Abstract
Lymph hearts are pulsatile organs, present in lower vertebrates, that function to propel lymph into the venous system. Although they are absent in mammals, the initial veno-lymphatic plexus that forms during mammalian jugular lymph sac development has been described as the vestigial homologue of the nascent stage of ancestral anterior lymph hearts. Despite the widespread presence of lymph hearts among vertebrate species and their unique function, extremely little is known about lymph heart development. We show that Xenopus anterior lymph heart muscle expresses skeletal muscle markers such as myoD and 12/101, rather than cardiac markers. The onset of lymph heart myoblast induction can be visualized by engrailed-1 (en1) staining in anterior trunk somites, which is dependent on Hedgehog (Hh) signaling. In the absence of Hh signaling and upon en1 knockdown, lymph heart muscle fails to develop, despite the normal development of the lymphatic endothelium of the lymph heart, and embryos develop edema. These results suggest a mechanism for the evolutionary transition from anterior lymph hearts to jugular lymph sacs in mammals.
Keywords: Xenopus, lymph heart, lymphatic, muscle, engrailed, Prox, Hedgehog, edema
Introduction
The lymphatic system is crucial for proper fluid homeostasis – it drains interstitial fluid (lymph) from tissues and returns it to the blood supply. While skeletal muscle contractions circulate lymph in mammals, lymph hearts have been documented in fish, amphibians, reptiles, and birds (Kampmeier, 1969). Lymph hearts are found at the junctions of the lymphatic and venous systems and serve as a mechanism to pump lymph throughout the lymphatic system and into the veins. In frogs, a pair of anterior lymph hearts arises dorso-caudal to the pronephroi, between the myotomes and the skin, during tailbud stages (Kampmeier, 1922). Four pairs of posterior lymph hearts develop during late tadpole stages and connect to the dorsal subcutaneous lymph sacs during metamorphosis, then regress; in adult Xenopus, only two pairs of posterior lymph hearts remain (Nieuwkoop and Faber, 1967). The development of anuran lymphatic vasculature begins at approximately the same time and location of the anterior lymph hearts (Kampmeier, 1922). A plexus of vessels appears on the outer face of the first four myotomes, as lateral outgrowths of the intersegmental veins. The primordium of the anterior lymph heart appears at the 3rd segmental vein. As the lymph stream begins to flow towards the exits in the venous system, the plexus progressively loses all connections with the veins except at the lymph hearts, through an undescribed mechanism. Although lymph hearts are absent in mammals, the initial veno-lymphatic plexus that forms during mammalian jugular lymph sac development has been described as the vestigial homologue of the nascent stage of the frog anterior lymph heart (Kampmeier, 1960). The plexus is the transient forerunner of the jugular lymph sac anlagen, which develops in the same location.
Lymph hearts consist of three tissue layers (Satoh and Nitatori, 1980). The inner tunica intima consists of an endothelial cell lining with supportive connective tissue. The middle tunica media contains the musculature of the lymph heart, while the outer tunica externa is made up of fibroelastic tissue. In mammals and Xenopus, lymphatic vessels (endothelium) have been shown to arise from the blood vasculature, and require the function of the homeobox transcription factor prox1 (Ny et al., 2005; Sabin, 1902; Sabin, 1904; Wigle and Oliver, 1999). On the other hand, the origin of lymph heart musculature has been controversial. Most notably, Knower (1908) described anuran lymph heart musculature arising from the adjacent myotomes, while Kampmeier (1969) specifically refutes this claim and describes lymph heart myoblasts as mesenchymal cells that lie lateral to the myotomes. Recent studies indicate that the endothelium and musculature of chick lymph hearts arise from the somites (Valasek et al., 2007; Wilting et al., 2006).
We have examined the development of anterior lymph hearts in Xenopus laevis, focusing on the origin and development of the musculature. We show that lymph heart myoblasts express en1 and are under distinct developmental control from the associated lymphatic endothelium, which expresses prox1. Inhibiting the Hh pathway leads to an absence of en1-positive myoblasts and a subsequent loss of lymph heart muscle, while prox1-positive lymphatic tissue is not affected, and embryos develop edema. Specific paralysis of the lymph heart with benzocaine also results in edema, emphasizing the importance of the lymphatic system for maintaining proper fluid balance. Timed cyclopamine administration reveals that the requirement for Hh signaling in lymph heart myogenesis coincides with the onset of en1 expression in lymph heart myoblasts, indicating that loss of En1 causes loss of lymphatic musculature upon blockade of Hh signaling. Indeed, we show that Engrailed-1 function is required for development of the lymph heart musculature but not endothelium. The results suggest that changes in the developmental pathway controlling lymph heart muscle development may be responsible for the evolutionary loss of lymph hearts.
Methods
General methods and microinjection
Xenopus laevis embryos were generated, microinjected, and cultured by standard methods (Sive et al., 2000). Microinjections (10 nL) were performed at the 2-cell stage, unilaterally or bilaterally, with 4 ng fluoresceinated standard control morpholino (Gene Tools, LLC) plus Engrailed-1 morpholinos as follows: En1 MO1 (5′ TGCAGCAGCAAAGTAAGTAGCCCCC) 20 ng, and En1 MO2 (5′ TCAGTTTGATCCTCCATGTTATCGC) 10 ng. Embryos were allowed to develop in 0.3X Marc’s Ringer (MR) solution and staged according to the normal table (Nieuwkoop and Faber, 1967).
Whole-mount in situ hybridization, antibody staining, and sectioning
Embryos were fixed for 2 h in MEMFA prior to immunohistochemistry or in situ hybridization, which was performed by standard methods (Sive et al., 2000). Probes were synthesized in vitro with plasmids digested and transcribed as follows: MyoD – XbaI/SP6 (Hopwood and Gurdon, 1990), GATA4 – EagI/T3 (Jiang and Evans, 1996), cardiac troponin – NotI/T7 (Drysdale et al., 1994), cardiac actin – PvuII/SP6 (Mohun et al., 1984), engrailed-1 – XbaI/SP6 (Eizema et al., 1994), prox1 – NcoI/SP6 (Schaefer et al., 1999), Msr – NotI/T7 (Devic et al., 1996), VEGF – BamHI, T7 and Flk-1 – NotI, T7 (Cleaver et al., 1997). The muscle specific 12/101 monoclonal antibody was used undiluted to visualize differentiated skeletal muscle (Kintner and Brockes, 1984), followed by goat anti-mouse IgG secondary antibody (HRP, Jackson ImmunoResearch, 1:100 or AlexaFluor 488, Invitrogen, 1:200). The Prox1 polyclonal antibody (Covance) was used at a 1:1000 dilution, followed by a goat anti-rabbit IgG secondary conjugated to HRP (BioRad) at a 1:1000 dilution. In cases where both in situ hybridization and antibody staining were carried out on embryos, in situ staining was performed first, followed immediately by immunohistochemistry. To aid in visualization of staining, some embryos were cleared with benzyl benzoate/benzyl alcohol (2:1) after thorough dehydration in methanol. For sectioning, processed embryos were embedded in 4% low melt agarose and sectioned at 50–100 microns using a vibratome (Oxford).
Cyclopamine treatment
A stock solution of cyclopamine (LC Laboratories) was made at 10mM in 100% ethanol and diluted to 100 μM in 1/3x MR for embryo treatment. Except for time-course experiments, when embryos were added to cyclopamine at the stages indicated, embryos at stage 8–11 were transferred from 1/3x MR + 100 μM cyclopamine or 1/3x MR with an equivalent volume of ethanol (solvent) as a control and allowed to develop to the desired stage, then fixed in MEMFA.
Benzocaine treatment
A stock solution of benzocaine (ethyl 4-aminobenzoate) was made at 10% in 100% ethanol. Tadpoles with beating lymph hearts (st. 42+) were incubated in 1/3x MR + 0.005–0.01% benzocaine or an equivalent volume of ethanol as a control. In benzocaine, lymph hearts stopped beating within one hour, while cardiac heartbeat was unaffected or modestly slowed. Edema developed within 24 hours in benzocaine, after which tadpoles were moved to 1/3x MR to restore lymph heart function. Recovery of edema occurred within 5 days of restored lymph heart function.
Lymphangiography
Tadpoles with beating lymph hearts (st.42+) were anaesthetized in 0.01% benzocaine and microinjected in the ventral tail fin (Ny et al., 2005) with 20–50 nl of 25mg/ml mini-Ruby rhodamine dextran (Invitrogen). Injected embryos were moved to 1/3x MR for one hour to label circulating lymph before being fixed in MEMFA. Embryos were dehydrated in MeOH and cleared in benzyl benzoate/benzyl alcohol (2:1) for visualization.
Results and Discussion
Lymph heart musculature expresses markers of skeletal, but not cardiac, muscle
The anterior bilateral lymph hearts in Xenopus, marked by prox1 expression in the lymphatic endothelium, develop at stage 32 at the 3rd segmental vein between trunk somites 3 and 4 (Nieuwkoop and Faber, 1967; Ny et al., 2005). EM analysis has shown that the structure of lymph heart myotubes shares characteristics of both cardiac and skeletal muscle (Rumyantsev and Krylova, 1990). We examined several markers to determine the molecular nature of lymph heart muscle. The 12/101 antibody, which marks differentiated skeletal muscle but not cardiac muscle (Kintner and Brockes, 1984), was used to visualize stage 42 tadpole lymph hearts (Figure 1A, C, M). At this stage, bilaterally symmetrical lymph hearts (Figure 1A) are found adjacent to trunk somites 3 and 4 (Figure 1C). In addition, differentiating lymph hearts also express myoD, another marker specific to skeletal and not cardiac muscle (Hopwood et al., 1989) (Figure 1B). Despite being labeled with skeletal muscle markers, the lymph heart myotubes have a thin branched structure similar to cardiac muscle (Figure 1C), and lymph heart muscles contract in a rhythmic manner (our observations and Ny et al., 2005). However, the lymph hearts do not express determinants of cardiac fate, such as GATA4, -5, and -6 (Figure 1D–E and not shown), nor markers of terminal cardiac differentiation, such as the contractile protein cardiac troponin (Figure 1F–G) during early (st.33–34) or late (st. 40) stages of lymph heart development. Cardiac actin is expressed in lymph hearts at stage 40 (Figure 1I, arrow), as expected, because this is the first α-actin expressed in skeletal muscle (Mohun et al., 1984), as shown by the signal in the paraxial mesoderm, hypaxial muscles, and head musculature, as well as the heart (Figure 1H, I). The skeletal nature of lymph heart musculature is consistent with the observation that lymph heart muscle originates from the somites in the chick, and demonstrates the plasticity of somite-derived myoblasts in their ability to form “cardiac-like” skeletal muscle.
Figure 1. Lymph heart myoblasts express skeletal muscle markers and not cardiac markers.
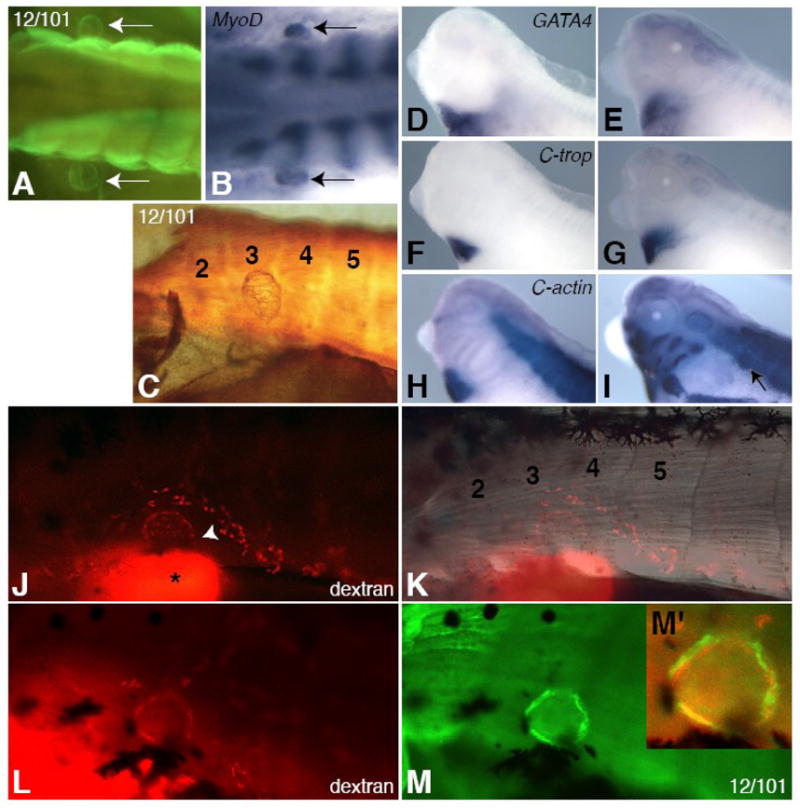
The 12/101 antibody marks lymph hearts in stage 42 tadpoles (A, C, M) and MyoD is expressed in differentiating lymph hearts at stage 40 (B, arrows). Two bilaterally symmetrical lymph hearts can be seen adjacent to the somites (A, B, arrows). The lymph heart forms in a region adjacent to somites 3 and 4 (C, K, trunk somites are numbered). Lymph heart muscle consists of a meshwork of myotubes (C). The lymph heart does not express cardiac markers GATA4 (D, E), cardiac troponin (F, G), or cardiac actin (H, I) at st. 33/34 (D, F, H) or st. 40 (E, G, I), except for cardiac actin at st. 40 (I, arrow), which marks all immature muscle (note expression in paraxial and head mesoderm in H, I). Rhodamine-dextran (mini-Ruby) injected subcutaneously into the posterior ventral tail fin drains anteriorly into the kidney (J, bright spot with asterisk) and into the lymph heart through vessels (J, arrowhead). The labeled lymph heart is located between somites 3 and 4 (K). Embryos injected with rhodamine-dextran (L, red) and then stained for the skeletal muscle antibody 12/101 (M, green) show co-expression (M′, yellow). All lateral views, anterior left, except for (A, B), which are dorsal views.
We used lymphangiography (Ny et al. 2005) to confirm that the structure labeled by the 12/101 antibody is the lymph heart. Rhodamine-dextran was injected subcutaneously into the ventral tail fin. Within one hour, the dye drained rostrally and most of the label was present in the kidney (Figure 1J, asterisk). However, labeled cells also were evident in streams that resembled vessels, leading to the lymph heart between somites 3 and 4 (Figure 1J, arrowhead; Figure 1K). The lymphatic tissue label (Figure 1L, red) colocalizes with 12/101 staining (Figure 1M, green; compare Figure 1L to 1M), with the muscle tissue encircling the dye-labeled lymphatic tissue, as expected (Figure 1M′).
The developing lymph heart is marked by en1 and prox1
We had previously examined en1 expression in tadpoles and found that it was expressed in anterior somites (Grimaldi et al., 2004). We further investigated the possibility that en1 positive cells may be marking myoblasts that give rise to the lymph heart musculature. To our surprise, we found that en1 specifically marks lymph heart myoblasts and not any other type of myoblast. Weak somitic expression is first seen at stage 28 in the clefts of anterior trunk somites 3 and 4 (Figure 2A, arrowheads). Transverse sections show en1 somite expression in a region lateral to the differentiated myotome at the D-V level of the notochord (Figure 2E, arrowhead). At later stages, en1 expression intensifies and then condenses into a small region ventral to the notochord, where the lymph heart will form (Figure 2, B–D, F–G). At stage 39, bilateral expression of en1 in the lymph heart region is very similar to later myoD and 12/101 staining (Figure 2D, Figure 1A–B). While en1 expression first appears on the same dorso-ventral plane as the notochord (Figure 2E, F), en1 positive cells progressively coalesce in a region ventral to the notochord, where the lymph heart forms (Figure 2G, compare to 2L). The mature lymph hearts are found directly above the pronephric tubules and glomus (Figure 2L), but arise from a distinct area in the somitic mesoderm, as opposed to intermediate mesoderm, from which the latter tissues form (Figure 2E and Supplemental Figure 1).
Figure 2. en1 and prox1 label the developing lymph heart musculature and endothelial tissue, respectively.
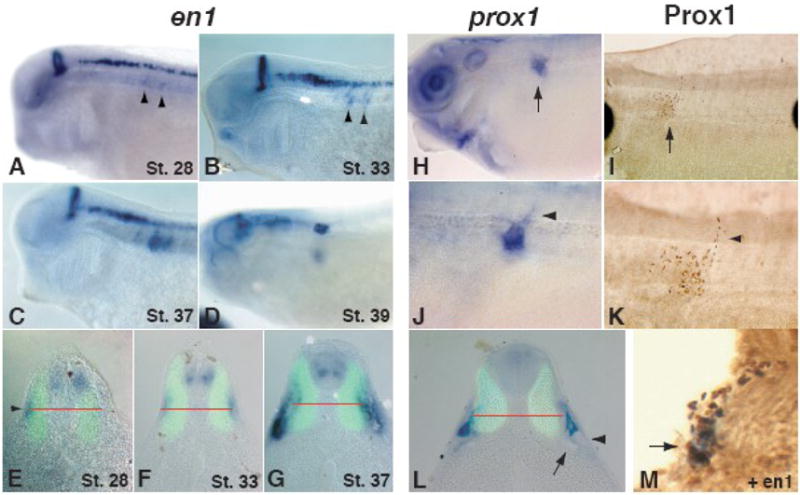
The expression of en1 is localized to the mid-hindbrain boundary, spinal interneurons, and anterior somites. The onset of somitic expression occurs at stage 28 (A) in a superficial region on a horizontal plane with the notochord (E, arrowhead, middle of notochord indicated by red line). The expression does not overlap with 12/101 staining in the differentiated muscle (green). Lateral views show early expression in the anterior somites (A–B, arrowheads), which intensifies and moves ventrally to occupy the final position of the lymph heart (C–D, G). A transverse section of a stage 33 tadpole (F) shows the intensity of expression increasing. By stage 37 (G), en1-positive cells have moved ventrally relative to the position of the notochord (red line) and are found directly above the glomus (arrow) and pronephric tubules (arrowhead). At stage 40, prox1 RNA (blue, H, J, L) and Prox1 protein (brown, I, K, M) are localized to the developing endothelial tissue of the lymph heart (H, I, arrows). (J, K) Higher magnification views of H and I illustrate the budding lymphatic vasculature dorsal to the lymph heart (arrowheads). (L) The expression of prox1 is slightly ventral to the notochord (red line) and dorsal to the pronephroi (arrow) and does not overlap with differentiated muscle (12/101, green). (M) Prox1 (brown) and en1 (blue) expressing cells co-localize at the site of lymph heart formation (arrow). The epidermis has sloughed off during the processing of this embryo.
The inner layer of lymph hearts consists of lymphatic endothelial cells (Satoh and Nitatori, 1980). The development of lymphatic endothelium in the mouse has been shown to depend on the activity of the homeobox transcription factor Prox1 (Wigle and Oliver, 1999), and the expression of prox1 RNA in Xenopus lymph hearts has been reported previously (Ny et al., 2005). We reexamined prox1 RNA expression as well as Prox1 protein in order to compare the timing and location of expression to that of en1. At stage 39, Prox1 RNA and protein are both detected in a region similar to en1 expression at this stage, adjacent to trunk somites 3 and 4 (Figure 2H, I) and slightly ventral to the notochord (Figure 2L). Lymphatic vessels leading out of the lymph heart are also forming at this stage (Figure 2J, K arrowheads). Prox1- and en1-expressing cells co-localize in the region of the developing lymph heart at this stage (Figure 2M, arrowhead).
Hh signaling is required for lymph heart muscle development but not lymphatic tissue
In zebrafish, En proteins mark a specific subset of somitic cells called muscle pioneers (Hatta et al., 1991). These form adjacent to the notochord and are dependent upon Hedgehog (Hh) signals for proper development (Currie and Ingham, 1996). Similar expression of en1 is seen in the chicken, where en1-positive cells mark the most lateral aspect of the epaxial myotome (Cheng et al., 2004; Davis et al., 1991). Expression of en1 in the chicken is also under positive control by Hh signaling (Cheng et al., 2004). In order to test whether en1-positive lymph heart myoblasts might be homologous to en1-positive cells in the zebrafish and chicken, we exposed embryos to the Hh signaling inhibitor cyclopamine and examined them for en1 expression and lymph heart formation. We have previously shown that cyclopamine can efficiently block Hh signaling in Xenopus embryos (Martin et al., 2007).
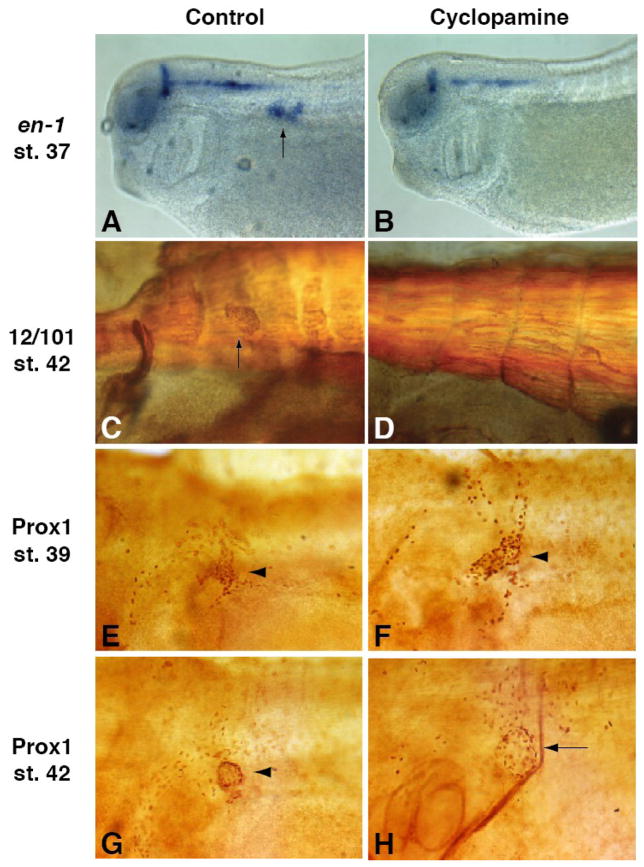
Exposure to cyclopamine had a drastic effect on both en1 expression and lymph heart formation. At stage 37, cyclopamine-treated tadpoles display a dramatic reduction of en1 in lymph heart myoblasts, while neural expression is relatively unaffected (Figure 3B, 96% with strong en1 reduction in lymph heart myoblasts, N=24), as compared to control embryos (Figure 3A, 4% with strong en1 reduction, N=23). Similarly, stage 42 tadpoles lack visibly beating lymph hearts and 12/101 staining reveals an absence of differentiated lymph hearts (Figure 3D, 88% missing 12/101-positive lymph hearts, N=45) as compared to controls (Figure 3C, 4% missing 12/101-positive lymph hearts, N=26). These results indicate that Xenopus lymph heart myoblasts undergo a similar developmental program as zebrafish pioneer muscle cells and en1 positive chicken myoblasts.
Figure 3. Lymph heart muscle but not lymphatic endothelium requires Hh signaling.
Cyclopamine treatment greatly reduces en1 expression in the somites (23 out of 24 embryos), while neural expression is relatively unaffected (B compared to A, arrow in A indicates lymph heart expression). At later stages, 12/101 staining shows that lymph heart musculature is missing in cyclopamine-treated tadpoles (D compared to C, arrow in C indicated lymph heart muscle). The presence of Prox1-positive lymphatic endothelial cells (arrowheads) is not affected by cyclopamine treatment at stage 39 (F compared to E) or stage 42 (H compared to G). (H) The loss of lymph heart musculature leads to a less compact distribution of Prox1-positive cells in the lymph heart (arrow).
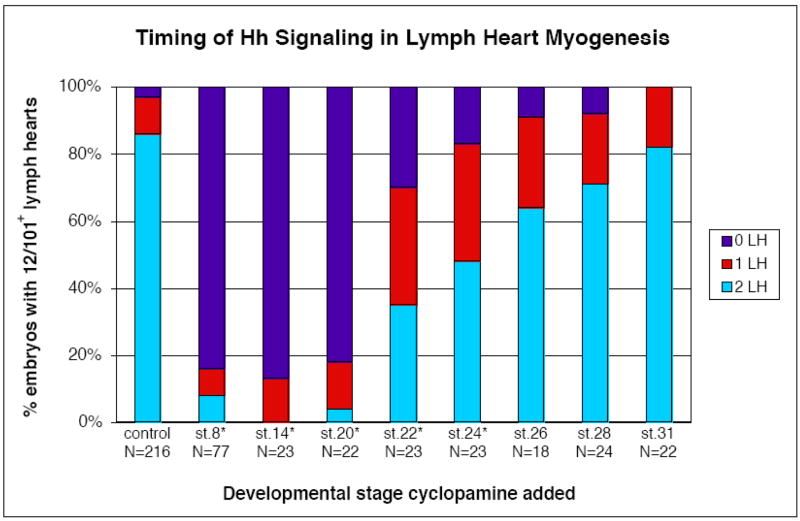
Figure 4. The timing of Hedgehog induction of lymph hearts corresponds to the onset of en1 expression.
Cyclopamine or ethanol (as a solvent control) was added to embryos at the specified developmental stages; tadpoles were fixed at stage 42–45, and stained with 12/101 for presence of lymph heart musculature. The ethanol controls for all stages are combined in this graph, but χ2 tests were performed between stage-matched cyclopamine and control samples for determination of statistical significance. Cyclopamine treatment has a less potent effect when added after stage 20 (4% wildtype at st.20 vs. 35% wildtype at st.22). Experimental and control samples are not significantly different when embryos are added to cyclopamine at stage 26, just prior to en1 expression in lymph heart myoblasts, or later in development (*p<0.01).
Surprisingly, cyclopamine-treated embryos exhibited a normal appearance of Prox1-positive endothelial cells in the lymph heart at stage 39 and 42 (Figure 3F, H compared to E, G, cyclopamine-treated N=9, control N=8). The only minor defect appears at stage 42, when Prox1-positive cells have condensed to form the tunica intima (Figure 3G, arrowhead). In cyclopamine-treated embryos, these cells are not as tightly packed (Figure 3H, arrow), indicating that the musculature of the tunica media is needed for the proper compaction of the tunica intima. The loss of lymph heart muscle, but not endothelial cells, in cyclopamine-treated embryos indicates that these two tissues are under distinct developmental control. This is interesting in light of the fact that lymph hearts have been lost in mammals, yet the endothelial lymphatic structure of the jugular lymph sac forms in the homologous location in mammals (Kampmeier, 1969).
Hh requirement for lymph heart myoblasts corresponds to the onset of en1 expression
To further address the requirement for Hh signaling in the formation of lymph heart myoblasts, we wanted to ascertain whether the timing of the Hh requirement correlates with the onset of en1 expression in lymph heart myoblasts. We therefore added cyclopamine to embryos at different stages of development and assayed them for lymph heart myogenesis by 12/101 staining at stage 45 (Figure 4). Inhibition of Hh signaling prior to stage 22 resulted in a drastic reduction in 12/101-positive lymph hearts (4% of embryos with 2 lymph hearts when cyclopamine added at st.20, N=22), and this loss was significantly different from controls until stage 26 (p<0.01, χ2 test). Addition of embryos at stage 26 or later resulted in significantly fewer defects in 12/101 staining, with over 80% (N=22) of embryos showing bilateral 12/101-positive lymph hearts when added to cyclopamine at stage 31 (Figure 4). These results demonstrate that Hh signaling is required slightly prior to the onset of en1 expression for the induction of lymph heart myoblasts, and that these en1-positive cells do not require continuous Hh signaling for later development. Therefore, the onset of en1 expression in lymph heart myoblasts coincides with the Hh-dependent induction of this tissue.
Loss of lymph heart muscle structure or function results in edema
While beating lymph hearts can be easily identified under the dissecting microscope in st.42+ wildtype tadpoles, those treated with cyclopamine from gastrula stages do not have beating lymph hearts. Cyclopamine-treated embryos have severe edema at tadpole stages (100%, N=79, Figure 5B), in addition to axial and gut-looping defects expected upon loss of Hh signaling (Figure 6J and not shown). Subcutaneously-injected dye, which is usually pumped into the pronephric sinus by the lymph hearts and cleared within an hour (Figure 5C), fills the coelomic cavity and is retained in cylopamine-treated embryos (Figure 5D). Our results indicate that Hh signaling is required to specify the musculature for functional lymph hearts; since lymphatic defects result in edema (Ny et al., 2005), we could potentially conclude that edema results in cyclopamine-treated embryos from non-functional lymph hearts. However, edema can be associated with impairment in cardioascular and renal function, in addition to lymphatic function, and Hh signaling is required for numerous developmental processes. Indeed, while most cyclopamine-treated embryos have beating hearts, in many the cardiac chambers do not fill with blood and no blood circulation is observed in the tail or gills. These observations implicate a vasculature defect upon inhibition of Hh signaling, which may contribute to the development of edema and/or the recruitment of lymph heart myoblasts from the somites. Thus, we examined expression of several vascular markers in cyclopamine-treated embryos (Supplemental Figure 2).
Figure 5. Lymph heart paralysis results in reversible edema.
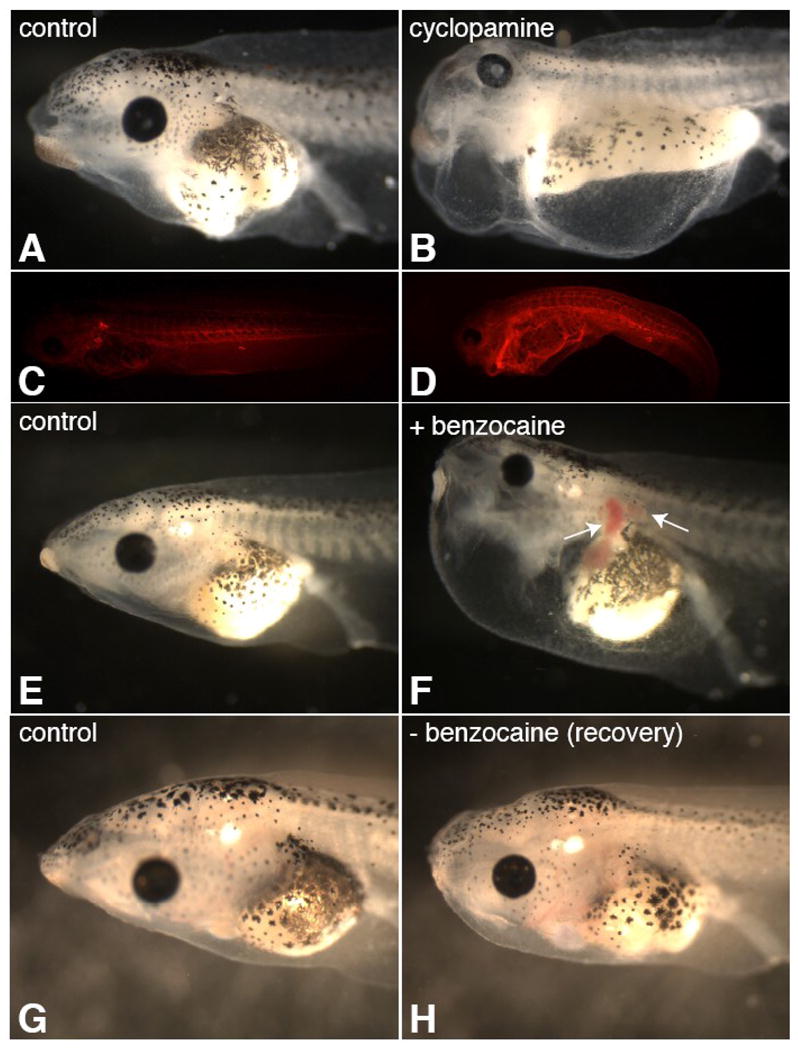
Cylopamine treatment from stage 8 results in loss of lymph heart beating and severe edema at stage 42 (B; 100%, N=79). Florescent dye injected subcutaneously into the fin of cyclopamine-treated tadpoles (stage 42) fills the body cavity and fails to clear the body in an hour (D), unlike in controls (C). Tadpoles with visibly beating lymph hearts (st.42+) were placed in a dilute benzocaine solution (0.005–0.01%), which resulted in lymph heart paralysis while leaving the cardiac heartbeat unaffected. After 24 hours of lymph heart paralysis, tadpoles developed severe edema with blood pooling in the kidney, lymph heart, and gills (F, arrows; 100%, N=60), while control tadpoles did not (E). Removal of these embryos from benzocaine restored lymph heart function and tadpoles were mostly recovered in 4 days (H; 58%, N=57, compare to G, control).
Figure 6. The development of edema upon cyclopamine treatment does not correspond to lymph heart beating.
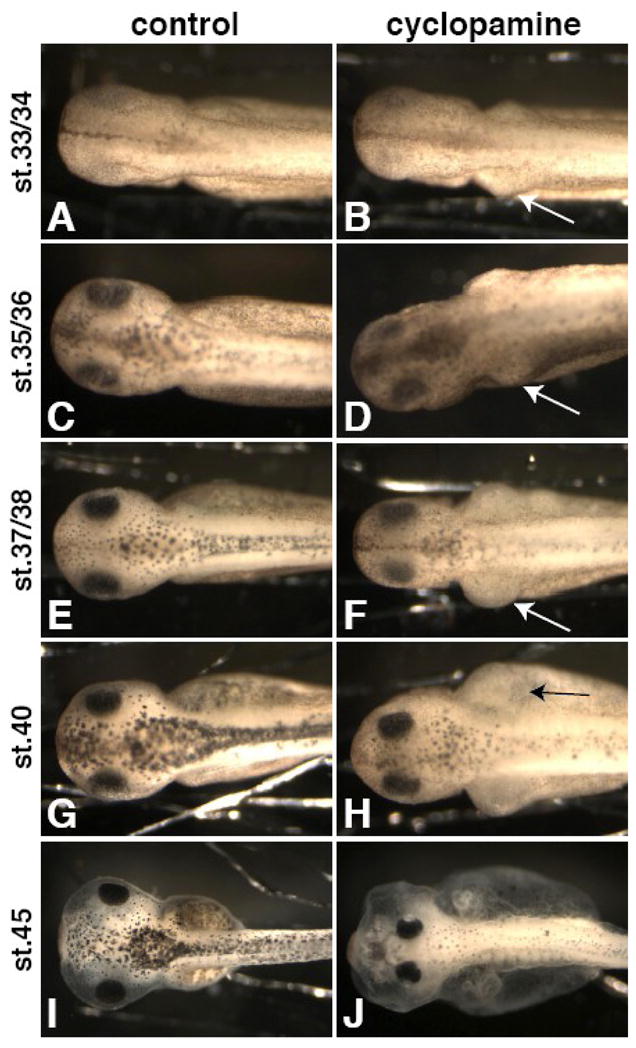
Embryos were added to cyclopamine (B, D, F, H, J) at stage 8 and compared to controls (A, C, E, G, I) at stage 33/34 (A, B), st. 35/36 (C, D), st. 37/38 (E, F), st. 40 (G, H), and st. 45 (I, J). Edema begins around the kidney area as early as st. 33/34 (B, arrow) and continues to worsen (D, F, H, arrows), long before the lymph hearts start beating (st. 41–42).
Cardiovascular markers (Msr, VEGF, and Flk-1) were unperturbed in the area of the lymph heart prior to en1 expression in cyclopamine-treated embryos (st.24–25, Figure S2A–B, E–F, I–J). In addition, development of the heart, aortic arch arteries, dorsal aorta, intersomitic vessels, pronephric sinus, and ventral blood islands appear normal. At the time of en1 onset in lymph heart myoblasts (stage 28, Figure S2C–D, G–H, K–L), cyclopamine-treated embryos have increased Msr and Flk1 expression in the posterior cardinal vein (PCV, Figure S2D, L, arrows) and decreased VEGF expression in the glomus (Figure S2H, arrowhead). However, all markers seem to be expressed normally in vasculature surrounding the lymph heart, indicating that lymph heart musculature does not fail to be recruited secondary to reduced blood supply in these embryos. Nonetheless, it is quite possible that the observed changes in the PCV architecture contribute to the edema and circulatory defects observed at tadpole stages in cyclopamine-treated embryos.
To address whether cyclopamine-induced edema might be specific to defective lymph heart function, we selectively paralyzed the lymph heart with a mild anesthetic (benzocaine) treatment. While high doses of benzocaine inhibit all muscle function, mild benzocaine treatment (0.01–0.005%) paralyzes skeletal and lymph heart muscle, while preserving cardiac muscle function. Over 24 hours of impaired lymph heart function, tadpoles develop severe edema, which resembles the edema phenotype in cyclopamine-treated embryos (Figure 5F; 100%, N=60). The cardiac hearts beat but are not filled with blood, and blood pools in the kidney, lymph heart, and gills (Figure 5F, arrows). As anesthetic was added after development of the vasculature and is not expected to affect vasculogenesis, blood pooling suggests that skeletal muscle contractions might be required for efficient cardiovascular circulation. The lymphedema and blood circulation defects were largely recovered within 4 days by returning embryos to culture media without benzocaine to allow the lymph heart to start beating again (Figure 5H; 58% recovered, N=57).
While these results support the conclusion that loss of lymph heart beating results in edema, they do not prove that the cyclopamine-induced edema results directly from loss of lymph heart myoblasts. In fact, we had seen vascular and renal capsule (glomus) defects upon loss of Hh signaling (Figure S2), which may cause edema or compound the effect of lymph heart dysfunction. To address this correlation, we examined whether the development of the edema phenotype in cyclopamine-treated embryos coincides with the onset of lymph heart beating. Embryos placed in cyclopamine from stage 8 onwards develop severe edema and other hallmark Hh loss-of-function phenotypes, such as narrowly-spaced eyes (Figure 6J). In a time-course observation of these embryos, it is clear that swelling begins earlier than the onset of lymph heart beating at stage 41–42; edema begins around the kidney area, as early as stage 33 (Figure 6B, arrow) and is clear at stage 35/36 (Figure 6D, arrow). Edema continues to develop so that it is severe at stage 40 (Figure 6H, arrow). Because the onset of edema in cyclopamine-treated embryos precedes lymph heart beating, it cannot be caused by solely by loss of lymph heart musculature, though our benzocaine experiments indicate that loss of lymph heart function would exacerbate pre-existing edema. Perhaps circulatory defects due to mispatterned vasculature are the primary cause of edema in cyclopamine-treated embryos. Alternatively, since swelling begins around the kidney, it is possible that kidney specification or function, as well as lymph heart myogenesis, is affected by loss of Hh signaling. We are currently investigating this possibility.
Engrailed-1 is required for lymph heart musculature
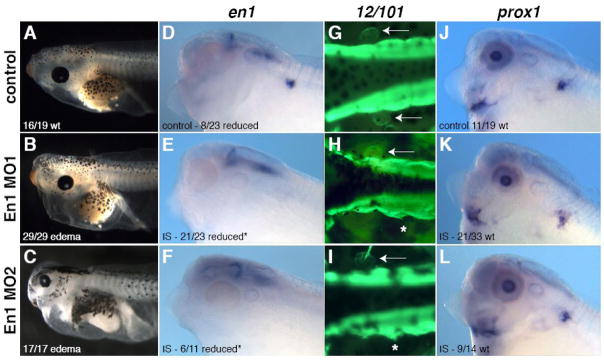
We have shown that Hh signaling is required for expression of engrailed-1, the earliest marker we have found of lymph heart myoblasts, and that the requirement for Hh signaling in lymph heart musculature coincides with the onset of en1 expression. These results suggest that loss of Engrailed-1 function in cyclopamine-treated embryos leads to failure of lymph heart myogenesis. To determine whether En1 is required for the specification of lymph heart musculature, we performed morpholino knockdown. With two non-overlapping MOs designed to block en1 translation, we obtained similar results to Hh signaling inhibition with cyclopamine. Bilateral injection of En1 MO led to loss of beating lymph hearts and subsequent edema (100%, N=46; Figure 7A–C), which began after stage 41 and was less severe than with cyclopamine or benzocaine treatment, without circulation defects. This specific result strengthens our hypothesis that loss of skeletal muscle contractions in benzocaine-treated tadpoles results in blood-pooling, as well as our conclusion that cyclopamine-induced edema results from additional causes other than lymph heart dysfunction. Unilateral injection of En1 MO resulted in loss of lymph heart musculature on the injected side, assayed by en1 (79%, N=34; Figure 7D–F) and MyoD expression (100%, N=13; data not shown) and 12/101 staining (95%, N=80; Figure 7G–I). However, paraxial mesoderm and prox1 expression in the endothelium were mostly unaffected (Figure 7K-L; 64% wt, N=47 for En1 morphants vs. 58% wt, N=19 for controls). These results from En1 knock-down recapitulate the phenotype of cyclopamine treatment.
Figure 7. Engrailed-1 is required for lymph heart myogenesis.
Two non-overlapping MOs were designed to block en1 translation. Bilateral injection of En1 MO leads to loss of lymph heart beat and edema (A–C). Unilateral injection of En1 MO leads to specific loss of lymph heart musculature assayed by en1 expression (D–F, *p<0.05 χ2 test) and 12/101 staining (G–I, dorsal view, arrow points to lymph heart, asterisk indicates injected side). In controls, 100% of embryos have two 12/101-positive lymph hearts (N=43), while 5% of En1 morphants do (En1 MO1 N=42, En1 MO2 N=38). Lymph heart endothelium (prox1, J–L) is not affected by En1 knockdown compared to controls (p>0.05, χ2 test).
Due to the highly specific spatio-temporal expression of en1 and its potent transcriptional repression activity, we were unable to perform traditional rescue experiments to determine the specificity of En1 MOs. However, we believe we have achieved specific En1 knockdown, as we obtained the same results with two non-overlapping MOs, and observed phenotypes were specific to the tissue that expresses en1, i.e. the lymph heart myoblasts, as opposed to lymphatic endothelium or other musculature. In addition, we observed edema that coincided in time with the onset of lymph heart beating, with no obvious effect on circulation or locomotion. Our results indicate that Engrailed-1 is required for specification of lymph heart myoblasts, downstream of Hh signaling.
Conclusions
Anterior lymph heart myoblasts represent a somitic cell lineage that shares similarity to teleost muscle pioneer cells, which also express en1 and require Hh signals. They also demonstrate the plasticity of somite-derived myoblasts in that they possess the ability to form “cardiac-like” skeletal muscle, despite not expressing cardiac specification or differentiation markers, such as GATA genes and cardiac troponin. The lymph heart musculature, which forms the tunica media, is under distinct developmental control from the endothelial tissue, which forms the tunica intima. Loss of Hh signaling or Engrailed-1 function causes an absence of lymph heart muscle, but does not affect the endothelial tissue. Mammals do not possess beating lymph hearts, but instead have a jugular (endothelial) lymph sac at the same anatomical position to that of the anterior lymph hearts in frogs. This evolutionary transition may have been facilitated due to the separate pathways regulating lymph heart myoblasts vs. endothelial tissue.
It is likely that Hh signaling is permissive for lymph heart myoblast induction, as there is no localized expression of Hh genes in somites 3 and 4, from which the anterior lymph heart myoblasts arise. In addition, Shh overexpression does not induce ectopic or larger lymph hearts (not shown), and the Hh receptor and target gene Patched is not prominently co-expressed with en1. Indeed, it is possible that inhibition of Hh signaling results in loss of lymph heart myoblasts indirectly, through its effects on somite patterning and differentiation. We have shown that loss of Hh signaling expands the dermamyotome (Martin et al., 2007), the area from which en1-expressing lymph heart myoblasts seem to arise (Figure 2E–F). Thus, loss of Hh signaling may keep the dermamyotome in a non-differentiated state at a crucial time-point for the lymph heart, not allowing for the differentiation of en1-positive lymph heart myoblasts.
Upon loss of lymph heart musculature (in cyclopamine-treated embryos and En1 morphants) or lymph heart beating (through benzocaine-induced paralysis), we observed edema in tadpole embryos. En1 MO-induced edema is likely specifically due to failure of lymph heart myogenesis, as we saw no obvious defects in tissues other than the lymph heart musculature, and edema resulted only after the time when the lymph hearts normally start to beat. Benzocaine treatment paralyzes skeletal muscle contractions and the lymph hearts, while leaving the cardiac heart beat intact. Benzocaine-induced edema was more severe than that observed in En1 morphants, with reduced blood circulation and pooling in the kidney, lymph heart, and gills. As the only difference between these embryos and En1 morphants is the paralysis of skeletal muscle, we hypothesize that skeletal muscle contractions help to circulate blood, and perhaps lymph, in Xenopus tadpoles. In mammals, which do not possess lymph hearts, skeletal muscle contractions serve to circulate lymph throughout the body.
Interestingly, blockade of Hh signaling induced edema earlier in development, prior to the onset of lymph heart beating at stage 41–42, indicating an alternate cause. Edema is associated with defects in cardiac, renal, and lymphatic systems. While the cardiac heart develops upon inhibition of Hedgehog signaling, we observed some abnormal expression of vasculature markers in the posterior cardinal vein, which may lead to circulatory defects and edema in these embryos. It is also possible that Hh signaling is required for kidney development in Xenopus laevis. Upon inhibition of Hh signaling, edematous swelling begins around the kidney at around the time it begins to be functional (stage 37–38), supporting this hypothesis. In addition, VEGF expression in the glomus is decreased in cyclopamine-treated embrhyos. Previously, ectopic Hh signaling has been shown to repress development of the pronephric duct and tubule through repression of Fgf8, which is required for condensation and epithelialization (Urban et al., 2006). We are currently performing loss-of-function experiments to establish whether kidney development is compromised in the absence of Hh signaling as well.
These results emphasize the importance of Hedgehog signaling in the organogenesis of lower vertebrates, in which there are differences in the Hh requirements for formation of somitic derivatives, such as muscle pioneers in teleosts and hypaxial musculature and lymph heart myoblasts in amphibians. We further establish the usefulness of Xenopus in the study of fluid balance through the action of the heart, lypmphatic system and pronephros.
Supplementary Material
(A) The lymph heart (en1, arrow) and glomus (WT1) occupy neighboring regions in the embryo at stage 35. (B–D) In situ hybridization at stage 28 for en1 (lymph heart, B), compared to glomus markers WT1 (C) and VEGF (D), shows that these tissues arise from distinct areas in the embryo. All panels are transverse vibratome sections after whole-mount in situ hybridization.
(A–D) Msr (E–H) VEGF (I–L) Flk1 in control (A, C, E, G, I, K) and cyclopamine-treated (B, D, F, H, J, L) embryos at stage 24–25 (A, B, E, F, I, J) and stage 28 (C, D, G, H, K, L). Early and anterior expression domains of these markers are unperturbed upon inhibition of Hh signaling. Msr and Flk1 are upregulated in the posterior cardinal vein in cyclopamine-treated embryos at st.28 (D, L, arrows) and VEGF expression is lost in the glomus at st.28 (H, arrow).
Acknowledgments
We thank P. Krieg for gifts of Msr and Flk1 plasmids, members of the Harland group for helpful comments and advice, and J. Young for technical assistance. This work was supported by the NIH (GM42341) and the Muscular Dystrophy Association (015164).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cheng L, Alvares LE, Ahmed MU, El-Hanfy AS, Dietrich S. The epaxial-hypaxial subdivision of the avian somite. Dev Biol. 2004;274:348–69. doi: 10.1016/j.ydbio.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Cleaver O, Tonissen KF, Saha MS, Krieg PA. Neovascularization of the Xenopus embryo. Dev Dyn. 1997;210:66–77. doi: 10.1002/(SICI)1097-0177(199709)210:1<66::AID-AJA7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Currie PD, Ingham PW. Induction of a specific muscle cell type by a hedgehog-like protein in zebrafish. Nature. 1996;382:452–5. doi: 10.1038/382452a0. [DOI] [PubMed] [Google Scholar]
- Davis CA, Holmyard DP, Millen KJ, Joyner AL. Examining pattern formation in mouse, chicken and frog embryos with an En-specific antiserum. Development. 1991;111:287–98. doi: 10.1242/dev.111.2.287. [DOI] [PubMed] [Google Scholar]
- Devic E, Paquereau L, Vernier P, Knibiehler B, Audigier Y. Expression of a new G protein-coupled receptor X-msr is associated with an endothelial lineage in Xenopus laevis. Mech Dev. 1996;59:129–40. doi: 10.1016/0925-4773(96)00585-0. [DOI] [PubMed] [Google Scholar]
- Drysdale TA, Tonissen KF, Patterson KD, Crawford MJ, Krieg PA. Cardiac troponin I is a heart-specific marker in the Xenopus embryo: expression during abnormal heart morphogenesis. Dev Biol. 1994;165:432–41. doi: 10.1006/dbio.1994.1265. [DOI] [PubMed] [Google Scholar]
- Eizema K, Koster JG, Stegeman BI, Baarends WM, Lanser PH, Destree OH. Comparative analysis of Engrailed-1 and Wnt-1 expression in the developing central nervous system of Xenopus laevis. Int J Dev Biol. 1994;38:623–32. [PubMed] [Google Scholar]
- Grimaldi A, Tettamanti G, Martin BL, Gaffield W, Pownall ME, Hughes SM. Hedgehog regulation of superficial slow muscle fibres in Xenopus and the evolution of tetrapod trunk myogenesis. Development. 2004;131:3249–62. doi: 10.1242/dev.01194. [DOI] [PubMed] [Google Scholar]
- Hatta K, Bremiller R, Westerfield M, Kimmel CB. Diversity of expression of engrailed-like antigens in zebrafish. Development. 1991;112:821–32. doi: 10.1242/dev.112.3.821. [DOI] [PubMed] [Google Scholar]
- Hopwood ND, Gurdon JB. Activation of muscle genes without myogenesis by ectopic expression of MyoD in frog embryo cells. Nature. 1990;347:197–200. doi: 10.1038/347197a0. [DOI] [PubMed] [Google Scholar]
- Hopwood ND, Pluck A, Gurdon JB. MyoD expression in the forming somites is an early response to mesoderm induction in Xenopus embryos. Embo J. 1989;8:3409–17. doi: 10.1002/j.1460-2075.1989.tb08505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Evans T. The Xenopus GATA-4/5/6 genes are associated with cardiac specification and can regulate cardiac-specific transcription during embryogenesis. Dev Biol. 1996;174:258–70. doi: 10.1006/dbio.1996.0071. [DOI] [PubMed] [Google Scholar]
- Kampmeier OF. The development of the anterior lymphatics and lymph hearts in Anuran embryos. Amer J Anat. 1922:30. [Google Scholar]
- Kampmeier OF. The developmental of the jugular lymph sacs in the light of the vestigial, provisional and definitive phases of morphogenesis. Amer J Anat. 1960:107. doi: 10.1002/aja.1001070205. [DOI] [PubMed] [Google Scholar]
- Kampmeier OF. Evolution and comparative morphology of the lymphatic system. Springfield, Illinois: Charles C. Thomas; 1969. [Google Scholar]
- Kintner CR, Brockes JP. Monoclonal antibodies identify blastemal cells derived from dedifferentiating limb regeneration. Nature. 1984;308:67–9. doi: 10.1038/308067a0. [DOI] [PubMed] [Google Scholar]
- Martin BL, Peyrot SM, Harland RM. Hedgehog signaling regulates the amount of hypaxial muscle development during Xenopus myogenesis. Dev Biol. 2007;304:722–34. doi: 10.1016/j.ydbio.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun TJ, Brennan S, Dathan N, Fairman S, Gurdon JB. Cell type-specific activation of actin genes in the early amphibian embryo. Nature. 1984;311:716–21. doi: 10.1038/311716a0. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis. Amsterdam: North-Holland Publishing Company; 1967. [Google Scholar]
- Ny A, Koch M, Schneider M, Neven E, Tong RT, Maity S, Fischer C, Plaisance S, Lambrechts D, Heligon C, et al. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nat Med. 2005;11:998–1004. doi: 10.1038/nm1285. [DOI] [PubMed] [Google Scholar]
- Rumyantsev PP, Krylova MI. Ultrastructure of myofibers and cells synthesizing DNA in the developing and regenerating lymph-heart muscles. Int Rev Cytol. 1990;120:1–52. doi: 10.1016/s0074-7696(08)61598-3. [DOI] [PubMed] [Google Scholar]
- Sabin FR. On the origin of the lymphatic system from the veins, and the development of the lymph hearts and thoracic duct in the pig. Amer J Anat. 1902;1:367–389. [Google Scholar]
- Sabin FR. On the development of the superficial lymphatics in the skin of the pig. Amer J Anat. 1904;3:183–195. [Google Scholar]
- Satoh Y, Nitatori T. On the fine structure of lymph hearts in amphibia and reptiles. Academic Press; 1980. [Google Scholar]
- Schaefer JJ, Oliver G, Henry JJ. Conservation of gene expression during embryonic lens formation and cornea-lens transdifferentiation in Xenopus laevis. Dev Dyn. 1999;215:308–18. doi: 10.1002/(SICI)1097-0177(199908)215:4<308::AID-AJA3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Press; 2000. [Google Scholar]
- Urban AE, Zhou X, Ungos JM, Raible DW, Altmann CR, Vize PD. FGF is essential for both condensation and mesenchymal-epithelial transition stages of pronephric kidney tubule development. Dev Biol. 2006;297:103–17. doi: 10.1016/j.ydbio.2006.04.469. [DOI] [PubMed] [Google Scholar]
- Valasek P, Macharia R, Neuhuber WL, Wilting J, Becker DL, Patel K. Lymph heart in chick--somitic origin, development and embryonic oedema. Development. 2007;134:4427–36. doi: 10.1242/dev.004697. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–78. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Wilting J, Aref Y, Huang R, Tomarev SI, Schweigerer L, Christ B, Valasek P, Papoutsi M. Dual origin of avian lymphatics. Dev Biol. 2006;292:165–73. doi: 10.1016/j.ydbio.2005.12.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The lymph heart (en1, arrow) and glomus (WT1) occupy neighboring regions in the embryo at stage 35. (B–D) In situ hybridization at stage 28 for en1 (lymph heart, B), compared to glomus markers WT1 (C) and VEGF (D), shows that these tissues arise from distinct areas in the embryo. All panels are transverse vibratome sections after whole-mount in situ hybridization.
(A–D) Msr (E–H) VEGF (I–L) Flk1 in control (A, C, E, G, I, K) and cyclopamine-treated (B, D, F, H, J, L) embryos at stage 24–25 (A, B, E, F, I, J) and stage 28 (C, D, G, H, K, L). Early and anterior expression domains of these markers are unperturbed upon inhibition of Hh signaling. Msr and Flk1 are upregulated in the posterior cardinal vein in cyclopamine-treated embryos at st.28 (D, L, arrows) and VEGF expression is lost in the glomus at st.28 (H, arrow).