Abstract
Localized and efficient gene transfer can be promoted by exploiting the interaction between the vector and biomaterial. Regulation of the vector-material interaction was investigated by capitalizing on the binding between lentivirus and phosphatidylserine (PS), a component of the plasma membrane. PS was incorporated into microspheres composed of the copolymers of lactide and glycolide (PLG) using an emulsion process. Increasing the weight ratio of PS to PLG led to a greater incorporation of PS. Lentivirus, but not adenovirus, associated with PS-PLG microspheres, and binding was specific to PS relative to PLG alone or PLG modified with phosphatidylcholine. Immobilized lentivirus produced large numbers of transduced cells, and increased transgene expression relative to virus alone. Microspheres were subsequently formed into porous tissue engineering scaffolds, with retention of lentivirus binding. Lentivirus immobilization resulted in long-term and localized expression within a subcutaneously implanted scaffold. Microspheres were also formed into multiple channel bridges for implantation into the spinal cord. Lentivirus delivery from the bridge produced maximal expression at the implant and a gradient of expression rostrally and caudally. This specific binding of lentiviral vectors to biomaterial scaffolds may provide a versatile tool for numerous applications in regenerative medicine or within model systems that investigate tissue development.
INTRODUCTION
Designer microenvironments are applied in vitro and in vivo either as model systems to molecularly dissect tissue formation, or to promote regeneration of lost or diseased tissues. A central feature of these microenvironments are a variety of natural and synthetic materials, which present adhesion sites to support cell interactions and have an architecture to pattern or organize the cells into structures. The physical properties (e.g., mechanics) of these materials are selected to support cellular processes such as migration or differentiation [1]. Delivery of gene therapy vectors provides a method to enhance the bioactivity of the structure. Induced expression of growth factors can exploit paracrine signaling [2], or vectors encoding transcription factors can direct differentiation [3].
The interaction between the vector and material has emerged as a critical design parameter for promoting and localizing gene transfer. Vector association with the biomaterial that supports cell adhesion places the vector into the cell microenvironment, and thus can overcome mass transport limitations [4]. The affinity of interaction between the material and vector is essential for retention and gene transfer, and is impacted by the mechanism of interaction. Vectors and biomaterials can often interact non-specifically though electrostatic, van der Walls and hydrophobic interactions [5]. These interactions are relatively weak and thus the vector can potentially be displaced. In contrast, materials and vectors have been designed to provide specific interactions, such as avidin-biotin interactions or antibody-mediated immobilization [6]. These specific interactions are strong; however modifying the vector or material with the complementary functional groups can be challenging and may limit the vector activity. Strategies that promote specific binding of viral vectors to materials could significantly enhance their efficacy and utility.
In this report, we investigate the immobilization of lentiviral vectors to biomaterials fabricated from the synthetic polymers of lactide and glycolide (PLG) by incorporation of phosphatidylserine (PS). PLG scaffolds are widely used for regenerative medicine, with applications such as nerve regeneration [7]. PS is a component of the plasma membrane that has been linked with lentivirus association to cell surfaces [8], and its use capitalizes on a natural binding site for this vector. PS is a hydrophobic compound that can readily incorporated into PLG microspheres, which are subsequently fused into an intact scaffold. PLG scaffolds can thus be fabricated in an appropriate geometry, and the vectors subsequently immobilized, thereby avoiding exposure of the vector to polymer processing conditions that could reduce vector activity. We characterize PS incorporation, and investigate the binding and release of lentiviral vectors. The potential to promote gene transfer in vitro and in vivo is examined, with in vivo studies performed in the spinal cord. Scaffolds that promote specific binding of lentiviral vectors may find enhanced utility for numerous regenerative applications and in model systems of tissue development.
MATERIALS AND METHODS
Microspheres formation and characterization
Microspheres were formed by an emulsion process using the copolymers of lactide and glycolide (PLG, 75:25 mole ratio of lactide to glycolide i.v.=0.6–0.8 dL/g) (Boehringer Ingelheim Chemical, Petersburg, VA). PLG was dissolved in dichloromethane to 3% (w/w) solution, which was then emulsified in 1% poly(vinyl alcohol) (PVA) at 7000 rpm to create microspheres. After 3 hours of stirring, microspheres were then washed with deionized water three times to remove PVA and lyophilized overnight. Phosphatidylserine (PS) or phosphatidylcholine (PC) modified PLG (PS-PLG or PC-PLG) microspheres were prepared using the same procedure except that PS was co-dissolved with PLG in dichloromethane with a weight ratio of 1:10 (PC or PS:PLG). Scanning electron microscopy (Hitachi 3500N) was used to image the surface morphology of the microspheres. For size measurements, a minimum of 5000 particles were collected, and the size was determined using a MultiSizer 3 Coulter Counter (Beckman, Fullerton, CA) with a 30 μm aperture tube. The PS content in microspheres was measured by the Stewart assay [9].
Virus Production
Lentivirus and adenovirus were prepared for the studies using established techniques. Lentivirus was produced in HEK-293T cells grown in DMEM plus 10% FBS at 37°C, and 5% CO2. The lentiviral packaging vectors (pMDL-GagPol, pRSV-Rev, pIVS-VSV-G), previously described by Dull et al [10], were co-transfected along with plenti-CMV-GFP, plenti-CMV-βgal, or plenti-CMV-luciferase into 293T cells using Lipofectamine 2000 (Roche Biosciences, Palo Alto, CA, USA). After 48 hours of transfection, the supernatant was collected and filtered (0.45 micron). Viruses were concentrated using PEG-it (System Biosciences, Mountain, CA, USA), with the precipitated lentiviruses resuspended with PBS. The titer of lentivirus encoding GFP (LV-GFP) was determined by counting GFP positive cells at 2 days after infection of serially diluted virus. Lentivirus titers were determined by HIV-1 p24 Antigen ELISA Kit (ZeptoMetrix Co., Buffalo, USA). Recombinant adenovirus carrying GFP (rAd-GFP) was constructed using Transpos-Ad™ method (Qbiogene, Carlsbad, CA, USA) according to the manufacturer’s protocol. Viruses were produced in HEK-293A cells and purified by ViraBind Adenovirus Purification Kit (Cell Biolabs, San Diego, CA, USA). The adenoviral titer was determined by TCID50 assay on HEK-293A cells.
Assay of active virus retained on the microspheres
Upon association with polymer microspheres, the quantity of active virus was determined. Lentivirus or adenovirus encoding GFP (107 of LV-GFP or rAd-GFP) were incubated with 1 mg of PLG, PC-PLG, and PS-PLG microspheres at room temperature for 30 min. and centrifuged to remove unbound virus. After 3 washes, the microspheres were mixed with HEK-293T cells and seeded. GFP expression was visualized 2 days after cell seeding. Viruses without microspheres were used as control. To determine lentivirus binding, multiple concentrations of LV-luc (107 to108 LP) were mixed with microspheres (1 mg). After washing, microspheres were mixed with HEK-293T cells and luciferase activity was measured at 2 days after cell seeding. To measure the activity on the microspheres, 2×107 of LV-luc were mixed with microspheres (1 mg) and incubated at room temperature. At each time point, HEK-293T cells were mixed with the microspheres, which were not washed, and seeded to measure the luciferase activity after 2 days.
In vitro transduction on lentivirus-loaded PS-PLG scaffold
Microspheres loaded with PS were subsequently processed into porous scaffolds using a gas foaming, particulate leaching process. The scaffold was constructed by mixing 1.5 mg of microspheres (PLG or PS-PLG) with 50 mg of NaCl (250 μm < d < 425 μm). The fabrication procedures have been described elsewhere [11]. In vitro cell transduction on the PLG or PS-PLG scaffold was analyzed by seeding and culturing cells on PLG scaffolds with immobilized virus. Lentivirus (10 μL) encoding β-galactosidase (LV-βgal, 5×108 LP) were incubated with PLG or PS-PLG scaffold for 30 min. Scaffolds were washed with PBS and 106 of HEK-293T cells were seeded on the top of the scaffolds. The expression of β-galactosidase on the cells attached to the scaffolds was visualized at 3 days after transduction by staining with X-gal [12].
Bioluminescence imaging
In vivo expression following implantation of virus loaded scaffolds was determined through bioluminescence imaging. Lentivirus encoding luciferase (LV-luc, 3×108 LP) was incubated with the PS-PLG scaffolds for 30 min. and implanted subcutaneously into male CD1 mice (20–22 g). Luciferase expression was monitored using an IVIS imaging system (Xenogen Corp., Alameda, CA, USA) as previously described [7]. Care and use of the laboratory animals followed the guidelines established by the Northwestern University Institutional Animal Care and Use Committee (IACUC).
Rat spinal cord hemisection model
For implantation into the spinal cord model, the PS-PLG microspheres were processed to form multiple channel bridges, as previously described [7]. Female Long-Evans rats (180–200 g; Charles River) were treated according to the Animal Care and Use Committee guidelines at Northwestern University.
Rats were euthanized 1 week and 4 weeks after implantation of virus-loaded PS-PLG and the spinal cord was retrieved. The implant and 5 segments of 0.5 cm length rostral and caudal of the implant were collected and luciferase readings were determined as previously described [7]. Luciferase readings were normalized with total protein measured by the enhanced test tube protocol of the bicinchoninic acid protein assay (Pierce, Rockford, IL).
Statistical analysis
Statistical significance between groups was determined by one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons. A level of P < 0.05 was accepted as significant.
RESULTS
Polymer modification with PS
The presentation of PS on scaffolds for regenerative medicine is based on the incorporation of PS into microspheres, which are subsequently employed as building blocks for fabrication of the scaffold. PLG microspheres are formed using an emulsion process, and PS is soluble in the organic solvent and was added to the polymer solution at 1:10 weight ratio. Visualization of the microspheres indicated that PS decreased the microsphere size (Fig. 1A). The diameter of the microspheres was substantially decreased, from 12.8 ± 2.5 μm to 3.6 ± 1.6 μm, with addition of PS (p<0.05). The PS content of the microspheres was approximately 10 μg PS per mg microsphere at a weight ratio of PS:PLG of 1:100. Increasing the PS:PLG weight ratio led to a greater incorporation of PS, with a PS content equal to 80 μg per mg microsphere at a weight ratio of 1:10 (Fig. 1B).
Fig. 1.
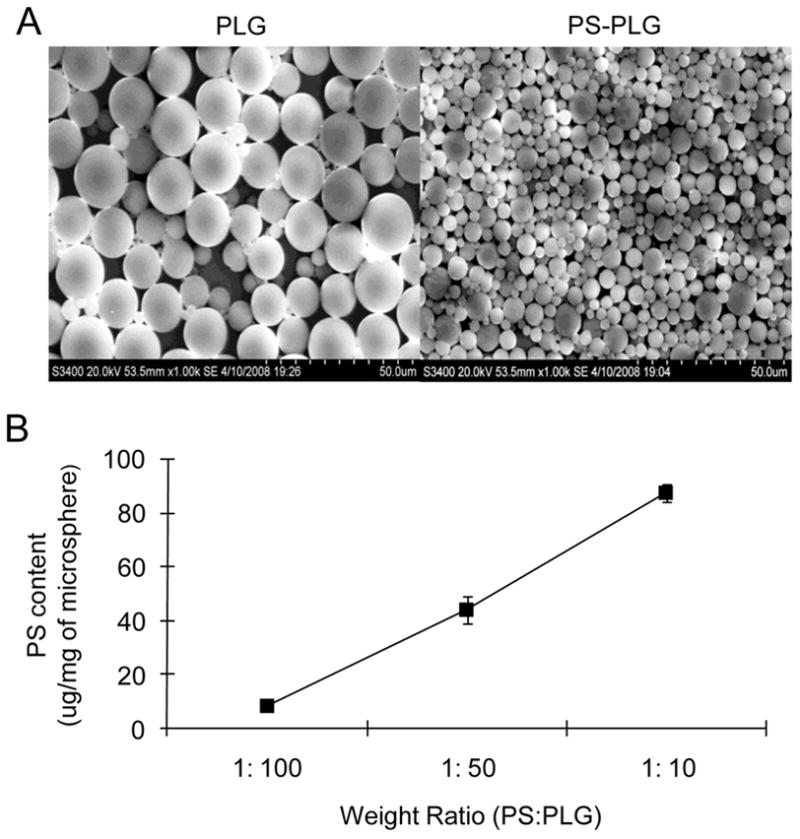
Characterization of PS-PLG microspheres (A) Scanning electron photomicrograph of PLG (Left) and PS-PLG (Right) microspheres. (B) Phosphatidylserine (PS) content in PS-PLG microspheres, prepared by the different weight ratio, was measured by the Stewart assay.
Specific binding of lentivirus to PS-PLG
Specific binding of lentivirus to PS modified microspheres was subsequently investigated. Microspheres were incubated with the virus and subsequently washed to remove unbound virus. Adenovirus was employed to confirm specificity of the lentivirus, and phosophatidylcholine (PC), another plasma membrane component, was used to control for the specificity of PS binding. Both adenovirus and lentivirus used alone resulted in substantial transduction, as expected (Fig. 2A, E). Incubation of the lentivirus and adenovirus with PLG or PC modified PLG resulted in few transduced cells (Fig 2B,C, F, G), consistent with previous reports [13]. Lentivirus association with the PS-PLG microspheres, however, produced substantial quantities of transduced cells (Fig. 2D), which was not observed with adenovirus (Fig. 2H).
Fig. 2.
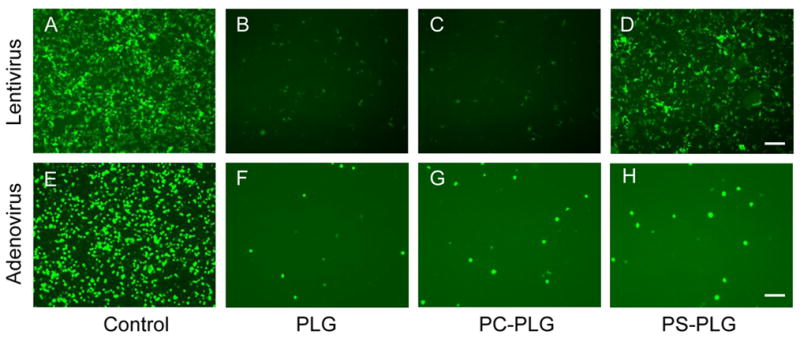
Specific binding of lentivirus to PS-PLG microspheres LV-GFP (A-D) and rAd-GFP (E-H) were incubated with PLG (B, F), PC-PLG (C, G), or PS-PLG (D, H) microspheres for 30 min and centrifuged to remove the unbound viruses. After wash with PBS, the microspheres were mixed with 293 cells and seeded on the plate. GFP expression was visualized at 2 days after seeding. Viruses without mixing with microspheres were used as control (A, E). Bar represents 100 μm.
The quantity of PS-PLG associated lentivirus was subsequently investigated by incubation of multiple virus doses and determination of the resulting activity (Fig. 3A). In the absence of polymer microspheres, the expression correlated with the dose of virus. For PS-PLG microspheres, increasing the dose of virus incubated with the microsphere produced a corresponding increase in expression. The highest virus dose (108 LP) produced maximal expression, though the increase from the 5×107 LP was modest and suggests that binding sites may be approaching saturation. The net activity following lentivirus association with PS-PLG was less than that obtained with the native virus, which likely results from virus loss during the wash steps. For PLG and PC-PLG, the expression levels following lentivirus association were low, consistent with the low binding of virus.
Fig. 3.
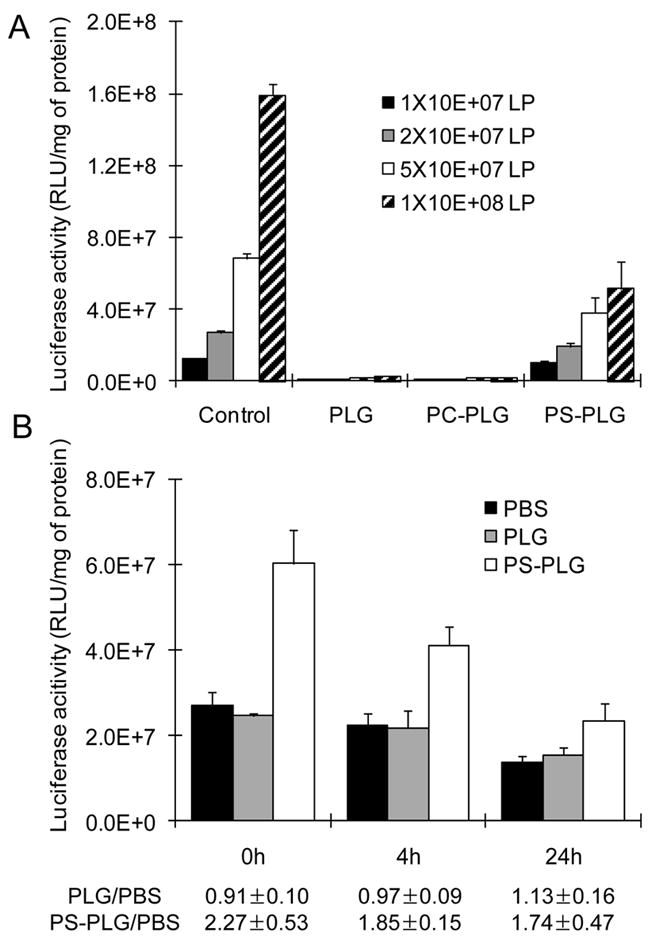
Retention of lentivirus on PS-PLG microspheres (A) Multiple doses of lentivirus encoding luciferase were incubated with 1 mg of PLG, PC-PLG, or PS-PLG microspheres for 30 min and centrifuged to remove the unbound viruses. After wash with PBS, the microspheres were mixed with 293 cells and seeded on the plate. Luciferase activity was measured at 2 days after seeding. Viruses without mixing with microspheres were used as control. (B) 2×107 of LV-luc were mixed with microspheres and incubated at room temperature. At each time point, HEK-293 cells were mixed with the microspheres, which were not washed, and seeded to measure the luciferase activity after 2 days.
Virus stabilization by association with PS-PLG
The activity of the virus associated with the polymer microspheres was subsequently investigated and increased the expression level relative to free virus. Lentivirus was incubated with the PS-PLG and PLG microspheres, and subsequently added (without washing) to cells for assessment of transduction at multiple time points, with free virus used as a control. The free virus and PLG-associated virus provided similar levels of activity (Fig. 3B). Interestingly, the PS-PLG associated lentivirus had a 2.3 fold increased activity immediately after binding. At 24 hours, the increase in activity between PS-PLG association and free virus declined to 1.7.
Scaffold-mediated lentiviral delivery in vitro and in vivo
Porous scaffolds were subsequently formed from PS modified microspheres using a gas foaming process. The processing conditions are mild, and avoid the use of organic solvents or high temperatures that could decrease the stability of PS. The scaffold was incubated with lentivirus encoding for β-galactosidase, washed, and then cells were seeded throughout the scaffold. PLG scaffolds lacking PS had few transduced cells (Fig. 4A), consistent with the microsphere results. Scaffolds containing the PS had large numbers of transduced cells that were distributed throughout the scaffold (Fig. 4B, C).
Fig. 4.
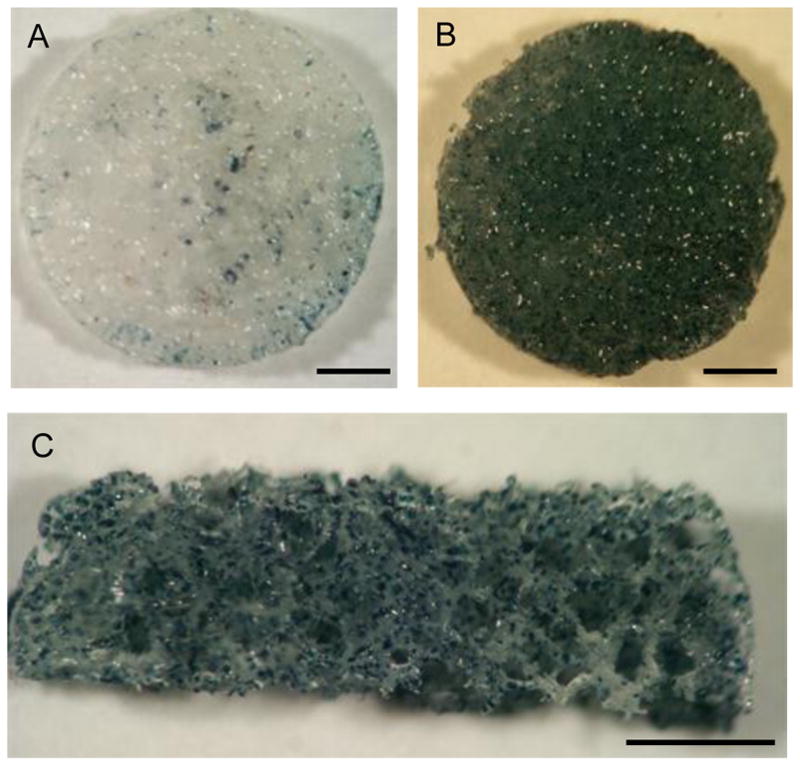
In vitro cell transduction on lentivirus-loaded PS-PLG scaffold Lentivirus encoding β-galactosidase was incubated with PLG (A) or PS-PLG (B) scaffold for 1hr. After wash with PBS, 293 cells were seeded on the scaffolds. β-gal staining was performed at 3 days after cell seeding. PS-PLG after β-gal staining was cut to see the cells in the middle layer of scaffold (C). Bars represent 1 mm.
Scaffolds with immobilized lentivirus encoding luciferase were subsequently implanted subcutaneously to investigate gene transfer in vivo. Bioluminescence imaging identified luciferase expression that was localized to the implant site (Fig. 5A), which persisted for the duration of the study, 12 weeks. Quantification of the photon flux indicated the greatest expression was observed during the initial 2 weeks, after which time the photon flux decreased 2 fold and remained at that level for the remaining 10 weeks (Fig. 5B).
Fig. 5.
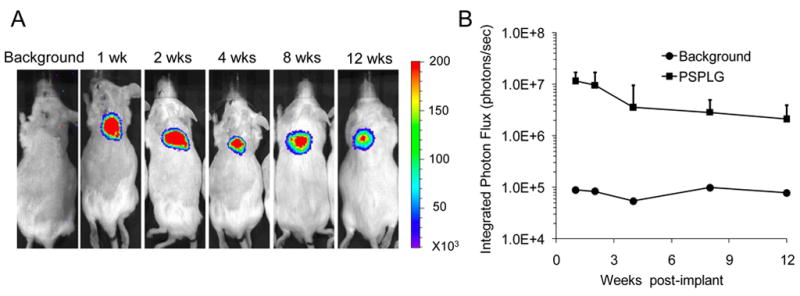
In vivo cell transduction on 3D PS-PLG scaffold Bioluminescence imaging for PS-PLG scaffolds delivering a lentiviral vector encoding luciferase (LV-luc, 3×108 LP). Scaffolds were implanted at the subcutaneous sites in CD-1 mice. Animals were injected with 150 mg/kg of luciferin prior to imaging. All images scaled to the same max/min levels.
Spinal cord implantation of lentivirus immobilized bridges
We subsequently investigated gene transfer following implantation of lentivirus-immobilized PLG multiple channel bridges into the injured spinal cord. We have previously reported that these bridges prevent the formation of a cyst-like cavity typical of many spinal cord injuries, likely due to stabilizing the injury following implantation [14]. Cells infiltrate into the pores and channels of the bridge, with the channels aligning cells along the major axis of the channel. Additionally, transgene expression within the spinal cord is challenging, generally leading to low levels of expression. Multiple channel spinal cord bridges were fabricated using the gas foaming process yet adapted to create a scaffold with dimensions appropriate for a lateral hemisection in a rat spinal cord (Fig. 6A). Bridges were incubated with lentivirus encoding luciferase and subsequently implanted. Luciferase activity was maximal at the implant site (T9/10) and the adjacent segment (T11) and persisted for the duration of the 4-week study (Fig. 6B). Expression in the segment immediately caudal to the implant site also had maximal expression. Expression levels dropped 4 to 6-fold in the spinal segments adjacent to those with maximal expression. Segments further from the injury contained less than 6% of the maximal luciferase expression. Transgene expression decreased approximately 20-fold between 1 week and 4 weeks of implantation. These studies indicate that lentivirus immobilized to bridges produces transgene expression within the spinal cord that forms a transient gradient along the spinal cord. Interestingly, this profile may encourage the initial ingrowth of axons into the bridge, and the declining expression over time may facilitate the re-entry of regenerating axons into the host tissue [15].
Fig. 6.
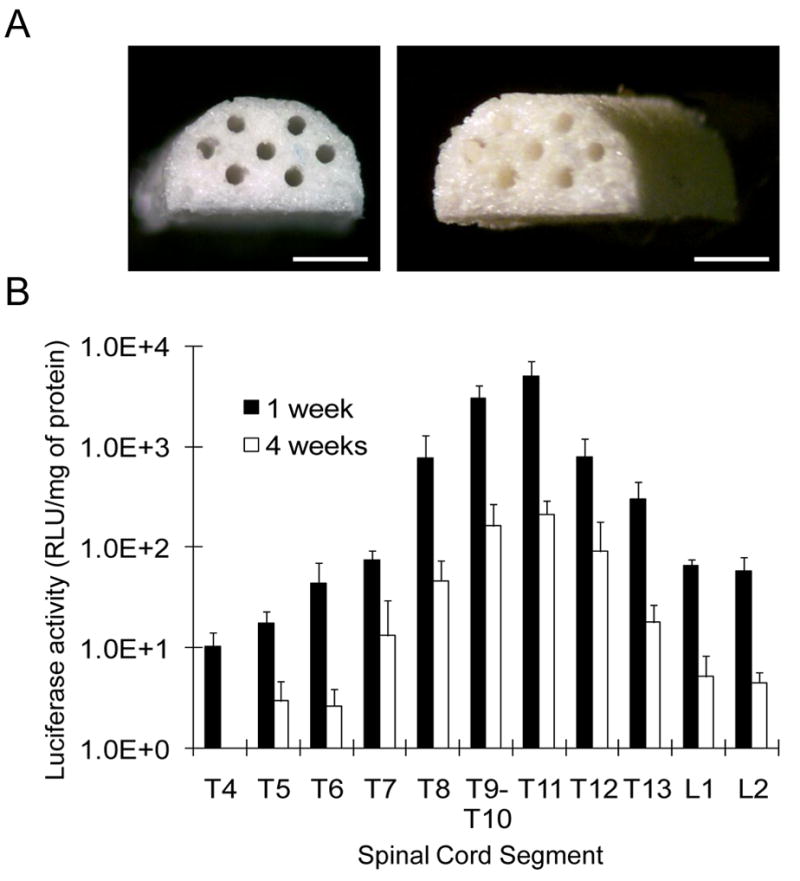
Spinal cord implantation of lentivirus immobilized bridges (A) Multi-channel spinal cord bridges were fabricated using the gas foaming process to create a scaffold with dimensions appropriate for a lateral hemisection in a rat spinal cord. Bar represents 1 mm (B) Bridges were incubated with lentivirus encoding luciferase(LV-luc, 3×108 LP) and subsequently implanted. Luciferase activity in the spinal cord segment including implanted site was measured at 1 week and 4 weeks (n=3) after implantation.
DISCUSSION
In this manuscript, we report on the specific binding of lentivirus to a PS modified PLG scaffold, which increases the quantities bound and the extent of transgene expression in vitro and in vivo. Immobilization of virus to the PS modified PLG significantly enhanced transgene expression in vitro and in vivo. A significant role of PS is to increase the amount of virus that associates with the material, thus a higher dosage of vector can be delivered locally. For the microspheres, we demonstrated an increase in lentivirus activity relative to virus alone, which likely results from microspheres overcoming mass transport limitation by depositing on the cells. For the scaffold, retention of the virus can co-localize the seeded cells with the immobilized vector. In vivo, the PS limits the rapid release that would normally lead to clearance from the target site that can lead to expression at off target sites. The in vivo expression persists for at least 4 weeks at both the subcutaneous and spinal cord implantation sites, and expression levels are significantly increased relative to unmodified PLG scaffolds.
PS for specific virus immobilization to the scaffold has several unique characteristics relative to the alternative approaches. Specific virus immobilization has employed vector biotinylation for immobilization to an avidin-modified surface [16], and the immobilization of antibodies specific for the virus [17]. Although these approaches have been effective, vector biotinylation can influence its activity [16]. Additionally, the consistent manipulation of biomaterial surfaces with large proteins such as ECM proteins, avidin and its derivatives, or antibodies is challenging. Additionally, antibody-antigen and biotin-avidin binding are strong interactions, and likely few binding interactions per vector due to the size of the antibody or avidin derivative. PS has a specific interaction with the VSV-G protein, which is influenced in part by electrostatic interactions. PS is a relatively low molecular weight lipid that is soluble in organic solvents, and can be incorporated into microspheres formed using the emulsion process, which is relatively easy compared with the immobilization of antibodies or avidin for virus binding. The small size of PS allows for substantial encapsulation, and can enable numerous interactions between the microsphere and vector. Importantly, this binding strength and number of binding interactions must be appropriately balanced to allow for efficient delivery [6, 18].
The combination of multiple channel bridges with gene therapy has the potential to provide architectural support and deliver vector to host cells, which then function as bioreactors to locally provide tissue inductive factors. For spinal cord regeneration, regenerative strategies have separately investigated the delivery of neurotrophic factors to stimulate neuron survival and axon outgrowth [19, 20], enzymes that block or degrade inhibitory factors [21], and anti-inflammatory factors [22]. The delivery of gene therapy vectors could enable multiple factors to be delivered simultaneously, with multiple vectors immobilized through the same PS interaction. Transgene expression was highest at the implant site with reduced expression observed in the segments adjacent to the implant, which may provide a directional cue for axonal growth. Expression in the adjacent segments likely results from transport of the vector into the adjacent segments due to diffusion or by the exchange of cerebrospinal fluid. Previously, immobilization of lipoplexes had the greatest expression in the segments adjacent to the implant site [23], which highlights the need to minimize release to transduce infiltrating cells. Additionally, maximal expression levels achieved with the lentivirus were approximately 80-fold greater than those obtained with the lipoplexes. Transgene expression in the adjacent segments may be beneficial as expression in these segments could promote neuron survival outside the injury site. Additionally, this expression pattern could create a concentration gradient, with the greatest concentration within the bridge and lower concentrations on either side. Axons are widely reported to extend towards the greater concentration. Thus, this spatial pattern of expression could direct axon elongation toward the injury, into and across the bridge [24].
Transgene expression in the spinal cord declined more than 10-fold between 1 and 4 weeks, which was not observed at the subcutaneous site. The different patterns of expression may reflect the cell types transduced by the vector. Gene delivery to the spinal cord has previously identified Schwann cells, fibroblasts, and macrophages as the primary targets of gene transfer [7]. In addition to the cell types, other factors that may contribute to the shorter duration of expression in the spinal cord are the inflammatory or immune response to the vector [25], which may lead to elimination of the transduced cells, or promoter silencing [26]. Both of these processes can be dependent upon the implantation site. Nonetheless, the initial expression of regenerative factors at the implant site may induce initial axonal growth into the bridge; however, persistently elevated concentrations of inductive factors have been associated with limited axonal re-entry. Thus, transgene expression should subside to encourage axonal re-entry into the host tissue [15].
CONCLUSIONS
We present a lentivirus delivery system for regenerative medicine that is based on virus immobilization to a scaffold using a mechanism previously implicated in cell association of the vector. PS is a phospholipid component that can be readily incorporated into synthetic polymer scaffolds to provide specific binding for lentivirus, and may be more versatile than the immobilization of large antibodies or proteins. Nano- or micro-particles common in drug delivery, or porous scaffolds used in regenerative medicine, can be designed for specific lentivirus immobilization. Lentivirus has recently been employed successfully in a clinical trial of hematopoietic stem cell gene therapy [27], and exploiting the specific binding of lentivirus to PS in this manner can be an enabling technology to expand the potential applications.
Acknowledgments
Financial support for this research was provided by grants from NIH (R01 EB005678 and RO1 EB003806).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 2.Guo T, Zhao J, Chang J, Ding Z, Hong H, Chen J, et al. Porous chitosan-gelatin scaffold containing plasmid DNA encoding transforming growth factor-beta1 for chondrocytes proliferation. Biomaterials. 2006;27:1095–1103. doi: 10.1016/j.biomaterials.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Itaka K, Ohba S, Miyata K, Kawaguchi H, Nakamura K, Takato T, et al. Bone regeneration by regulated in vivo gene transfer using biocompatible polyplex nanomicelles. Mol Ther. 2007;15:1655–1662. doi: 10.1038/sj.mt.6300218. [DOI] [PubMed] [Google Scholar]
- 4.De Laporte L, Shea LD. Matrices and scaffolds for DNA delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:292–307. doi: 10.1016/j.addr.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pannier AK, Anderson BC, Shea LD. Substrate-mediated delivery from self-assembled monolayers: effect of surface ionization, hydrophilicity, and patterning. Acta Biomater. 2005;1:511–522. doi: 10.1016/j.actbio.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segura T, Volk MJ, Shea LD. Substrate-mediated DNA delivery: role of the cationic polymer structure and extent of modification. J Control Release. 2003;93:69–84. doi: 10.1016/j.jconrel.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 7.De Laporte L, Yang Y, Zelivyanskaya ML, Cummings BJ, Anderson AJ, Shea LD. Plasmid releasing multiple channel bridges for transgene expression after spinal cord injury. Mol Ther. 2009;17:318–326. doi: 10.1038/mt.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carneiro FA, Bianconi ML, Weissmuller G, Stauffer F, Da Poian AT. Membrane recognition by vesicular stomatitis virus involves enthalpy-driven protein-lipid interactions. J Virol. 2002;76:3756–3764. doi: 10.1128/JVI.76.8.3756-3764.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart JC. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1980;104:10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- 10.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang JH, Rives CB, Shea LD. Plasmid delivery in vivo from porous tissue-engineering scaffolds: transgene expression and cellular transfection. Mol Ther. 2005;12:475–483. doi: 10.1016/j.ymthe.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bondi A, Chieregatti G, Eusebi V, Fulcheri E, Bussolati G. The use of beta-galactosidase as a tracer in immunocytochemistry. Histochemistry. 1982;76:153–158. doi: 10.1007/BF00501918. [DOI] [PubMed] [Google Scholar]
- 13.Shin S, Salvay DM, Shea LD. Lentivirus delivery by adsorption to tissue engineering scaffolds. J Biomed Mater Res A. 2009 doi: 10.1002/jbm.a.32619. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Y, Laporte LD, Zelivyanskaya ML, Whittlesey KJ, Anderson AJ, Cummings BJ, et al. Multiple channel bridges for spinal cord injury: cellular characterization of host response. Tissue Eng Part A. 2009;15:3283–3295. doi: 10.1089/ten.tea.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geller HM, Fawcett JW. Building a bridge: engineering spinal cord repair. Exp Neurol. 2002;174:125–136. doi: 10.1006/exnr.2002.7865. [DOI] [PubMed] [Google Scholar]
- 16.Hu WW, Lang MW, Krebsbach PH. Development of adenovirus immobilization strategies for in situ gene therapy. J Gene Med. 2008;10:1102–1112. doi: 10.1002/jgm.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy RJ, Song C, Tallapragada S, DeFelice S, Hinson JT, Vyavahare N, et al. Localized adenovirus gene delivery using antiviral IgG complexation. Gene Ther. 2001;8:659–667. doi: 10.1038/sj.gt.3301452. [DOI] [PubMed] [Google Scholar]
- 18.Segura T, Shea LD. Surface-tethered DNA complexes for enhanced gene delivery. Bioconjug Chem. 2002;13:621–629. doi: 10.1021/bc015575f. [DOI] [PubMed] [Google Scholar]
- 19.Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- 20.Bradbury EJ, Khemani S, Von R, King, Priestley JV, McMahon SB. NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur J Neurosci. 1999;11:3873–3883. doi: 10.1046/j.1460-9568.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- 21.Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willerth SM, Sakiyama-Elbert SE. Approaches to neural tissue engineering using scaffolds for drug delivery. Adv Drug Deliv Rev. 2007;59:325–338. doi: 10.1016/j.addr.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Laporte L, Yan AL, Shea LD. Local gene delivery from ECM-coated poly(lactide-co-glycolide) multiple channel bridges after spinal cord injury. Biomaterials. 2009;30:2361–2368. doi: 10.1016/j.biomaterials.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houchin-Ray T, Swift LA, Jang JH, Shea LD. Patterned PLG substrates for localized DNA delivery and directed neurite extension. Biomaterials. 2007;28:2603–2611. doi: 10.1016/j.biomaterials.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao X, Li J, McCown TJ, Samulski RJ. Gene transfer by adeno-associated virus vectors into the central nervous system. Exp Neurol. 1997;144:113–124. doi: 10.1006/exnr.1996.6396. [DOI] [PubMed] [Google Scholar]
- 26.Kasparov S. Suitability of hCMV for viral gene expression in the brain. Nat Methods. 2007;4:379. doi: 10.1038/nmeth0507-379a. [DOI] [PubMed] [Google Scholar]
- 27.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]


