breast cancer patients (n = 14).
Changes in White Blood Cell and Differential Counts Associated with RT
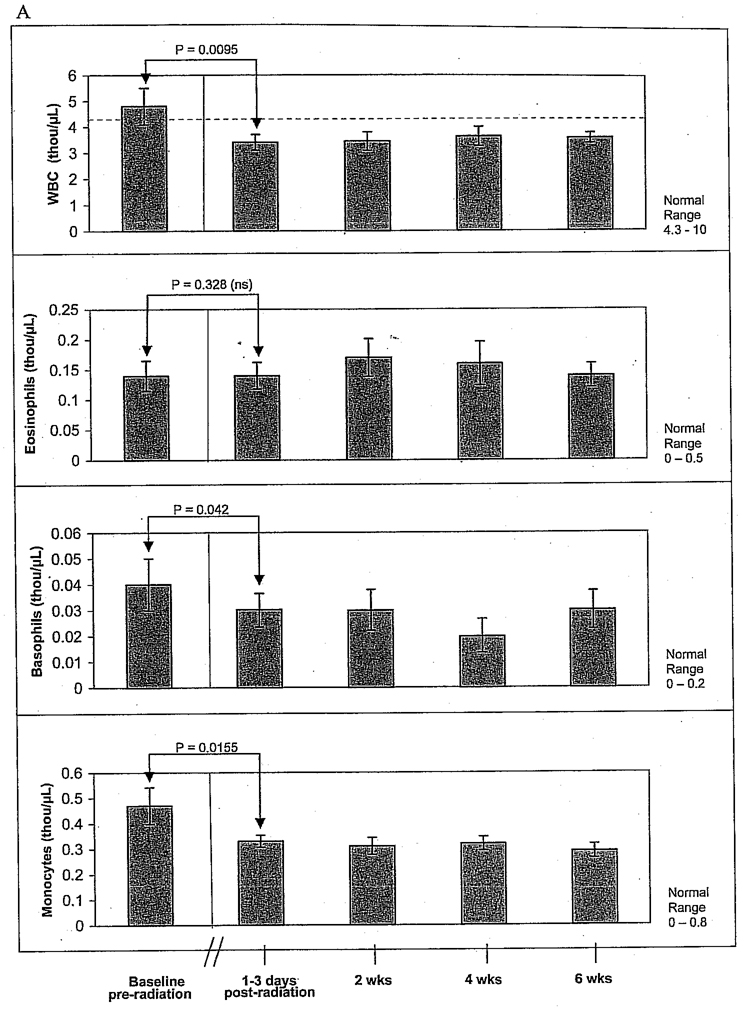
Figure 3A shows that WBC had recovered from chemotherapy and was normal in most of the participants (mean WBCs 4.81 thousand/µL + 2.63 SEM) just prior to starting radiation therapy. However, there was a significant decrease in mean total WBCs to 3.4 thou/µL ± 1.13 (p = .0095) immediately following completion of RT. WBC count had not returned to normal at 6 weeks post-RT. Basophils and monocytes, but not eosinophils, were significantly reduced after radiation but did not decline into the subnormal range.
Figure 3.
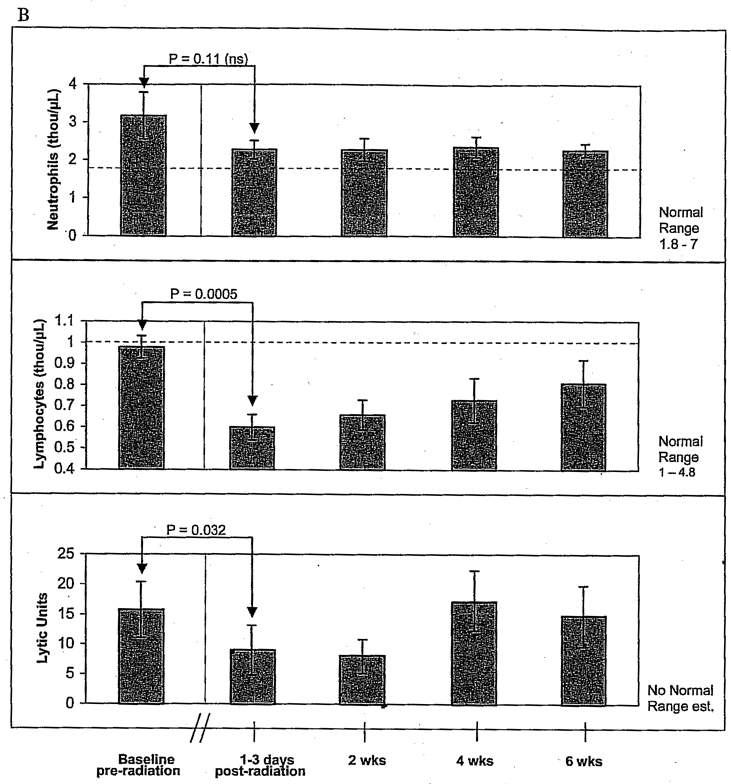
A, Pre- and postradiotherapy total white blood cell and differential count for neutrophils, eosinophils, basophils, and monocytes in stage I–III breast cancer patients (n = 14). There were significant decreases in mean total white blood cells (WBC), monocytes, and basophils after radiotherapy (RT). WBC levels failed to return to baseline by the end of the 6-week study. B, Lymphocytes and natural killer cell activity pre- versus postRT (n = 14). Neutrophil counts decreased after RT but did not drop below normal levels. Postradiation lymphopenia and low natural killer cell activity were observed and persisted throughout the 6-week post-RT period. The red dotted line indicates normal ranges. Normal ranges for natural killer cell activity have not been established. ns = no significance.
Lymphopenia and low NK cell activity were detected immediately following RT and persisted throughout the 6-week post-RT period (Figure 3B). Post-RT lymphopenia was observed in 93% of the subjects. There was a statistically significant decline in absolute lymphocyte count following RT (Wilcoxon signed rank test p = .0005). Mean lymphocytes prior to RT were near normal but fell by 39% immediately following RT, declining into the subnormal range. The absolute number of lymphocytes increased within the 6-week post-RT observation period but did not return to pre-RT levels. Although neutrophil counts dropped slightly after RT, they did not drop below normal limits.
Changes in NK Cell Activity Associated with RT
NK cell lyric activity against K562 tumor targets was also decreased after RT (see Figure 3B). Mean NK cell activity was 15.7 ± 4.6 SEM LU 20 before RT and dropped to 9.01 ± 4.2 SEM LU20 immediately after completion of RT (two-tailed t-test; p = .032). NK cell activity returned to pre-RT baseline levels by the end of the 6-week post-RT observation period.
Severity of Lymphopenia and Low NK Cell Activity Was Related to RT Area but Not Dose
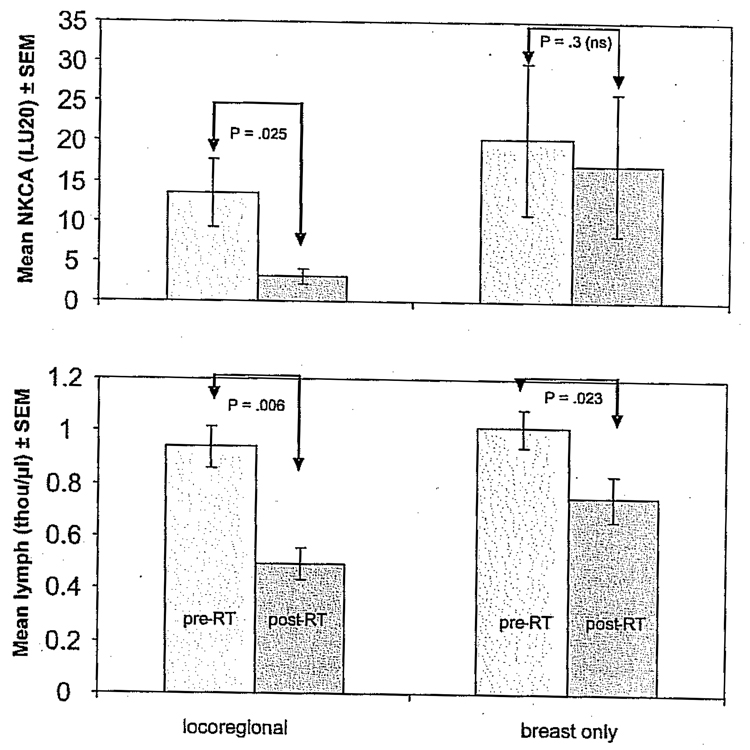
Total radiation dose in the 14 subjects was similar among patients, ranging between 60 and 65 Gy, which was administered in fractions over a narrow range of 30 to 36 days. No clear relationship between loss of NK cell activity and total RT dose was detected (Table 2 and Table 3). However, loss of NK cell activity and lymphocyte counts differed when comparing patients who received RT only to the breast (n = 6) versus those patients who received both local and regional treatment (n = 8). We compared patients who received RT only to the breast (n = 6) versus those who received wider area locoregional RT (n = 8). Patients who received locoregional RT showed greater lymphopenia and NK cell activity loss following RT compared with those who received only breast RT. Figure 4 shows a mean change in NK cell activity (LU20) and absolute lymphocyte count before and after standard external beam RT. The loss of NK cell activity (p = .025) was statistically significant only for those patients who received wider field locoregional RT (Wilcoxon test), and RT-related depletion of lymphocytes was greater in patients who underwent locoregional RT (46% decrease) compared with patients who only had breast RT (22% decrease). These data suggest that locoregional RT, involving a larger field and more lymphatic exposure, may have more immunologic consequences compared with RT to the breast alone.
Table 2.
Effect of Radiation Dose on Lymphocyte Counts (thou/µL)
| Dose (cGy) | Stage | Surgery | HER2 | Pretreatment Lymphocytes |
Posttreatment Lymphocytes |
Δ Lymphocytes |
|---|---|---|---|---|---|---|
| 5,400 | 1 | M | I | 0.7 | 0.34 | −0.36 |
| 6,000 | 2 | M | N | 1.07 | 0.84 | −0.23 |
| 6,000 | 2 | L | N | 1.22 | 0.68 | −0.54 |
| 6,000 | 2 | M | N | 0.85 | 0.62 | −0.23 |
| 6,040 | 3 | M | P | 1 | 0.57 | −0.43 |
| 6,040 | 3 | M | N | 0.83 | 0.25 | −0.58 |
| 6,040 | 3 | M | N | 1.24 | 0.79 | −0.45 |
| 6,300 | 2 | L | P | 0.7 | 0.5 | −0.2 |
| 6,400 | 1 | L | N | 1.06 | 0.8 | −0.26 |
| 6,440 | 2 | L | P | 1 | 1.1 | 0.1 |
| 6,520 | 3 | L | N | 1.1 | 0.5 | −0.6 |
| 6,520 | 3 | M | I | 0.7 | 0.5 | −0.2 |
| 6,640 | 1 | L | N | 1.04 | 0.58 | −0.46 |
| 6,640 | 3 | M | N | 1.25 | 0.39 | −0.86 |
I = indeterminant; L = lumpectomy; M = mastectomy; N = negative; P = positive.
Table 3.
Effect of Radiation Dose on Natural Killer Cell Activity
| Dose (cGy) | Stage | Surgery | HER2 | Presentment LU20 | Posttreatment LU20 | Δ LU |
|---|---|---|---|---|---|---|
| 5,400 | 1 | M | I | 17.45 | 0.78 | −16.67 |
| 6,000 | 2 | M | N | 48.63 | 53.52 | 4.89 |
| 6,000 | 2 | L | N | 11.42 | 1.74 | −9.68 |
| 6,000 | 2 | M | N | 20.82 | 2.51 | −18.31 |
| 6,040 | 3 | M | P | 9.20 | 1.08 | −8.12 |
| 6,040 | 3 | M | N | 37.09 | 3.8 | −33.29 |
| 6,040 | 3 | M | N | 3.72 | 1.94 | −1.78 |
| 6,300 | 2 | L | P | 5.96 | 9.26 | 3.3 |
| 6,400 | 1 | L | N | 50.79 | 34.61 | −16.18 |
| 6,440 | 2 | L | P | 0.26 | 1.45 | 1.19 |
| 6,520 | 3 | L | N | 3.66 | 1.45 | −2.21 |
| 6,520 | 3 | M | I | 3.47 | 2.49 | −0.98 |
| 6,640 | 1 | L | N | 4.93 | 2.16 | −2.77 |
| 6,640 | 3 | M | N | 2.54 | 9.35 | 6.81 |
I = indeterminant; L = lumpectomy; LU = lytic units; M = mastectomy; N = negative; P = positive.
Lytic units = number of natural killer cells required to cause 20% target cell lysis relative to 107 effector cells (K562), determined by the equation 107/LU 20.
Figure 4.
Mean change in natural killer cell activity (NKCA) (LU20) and absolute lymphocyte count before and after standard external beam radiotherapy (RT). Here we compare patients who received RT only to the breast (n = 6) versus those who received wider area locoregional RT (n = 8). Patients who received locoregional RT showed greater lymphopenia and NKCA loss compared with those who received only breast RT.
Changes in Monocyte and PMN Phagocytic Activity Associated with RT
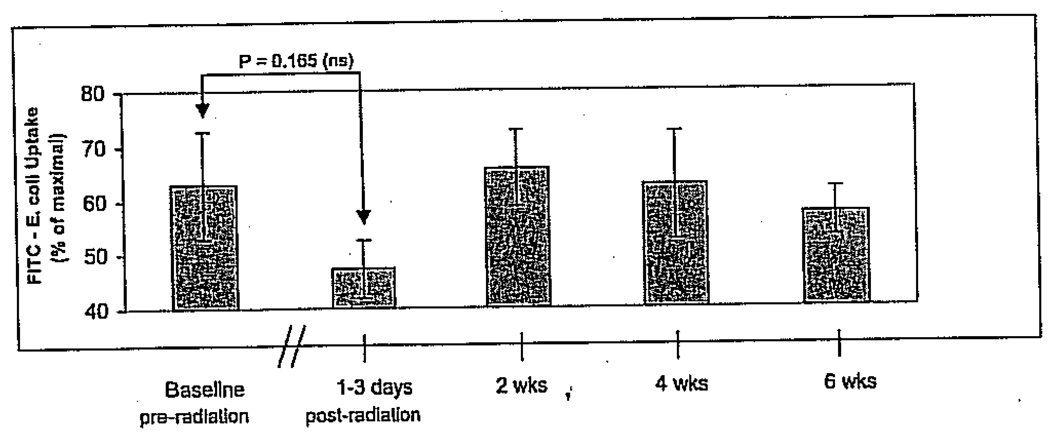
Mean phagocytic activity of monocytes, but not PMN leukocytes, declined markedly after completion of RT (Figure 5; PMN data not shown). Despite the clear drop in the mean monocyte phagocytic activity, the difference between pre-RT baseline and the first post-RT measure taken within the first 72 hours after completion of RT, this trend in decreasing phagocytic activity was not statistically significant. Some recovery of monocyte phagocytic activity appears to occur within the 6 weeks post-RT observation period.
Figure 5.
Pre- and postradiotherapy mean percent phagocytic activity of monocytes showing a trend toward decreasing phagocytic activity after radiotherapy (RT). Some recovery of monocyte phagocytic activity appears to occur within the 6-week post-RT observation period. FITC = fluorescein isothiocyanate.
Ex Vivo Cytokine Measurements: TNF-α and IFN-γ
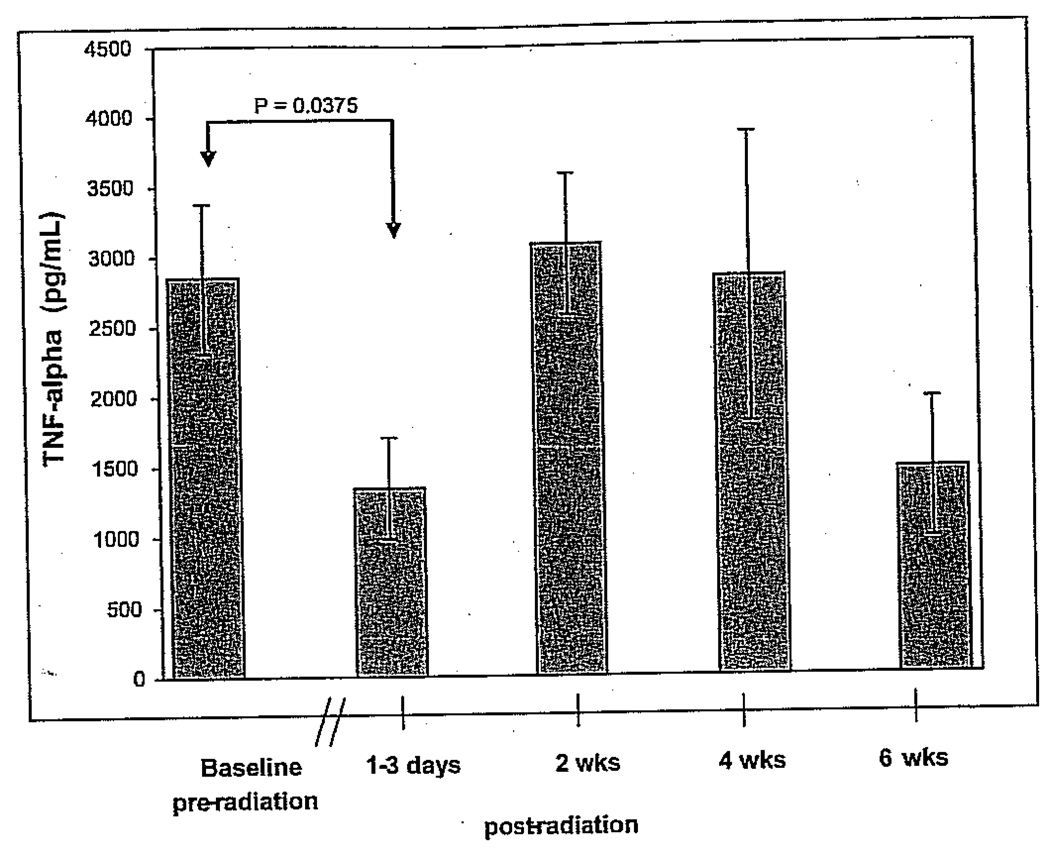
RT resulted in a measurable reduction in the capacity to produce TNF-α following PHA stimulation (Figure 6), yet IFN-γ production was unaffected. Between study participants’ pre-RT and post-RT blood draw, the average adjusted TNF-α production in PHA-stimulated PBMCs decreased by 46% from 2,847.9 to 1,309.9 pg/mL (p = .035, Wilcoxon rank signed test, 95% confidence interval). Differences in IFN-γ production were not significant at any time point (data not shown).
Figure 6.
PHA-stimulated tumor necrosis factor α (TNF-α) production in peripheral blood mononuclear cells at each study time point (mean ± SEM). Production levels between visit 1 (preradiation baseline) and visit 2 (postradiation) were significantly different. (When the four subjects recruited from the University of Minnesota were removed from analysis, the p value was greated than .05.)
Fatigue Scores Pre- and Post-RT
Using the Functional Assessment of Cancer Therapy (FACT)–Functional Assessment of Chronic Illness Therapy (FACIT) questionnaire, the breast cancer patients in this study did not report significant fatigue at any time point. The maximum score on this instrument is 52, which indicates the absence of fatigue symptoms. Mean post-RT scores over the 6 weeks following RT had a narrow range from 38 to 43.
Conclusions and Discussion
The main findings of this study are that women with stage I, II, or III breast cancer after completing RT showed (1) lymphopenia and low NK cell activity throughout the 6 weeks after the completion of RT, (2) depressed phagocytic activity of monocytes, and (3) a transient but measurable depression of TNF-α (but not IFN-γ) production by PHA-stimulated PBMCs. Patients who received RT to both breast and regional RT showed more immune deficits compared with those who received only breast RT.
The interpretation of these data may be limited by the fact that all study subjects received myelosuppressive systemic chemotherapy prior to RT. However, mean RBCs, hemoglobin, hematocrit, and WBCs were normal at the start of RT. What is striking about the results of this study is that although the mean WBCs were in the normal range prior to starting RT, total WBC and lymphocyte populations decreased after 6 weeks after RT, as did NK cell functional activity.
Persistent lymphopenia and low NK cell activity may have consequences for risk of relapse. Low NK cell activity and low TNF-α levels are associated with increased risk of recurrent cancer.1,4,5,7,10,12–15 Our data suggest that women may be at immunologic risk following RT. The immune defect was restricted to lymphocytes and monocytes and their functional activity. The fact that lymphocytes and monocytes were higher after chemotherapy that was completed at least 4 to 6 weeks before RT began suggests that these changes are related to a specific immunologic effect of RT. The patients in this study did not report significant changes in fatigue, despite depression of TNF-α levels in the post-RT period.
These data may shed light on potential clinical implications of the immune defects of women after standard breast cancer therapy and contribute to the development and evaluation of adjunct immune therapies in the oncologic treatment sequence. Medicinal mushrooms, especially Trametes versicolor (turkey tail mushroom), show therapeutic promise as immune modulators.23–26
This study has several limitations, including (1) a small sample size of 14 breast cancer patients who were observed just before and after RT and (2) the potential confounding effect of previous systemic chemotherapy and growth factor therapy during chemotherapy. However, a consistent pattern of immune deficits in NK cell and cytokine activity, as well as persistent lymphopenia, emerged in more than 70% of the subjects and appeared to be temporally related to RT. Ideally, a study identifying radiologic effects on immune function would include participants who only received RT as an adjuvant therapy. However, a large proportion of early-stage breast cancer patients currently receive adjuvant chemotherapy prior to RT. These data describe the immune consequence of the standard of care sequence in oncology care in Seattle and Minneapolis. The immune defects detected in these breast cancer patients were not present after completion of chemotherapy but rather occurred after the initiation of RT.
This study is the first to describe changes in multiple hematologic and immune functional and immune cell population subset parameters in breast cancer patients before and after RT for early-stage breast cancer. Although the mechanisms underlying the immunologic effects of RT are poorly understood, these results help characterize the nature of the immune defects observed during the course of conventional therapy. Both low NK cell activity and TNF-α levels have been associated with poorer prognosis and worse symptomatology in cancer patients.27
Lymphopenia, low NK cell activity and low phagocytic activity of monocytes, and depressed TNF-α were observed following standard external beam chest RT for breast cancer and may have consequences for anticancer immune competence in the weeks and months following completion of standard treatment. The decreases observed in NK cell activity after RT could be due to a decrease in NK cell numbers in peripheral blood and/or in NK cell functional activity. This will be an important question to address in future studies. These novel data emphasize the importance of research on immunomodulators to maintain white cell function in breast cancer patients. Immune therapies that increase lymphocyte counts, NK cell activity, TNF-α production, and phagocytic activity may be warranted in the post-RT period.
Acknowledgments
Grateful acknowledgment to Peter Johnstone, MD, and Vivek Mehta, MD, for consultation on breast cancer radiotherapy and to Ana Nelson for bibliographic assistance.
Supported by National Center for Complementary and Alternative Medicine/National Institutes of Health grant no. 5 U19-AT001998 and Cancer Treatment Research Foundation (Gateway) grant no. G-04-02.
Contributor Information
Leanna J. Standish, Developmental Center for Research on Complementary and Alternative Medicine, Bastyr University, Kenmore, WA.
Carolyn Torkelson, Center for Spiritual Healing, University of Minnesota, Minneapolis, MN.
Frank A. Hamill, Developmental Center for Research on Complementary and Alternative Medicine, Bastyr University, Kenmore, WA.
Daesong Yim, Developmental Center for Research on Complementary and Alternative Medicine, Bastyr University, Kenmore, WA.
Alicia Hill-Force, Developmental Center for Research on Complementary and Alternative Medicine, Bastyr University, Kenmore, WA.
Annette Fitzpatrick, Developmental Center for Research on Complementary and Alternative Medicine, Bastyr University, Kenmore, WA.
Monica Olsen, Developmental Center for Research on Complementary and Alternative Medicine, Bastyr University, Kenmore, WA.
Sandi Schildt, Developmental Center for Research on Complementary and Alternative Medicine, Bastyr University, Kenmore, WA.
Erin Sweet, Developmental Center for Research on Complementary and Alternative Medicine, Bastyr University, Kenmore, WA.
Cynthia A. Wenner, Developmental Center for Research on Complementary and Alternative Medicine, Bastyr University, Kenmore, WA.
Mark R. Martzen, Developmental Center for Research on Complementary and Alternative Medicine, Bastyr University, Kenmore, WA.
References
- 1.Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer. 1999;80:880–888. doi: 10.1002/(sici)1097-0215(19990315)80:6<880::aid-ijc14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.Hacene K, Desplaces A, Brunet M, et al. Competitive prognostic value of clinicopathologic and bioimmunologic factors in primary breast cancer. Cancer. 1986;57:245–250. doi: 10.1002/1097-0142(19860115)57:2<245::aid-cncr2820570210>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Mohanty I, Nayak M, Nanda BK. Cell mediated immune status in carcinoma breast. Indian J Pathol Microbiol. 1991;34:1–6. [PubMed] [Google Scholar]
- 4.Pross HF, Sterns E, MacGillis DR. Natural killer cell activity in women at “high risk” for breast cancer, with and without benign breast syndrome. Int J Cancer. 1984;34:303–308. doi: 10.1002/ijc.2910340303. [DOI] [PubMed] [Google Scholar]
- 5.Claude L, Perol D, Ray-Coquard I, et al. Lymphopenia: a new independent prognostic factor for survival in patients treated with whole brain radiotherapy for brain metastases from breast carcinoma. Radiother Oncol. 2005;76:334–339. doi: 10.1016/j.radonc.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Lowdell MW, Craston R, Samuel D, et al. Evidence that continued remission in patients treated for acute leukaemia is dependent upon autologous natural killer cells. Br J Haematol. 2002;117:821–827. doi: 10.1046/j.1365-2141.2002.03495.x. [DOI] [PubMed] [Google Scholar]
- 7.Strender LE, Blomgren H, Wasserman J, et al. Influence of adjuvant radiation therapy in breast cancer on PWM induced Ig-secretion by blood lymphocytes in vitro. Anticancer Res. 1983;3:41–45. [PubMed] [Google Scholar]
- 8.Shukla HS, Hughes LE, Whitehead RH, Newcombe RG. Long-term follow-up of general immune competence in breast cancer. II. Sequential pre- and post-treatment levels: a 10 year study. Cancer Immunol Immunother. 1986;21:6–11. doi: 10.1007/BF00199370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rotstein S, Blomgren H, von Stedingk LV, et al. Long-term effects on the immune system following local irradiation for breast cancer. Pokeweed mitogen induced immunoglobulin secretion by blood lymphocytes and serum immunoglobulin levels. Eur J Surg Oncol. 1985;11:137–141. [PubMed] [Google Scholar]
- 10.Campbell MJ, Scott J, Maecker HT, et al. Immune dysfunction and micrometastases in women with breast cancer. Breast Cancer Res Treat. 2005;91:163–171. doi: 10.1007/s10549-004-7048-0. [DOI] [PubMed] [Google Scholar]
- 11.Petrini B, Wasserman J, Glas U, Blomgren H. T lymphocyte subpopulations in blood following radiation therapy for breast cancer. Eur J Cancer Clin Oncol. 1982;18:921–924. doi: 10.1016/0277-5379(82)90238-3. [DOI] [PubMed] [Google Scholar]
- 12.Zielinski CC, Mueller C, Tyl E, et al. Impaired production of tumor necrosis factor in breast cancer. Cancer. 1990;66:1944–1948. doi: 10.1002/1097-0142(19901101)66:9<1944::aid-cncr2820660916>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 13.Marucha PT, Crespin TR, Shelby RA, Andersen BL. TNF-alpha levels in cancer patients relate to social variables. Brain Behav Immun. 2005;19:521–525. doi: 10.1016/j.bbi.2005.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolini A, Carpi A, Rossi G. Cytokines in breast cancer. Cytokine Growth Factor Rev. 2006;17:325–337. doi: 10.1016/j.cytogfr.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Zielinski CC, Budinsky AC, Wagner TM, et al. Defect of tumour necrosis factor-alpha (TNF-alpha) production and TNF-alpha-induced ICAM-1-expression in BRCA1 mutations carriers. Breast Cancer Res Treat. 2003;81:99–105. doi: 10.1023/A:1025761716283. [DOI] [PubMed] [Google Scholar]
- 16.Perik PJ, Van der Graaf WT, De Vries EG, et al. Circulating apoptotic proteins are increased in long-term disease-free breast cancer survivors. Acta Oncol (Stockh) 2006;45:175–183. doi: 10.1080/02841860500482225. [DOI] [PubMed] [Google Scholar]
- 17.Turriziani A, Mattiucci GC, Montoro C, et al. Radiotherapy-related fatigue: incidence and predictive factors. Rays. 2005;30:197–203. [PubMed] [Google Scholar]
- 18.Cleeland CS, Bennett GJ, Dantzer R, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 19.Collado-Hidalgo A, Bower JE, Ganz PA, et al. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 20.Geinitz H, Zimmermann FB, Stoll P, et al. Fatigue, serum cytokine levels, and blood cell counts during radiotherapy of patients with breast cancer. Int J Radiat Oncol Biol Phys. 2001;51:691–698. doi: 10.1016/s0360-3016(01)01657-1. [DOI] [PubMed] [Google Scholar]
- 21.Parker R, Berman N. Sample size: more than calculations. Am Statistician. 2003;57:166–170. [Google Scholar]
- 22.Johann S, Blumel G, Lipp M, Forster R. A versatile flow cytometry-based assay for the determination of short- and long-term natural killer cell activity. J Immunol Methods. 1995;185:209–216. doi: 10.1016/0022-1759(95)00116-r. [DOI] [PubMed] [Google Scholar]
- 23.Hirai R, Oguchi Y, Sugita N, et al. Enhancement of T-cell proliferation by PSK. Int J Immunopharmacol. 1993;15:745–750. doi: 10.1016/0192-0561(93)90147-q. [DOI] [PubMed] [Google Scholar]
- 24.Kidd PM. The use of mushroom glucans and proteoglycans in cancer treatment. Altern Med Rev. 2000;5:4–27. [PubMed] [Google Scholar]
- 25.Toi M, Hattori T, Akagi M, et al. Randomized adjuvant trial to evaluate the addition of tamoxifen and PSK to chemotherapy in patients with primary breast cancer. 5-Year results from the Nishi-Nippon Group of the Adjuvant Chemoendocrine Therapy for Breast Cancer Organization. Cancer. 1992;70:2475–2483. doi: 10.1002/1097-0142(19921115)70:10<2475::aid-cncr2820701014>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 26.Morimoto T, Ogawa M, Orita K, et al. Postoperative adjuvant randomised trial comparing chemoendocrine therapy, chemotherapy and immunotherapy for patients with stage II breast cancer: 5-year results from the Nishinihon Cooperative Study Group of Adjuvant Chemoendocrine Therapy for Breast Cancer (ACETBC) of Japan. Eur J Cancer. 1996;32A:235–242. doi: 10.1016/0959-8049(95)00579-x. [DOI] [PubMed] [Google Scholar]
- 27.Ashamalla H, Marella V, Feliberti J, et al. Correlation of cytokine levels and response to radiation therapy and/or brachytherapy in low/intermediate risk prostate cancer. J Clin Oncol. 2005;23:13–17. [Google Scholar]