Abstract
Human adolescence has been characterized by increases in risk-taking, emotional lability, and deficient patterns of behavioral regulation. These behaviors have often been attributed to changes in brain structure that occur during this developmental period, notably alterations in gray and white matter that impact synaptic architecture in frontal, limbic, and striatal regions. In this review, we provide a rationale for considering that these behaviors may be due to changes in dopamine system activity, particularly overactivity, during adolescence relative to either childhood or adulthood. This rationale relies on animal data due to limitations in assessing neurochemical activity more directly in juveniles. Accordingly, we also present a strategy that incorporates molecular genetic techniques to infer the status of the underlying tone of the dopamine system across developmental groups. Implications for the understanding of adolescent behavioral development are discussed.
Keywords: Dopamine, COMT, Brain Development, Adolescence, Working Memory
Introduction
Adolescence is a transitional period between childhood and adulthood characterized by behavioral, hormonal, and neurochemical changes designed to prepare organisms for independent survival (Casey et al., 2008; Doremus-Fitzwater et al., 2010; Spear, 2003). Although these transitions are largely positive, adolescence can be conceptualized as a period of vulnerability. Risk-taking increases during this time (Steinberg, 2008), as does vulnerability to psychiatric disorders (Kessler et al., 2005; Paus, Keshaven & Giedd, 2008). Theories abound to explain these patterns, most of which suggest that adolescent behavior patterns are attributable to changes in brain maturation during this period of the lifespan (Casey et al., 2008; Fareri et al., 2008; Steinberg, 2008). Many frameworks focus on one of two neural substrates, considering one or both. These include (a) the prefrontal cortex (PFC), which is structurally and functionally under-developed, leading to deficiencies in behavioral regulation, as well as (b) limbic and striatal structures, which may be characterized by relatively heightened patterns of activation, conferring a state of motivational “over-drive”. Simplistically put, it is hypothesized that “go” signals are strong, while regulatory ‘stop” or monitoring signals are weak. These processes may be inter-related in that sub-cortical signaling can be excessive due to a lack of overarching cortical control. Accordingly, it is unknown whether adolescents’ behavior patterns are due to deficiencies in the PFC’s structural and functional maturation, to a subcortical system that is in over-drive and could not be controlled even in the presence of an adequately functioning PFC, or to some combination of both factors. It should also be mentioned at the outset that not all models adhere to the formulation that motivational circuits are in a state of over-drive; some propose that the opposite is true (Comings & Bloom, 2000; Volkow et al., 2007). Although the brain is undergoing structural refinement during adolescence, adolescents’ difficulties with behavioral regulation may be exacerbated by other neurodynamic processes.
We have offered a unifying account of adolescent behavior that explains it in terms of the development of the dopamine (DA) system (Wahlstrom et al., 2010). Briefly, our perspective builds upon the theory that DA underlies a behavioral activation system that modulates incentive-motivated approach behavior (Depue & Collins, 1999). This system promotes reward-seeking through activity in limbic, striatal, and frontal networks, facilitating an individual’s ability to translate positive motivations into adaptive actions. The adaptive pursuit of positive incentives is critical to independent future-directed behavior. Our perspective is that activity in this system increases during adolescence to meet the demands associated with the transition to independent living. The increase occurs via a tonic increase in DA availability and impacts both subcortical (limbic and striatal) and cortical (prefrontal) circuits.
Operating within the context of continued structural brain development, heightened DA activity within this system may result in an apparent over-activation of incentive motivation in the absence of reliable levels of behavioral control. Unfortunately, the assessment of neurochemistry in human adolescents has proven elusive due to methodological limitations and human subjects concerns. The purpose of the current review is to describe developmental changes in the DA system through adolescence and to suggest a genetic model for how the integrity of the system can be assessed non-invasively in healthy adolescents. In addressing this aim, we will (i) provide an overview of brain development during adolescence; (ii) provide a brief overview of the DA system from a neurophysiological perspective, (iii) review the development of the DA system, presenting data from the animal literature; this review represents the major portion of this paper and is intended to provide a rationale for why this system should be scrutinized in humans for its role in adolescent behavior; finally, we will (iv) present a molecular genetic strategy for the indirect assessment of human neurochemical development. We have chosen DA, the COMT Val158Met single nucleotide polymorphism, and working memory to illustrate this strategy. Although working memory does not necessarily reflect the risk taking behaviors that are of primary interest in adolescents, it was chosen because there are well-established literatures regarding inter-relationships between it, COMT, and dopamine, allowing for more straightforward developmental inferences. However, similar models could be derived for other behavioral domains, particularly those associated with the processing of motivational signals, and other neurochemical systems.
An Overview of Behavioral and Brain Development in Adolescence
Much of the literature on adolescent brain development focuses on the development of prefrontal systems, a topic that can be considered from both behavioral and brain imaging perspectives. In terms of behavioral approaches, executive functions that have been linked to prefrontal substrates are evident late in infancy, coincident with independent locomotion (Bell & Fox, 1992; Diamond, 1990a,b) and show a steady course of improvement through childhood (Luciana & Nelson, 2002; Kerr & Zelazo, 2004; Levin et al., 1991; Ridderinkoff et al., 1997; Welsh & Pennington, 1991). These functions primary include rudimentary working memory skills, behavioral approach in the face of interference, and flexibility in shifting responses in response to feedback contingencies. With respect to all of these domains, studies conducted over the past several decades have generally concluded that adolescence is a period of continued PFC development. Adolescence is characterized by the continued maturation of a wide variety of PFC-mediated behaviors including planning (Asato et al., 2006; Luciana et al., 2009), concept formation (Chelune & Baer, 1986), working memory (Conklin et al., 2007; DeLuca et al., 2003; Luna et al., 2004; Luciana et al., 2005), inhibitory control (Hooper et al., 2004; Luna et al., 2010), delay discounting (Olson et al., 2007; Steinberg et al., 2008) and motivated decision-making as measured by gambling tasks (Crone & van der Molen, 2004; Hooper et al., 2004; Overman, 2004; Cauffman et al., 2009). When the age of maturation is reached varies by task demand (Luciana et al., 2005; Steinberg et al., 2009) and may depend on the amount or complexity of information that has to be processed within a given context (Luciana et al., 2005). Depending on the task, performance improves into the late teens and perhaps early twenties (DeLuca et al., 2003; Luciana et al., 2009; Steinberg et al., 2009). An exhaustive review of this literature is outside the scope of the current review, but this sampling of studies is representative of the literature as a whole in terms of the finding that executive skills continue to develop during adolescence.
These maturational trajectories are generally interpreted as reflecting the structural maturation of various tissue compartments that comprise the prefrontal cortex and associated networks. For instance, human neuroimaging studies indicate decreases in cortical gray matter during adolescence (Giedd et al., 1999; Giedd, 2004; Gogtay et al., 2004; Gogtay & Thompson, 2009; Jernigan, Trauner, Hesselink, & Tallal, 1991; Luders et al., 2005; Thompson et al., 2005; Sowell et al., 2003). Collectively, these studies indicate that the total volume of gray matter increases across the cortex prior to puberty (generally inferred by the age of the participant sample), reaching a peak somewhere in the early-to-mid pubertal period after which there is a post-pubertal decline. The post-pubertal changes in gray matter are non-linear and vary with respect to brain region. For instance, a recent longitudinal study, using time-lapsed images of participants studied repeatedly over time, indicates that the primary sensorimotor cortices and the frontal and occipital poles mature first. The remainder of the cortex develops in a parietal to frontal direction with the superior temporal cortex maturing last (Gogtay et al., 2004). Gray matter volumes may peak earlier in males versus females in most cortical regions (Lenroot & Giedd, 2010). The decline in gray matter that occurs after these peak values are reached is generally attributed to the pruning (elimination) of synaptic contacts.
As this elimination is occurring, there are concomitant increases in white matter volume and density (Bartzokis et al., 2008; Giedd et al., 1999; Paus, 2001; Pfefferbaum et al., 1994). These age-related increases appear to be more linear as compared to the non-linear changes in gray matter volumes. Indeed, white matter volumes accelerate through adolescence and through early-to-middle adulthood. As with gray matter, there is a suggestion of sexual dimorphism, with males showing steeper increases in white matter volumes across adolescence as compared to females (Paus, 2010). In addition to the assessment of white matter volumes, age-related increases in the directional organization of white matter, as assessed through diffusion tensor imaging (DTI), have been observed (Ashtari et al., 2007; Barnea-Goraly, 2005; Ben Bashat et al., 2005; Bonekamp et al., 2007; Eluvathingal, et al., 2007; Klingberg et al., 1999; Hasan et al., 2007; Lebel et al., 2008; Li & Noseworthy, 2002; Muetzel et al., 2008; Qui et al., 2008; Schmithorst & Yuan, 2009; Schneider, et al., 2004; Snook et al., 2005, 2007; Zhang et al., 2005). DTI measures the relative diffusion of water within voxels that have been characterized on the basis of automated tissue parcellations as white matter. Based on the observed diffusion patterns, inferences are made regarding the structural properties of the white matter within which the water molecules are located, given that water cannot diffuse in an unobstructed manner in the presence of barriers to diffusion (Basser et al., 1994). Directionally oriented fibers represent one such barrier. Overall, these studies indicate that with increasing age, diffusion decreases in a manner that is directionally sensitive. These decreases have been attributed to increases in myelination or axonal caliber (Paus, 2010), supporting the notion that functional networks are being sculpted in a manner that increases information processing efficiency. Moreover, they indicate that with increasing development, frontal systems become increasingly capable from a functional neuroanatomical perspective of assuming greater regulation over subcortical, primary sensory, and motor processing (Eluvathingal et al., 2007).
Diffusion tensor imaging studies of adolescents have suggested that increases in white matter integrity are associated with better performance on measures of general intelligence (Schmithorst, 2005; Shaw et al., 2006), delay discounting (Olson et al., 2009), interhemispheric transfer (Muetzel et al., 2008), working memory (Nagy et al., 2004; Olesen, et al., 2003), inhibitory control (Liston et al., 2006), and reading ability (Qiu et al., 2008). In a departure from this general pattern, Berns et al. (2009) reported that as risk-taking tendencies increase during adolescence, frontal lobe white matter integrity is better developed, a pattern that was interpreted as suggesting that risk-takers have more mature brains. Despite this inconsistency, these studies suggest regional associations between white matter development and behavior and also support, to a more limited extent, the notion that the structural integrity of broadly distributed functional networks supports behavioral development. This latter conclusion is more tentative, because studies have not always considered the extent to which behavior-white matter associations change with age. Because white matter integrity underlies connectivity between regions, these studies are particularly important in suggesting the manner in which functional networks mature across development.
In a more direct assessment of this functional capacity, several groups have used functional MRI to document developmental differences in brain activation patterns. These studies have found age-related differences in orbitofrontal cortex (OFC) and ventral striatum activation when individuals respond to rewards or when they make reward-relevant decisions, with the general pattern of heightened activity during adolescence (Van Leijenhorst, Zanolie1, Van Meel, Westenberg, Rombouts & Crone, 2009; Ernst et al., 2005; Galvan et al., 2006; May et al., 2004). Additionally, adolescents exhibit unique patterns of amygdala activation when emotional facial expressions are viewed (Baird et al., 1999; Thomas et al., 2001). With respect to more purely cognitive functions, adolescents as compared to adults show inefficient patterns of neural activation during the performance of cognitive control tasks. Interpretation of this literature is complicated by disagreements regarding how more versus less focal patterns of BOLD activation should be interpreted (see Luna et al., 2010 for a comprehensive review).
However, based on this work, “dual system” models have been invoked to explain adolescent behavior (Casey et al., 2008; Fareri et al., 2008). These models advocate that there is poor regulatory control exerted by cortical structures, particularly the PFC, due to immaturity while at the same time there is unrestrained activity within limbic and striatal circuits. While it is compelling to attribute adolescents’ relative behavioral immaturities to deficiencies in the structural integrity of PFC circuits, it could be that neurochemical systems are also changing in a manner that impacts not only motivational circuits (see Spear, 2003 for review), but also cognitive skills (Goldman-Rakic, 1987a). Neurochemical changes would necessarily interact with structural ones in determining the course of behavioral development and may help to explain inconsistencies such as the data of Berns and colleagues (2009). In considering which neurochemical systems would best map onto the behavioral patterns observed in adolescence, we and others have suggested that the dopamine system is of interest given its known role in modulationg reward responding, reinforcement learning, and high-level cognition (Frank & Hutchinson, in press; Schultz et al., 2008; Spear, 2003; Wahlstrom et al., 2010). What, then, do we know about the status of the DA system during childhood and adolescence? Much of the available knowledge is derived from rodent and non-human primate studies. Although one could question the merits of generalizing findings from the animal literature to humans, these findings represent virtually all that is known in a direct sense about the development of this system.
Overview of the Dopamine System
Synthesis Pathway and Inactivation
As a member of the catecholamine class of neurotransmitters, DA is defined by the presence of a catechol nucleus and ethylamine side-chain (Feldman, Meyer, & Quenzer, 1997). Its synthesis begins with the non-essential amino acid tyrosine and ends with the production of epinephrine. Tyrosine is converted to L-3,4-dihydroxyphenylalanine (L-DOPA) by tyrosine hydroxylase (TH), the rate-limiting enzyme in the synthetic pathway. L-DOPA is catalyzed by L-amino acid decarboxylase (AADC) into DA, which is converted to norepinephrine by dopamine β-hydroxylase (DBH) and then to epinephrine by phenylethanolamine N-methyltransferase (PNMT). Specific cell types express only those enzymes necessary to produce the end-product neurotransmitter. Thus, DA cells can be differentiated from those that produce other catecholamines by their lack of DBH.
Within the synaptic cleft, excess DA either diffuses or is regulated by reuptake or catabolic mechanisms. Reuptake is the process by which DA is taken back into the pre-synaptic terminal via transporter receptors. Some proportion is then repackaged into presynaptic vesicles for later release. Transporter knockout mice have dramatically slow rates of synaptic clearance, indicating the importance of reuptake for DA inactivation and of the DA transporter in regulating this process (Giros et al., 1996). DA transporters are abundant in subcortical regions but virtually absent in the PFC (Sesack et al., 1998). Accordingly, the PFC relies on other mechanisms, particularly enzymatic inactivation, for DA’s regulation. Monoamine oxidase (MAO) and catechol O-methyltransferase (COMT) are the primary enzymes responsible for this inactivation, with MAO accounting for approximately 90% of DA catabolism and COMT accounting for the other 10% (Westerink, 1985). MAO catabolizes all monoamines and is expressed in one of two forms: MAO-A or MAO-B. Differences between the two are attributed to substrate selectivity and the fact that each is coded for by a unique gene. MAO-A is primarily responsible for the inactivation of DA in rats (Butcher et al., 1990), though similar findings have not been documented in humans. Conversely, COMT catalyzes catecholamines and appears to be most important in the PFC (Gogos et al., 1998), since COMT knockout mice exhibit increases in PFC DA despite unchanged striatal levels.
Cell Bodies and Projections
DA is synthesized in several regions of the mammalian midbrain. Several major cell groups have been identified (Bjorklund & Dunnett, 2007), which are located mainly in mesencephalic and diencephalic regions. They can be divided into ascending, descending, and local neuron systems (Fuxe et al., 1974). The mesencephalic DA cell groups are composed largely of ascending projections; two of these projection systems are of primary interest in understanding DA’s modulation of behavior. These systems originate in the midbrain substantia nigra and ventral tegmental area (VTA). The first system projects primarily to dorsal and ventral striatal regions. The second system projects to limbic structures, including the olfactory tubercle, nucleus accumbens, amygdala, hippocampus, and septum. These projections have been labeled collectively as the “mesolimbic” DA pathway (Moore and Bloom, 1978; Oades & Halliday, 1987; Simon, Scatton & LeMoal, 1980). The second system also projects to the cortex. Cortical dopamine projections are relatively sparse, with the exception of those that reach prefrontal and primary motor regions. These cortical projections are collectively labeled the “mesocortical” DA pathway. The mesocortical DA system includes the transitional entorhinal, cingulate, and orbitofrontal cortices as well as dorsolateral PFC (Goldman-Rakic, 1987; Beckstead, Domesick, & Nauta, 1979). The prefrontal DA projection system is distinguishable from the mesolimbic pathway in terms of the basal firing rate and degree of burst activity of DA neurons, the amount of DA turnover, the sensitivity to DA agonists and antagonists, the absence of impulse-and nerve-terminal autoreceptors, and the development of tolerance following chronic neuroleptic administration (Bannon & Roth, 1983; Bjorklund & Dunnett, 2007; Tam & Roth, 1997).
Receptors
Six subtypes of DA receptors have been identified and designated D1 through D5 (two isoforms of the D2 subtype exist). All are G protein-coupled and have been categorized as belonging to one of two classes designated as D1-like (D1 and D5) or D2-like (D2, D3, and D4). Autoreceptors, which are D2-like, have been identified on the presynaptic terminals of dopaminergic cells (see below). D1-like receptors can be differentiated from D2-like receptors because of their ability to stimulate adenylyl cyclase activity and increase cyclic adenosine monophosphate (cAMP), which results in an overall excitatory effect within the cell (Kebabian & Calne, 1979). Conversely, D2-like receptor activation either inhibits or has no effect on cAMP levels. D1 and D2 receptors are distributed throughout the brain. Autoradiographic techniques in rats have demonstrated D1 and D2 binding in the forebrain, striatum, nucleus accumbens (nAcc), olfactory tubercles, and substantia nigra (SN), with D1 density higher in all areas assessed (Boyson, McGonigle, & Molinoff, 1986). Similar to rodents, D1 and D2 receptors in primates and humans are distributed throughout subcortical and cortical regions (Hall et al., 1994; Lidow et al., 1991; Madras et al., 1988). D1 density appears to be higher compared to D2 density in limbic and cortical regions (Hall et al., 1994), although the densities of both receptor types are greater in striatal and limbic regions as compared to the cortex (Meador-Woodruff et al., 1991). Cortically, DA receptor density is relatively greater in anterior versus posterior regions (Lidow et al., 1991). Autoreceptors are found on the dendrites, cell bodies, and terminals of DA cells. Autoreceptor stimulation inhibits DA synthesis and release. Autoreceptors have not been found on DA cells that terminate in the PFC or amygdala (Kilts et al., 1987).
Genes that Regulate Dopamine Activity
There are genetic contributions to individual differences in DA signaling, quantified through the measurement of genetic polymorphisms (genetic variants appearing in at least 1 percent of the population). Some of the most prominent within the literature include the COMT108/158Val-Met single nucleotide polymorphism (SNP) (Grossman, Emanuel, & Budaf, 1992), dopamine transporter (DAT1) variable number of tandem repeats (VNTR) (Vandenbergh et al., 1992), the Taq1A SNP, which appears to impact the D2 receptor system (Neville et al., 2004; Noble, 2003), D3 receptor Ser9Gly SNP (Crocq et al., 1992), and the MAO-A tandem repeat (Zhu et al., 1992). All influence DA signaling by altering the activity or effectiveness of the individual components they govern (Heinz et al., 2000; Lachman et al., 1996), and all have been linked to DA-mediated behaviors (Malhotra et al., 2002; Bellgrove et al., 2005), as well as psychiatric disorders such as ADHD, schizophrenia, and alcoholism (Brookes et al., 2006; Shifman et al., 2002). The proportion of phenotypic variance accounted for by genetic polymorphisms is typically low, which is unsurprising given the complexity of most phenotypes of interest to behavioral researchers. It is thus important to note within this context that interactions between genes may account for more variance than would be predicted by additive effects. For example, Reuter and colleagues (2005) demonstrated that an interaction between COMT Val158Met and DRD2 Taq1A polymorphisms accounted for 13% of the variance in performance on the Stroop interference task, more than what was accounted for by either SNP alone. Similar findings have been revealed with respect to disease risk, as Zappia and colleagues (2004) have demonstrated that two polymorphisms impacting the deposition of β-amyloid significantly increased risk for Alzheimer’s disease beyond what would be expected by additive effects. Other gene-gene interactive effects whereby the effect of one polymorphism is mediated by a second polymorphism (i.e., the effect of the first is evident in only in the presence of a certain allele in the second) are also emerging in the cognitive literature (Gosso et al., 2008; Reuter, Schmitz, Corr, & Hennig, 2006; Roffman et al., 2008; Stelzel et al., 2009; Tan et al., 2007).
This overview is presented primarily to convey the complexities involved in considering developmental changes within any given neurochemical system; in evaluating the nature of developmental changes, one must consider potential alterations in precursors, synthesis, projections, receptor molecules, and enzymatic processes that regulate synaptic activity.
Cross-species Considerations
Although DA cell groupings and projections have been largely conserved across mammalian species, rodents and primates differ with respect to certain aspects of the system. This evidence is important to consider because later sections of this review will present findings that suggest the developmental trajectory of the DA system differs across species. For example, the primate brain is characterized by extensive cortical DA projections, which are either minor or absent in the rat (Berger, Gaspar, Verney, 1991). In addition, primates unlike rodents exhibit variations in DA concentrations across regions, with higher concentrations measured in the frontal cortex compared to more posterior regions (Goldman-Rakic & Brown, 1982; Kehr, Lindqvist, & Carlsson, 1976). Finally, DA afferents in the adult primate cortex, but not the rodent cortex, are distributed in a laminar specific manner (Berger et al., 1988).
Dopamine System Development From Fetal Life to Adulthood
Prenatal and Early Postnatal Development
In rats, which have a total gestation of approximately 21 days, the neurogenesis of TH-containing cells in the mesencephalon begins on embryonic day (E) 12 (Altman & Bayer, 1981). These cells migrate to the striatum by E15 (Voorn et al., 1986) and reach the sub-plate of the future PFC before birth (Kalsbeek et al., 1988). Initially, DA projections from the midbrain to the striatum are undifferentiated, but nigrostriatal and mesolimbic specificity is established sometime between E15 and birth (Hu et al., 2004), as are projections to the PFC and ACC (Berger, Gaspar, & Verney, 1991). Similar to rodents, the development of DA cells and the innervation of cortical regions in primates is largely complete by birth. However, the developmental changes that occur prenatally in non-human primates are protracted as compared to rodents, presumably to meet the needs of a more complicated neurochemical infrastructure as evidenced by the more widespread innervation and laminar-specific differences in density described above (Berger et al., 1988; Berger, Gaspar, Verney, 1991). For example, the origin of DA cells begins in the first third of gestation in primates (Berger, Alvarez, & Goldman-Rakic, 1983; Levitt & Rakic, 1982).
The developmental trajectory in humans is largely similar to that of non-human primates, where DA-containing cells reach the subplate by 13 weeks gestation and are distributed throughout the cerebral wall at 24 weeks (Verney et al., 1993; Zecevic & Verney, 1995), corresponding to the end of neuronal migration. Across species, the elaboration of DA projections takes place throughout the dorsal PFC and anterior cingulate cortex during the first few days of postnatal life (Berger et al., 1985; Kalsbeek et al., 1988).
The neurogenesis of TH-containing cells precedes that of dopamine receptors. In rats, D1 and D2 receptors have been stained as early as days E14 and E15 in the striatum and reach the frontal cortex by E18 (Caille et al., 1995; Jung & Bennett, 1996; Schambra et al., 1994). D2 receptors are more densely concentrated in the striatum compared to D1 receptors in the prenatal brain (Jung & Bennett, 1996), and the density and affinity of both appears to increase with age (Jung & Bennett, 1996; Sales et al., 1989). Similar patterns in the general timing of DA receptor ontogeny have been demonstrated in primates. D1 and D2 receptors reach the marginal zone by E73 and begin to show regional differences across cortical regions by E107 (Lidow, 1995). The distribution of D1 receptors reaches maturity by E128 in the PFC and somatosensory cortex but the 8th postnatal month in the motor cortex. D2 receptors follow a similar pattern (Lidow, 1995). Human data are inconsistent given methodological difficulties in measuring receptor binding in post-mortem samples; however, evidence indicates that D1 and D2 receptors are present in both cortical and subcortical regions by gestational week 20 (Meng et al., 1999; Unis et al., 1998).
As would be expected given the proliferation of DA-containing cells in the prenatal brain, the activity of DA precursors can be measured early in development. TH has been identified as early as E11 in the rat brain (Teitelman et al., 1979) and gestational week 5 in humans (Pearson, Brandeis, & Goldstein, 1980). DBH and PNMT can be identified shortly after (Teitelman et al., 1979), as can COMT (Parvez et al., 1979) and MAO (Fiszman et al., 1991). Precursors (e.g. TH) are identified prior to degradation enzymes (e.g. COMT and MAO), suggesting that the developmental time course mirrors the synthetic chain described in adults (Fiszman et al., 1991).
The early onset of DA signaling may play a functional role in the developing brain. For example, DA may influence neuronal migration and differentiation given its modulation of growth cone activity and axonal outgrowth (Lankford, DeMello, & Klein, 1988). DA also impacts the morphology of cortical cells, since the dendritic arbors of cortical pyramidal cells are decreased in rats with VTA lesions (Kalsbeek, Matthijssen, & Uylings, 1989). Part of DA’s role in these developmental processes might relate to nerve growth given that DA cells contain neuro-trophins such as brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3)(Seroogy et al., 1994; Tucker, Meyer, & Barde, 2001).
Thus, the DA system appears to be well developed at birth. DA-containing neurons have reached all areas of the cortex, both D1 and D2 receptors can be identified across cortical regions, and the infrastructure required for DA transmission is intact. With respect to postnatal changes within the DA system, the primary events of interest are likely to comprise shifts within this predefined architecture (i.e. changes in receptor number; increases or decreases in synaptic density) as opposed to broader changes in neuroanatomical structure or function.
Juvenile and Adolescent Development
Innervation density and tissue concentrations
Unlike the prenatal period, which is characterized by cross-species similarities in DA signaling, developmental changes in DA concentrations and innervation during childhood and into adolescence are distinct in primates and rats.
In rhesus monkeys, DA concentrations are evenly distributed throughout the cortex in early childhood but gradually shift in an anterior direction until adolescence, at which point the highest concentrations of DA can be found in the PFC (Goldman-Rakic & Brown, 1982). Concentrations decrease in the orbitofrontal cortex (OFC) and DLPFC between birth and 8 months but increase steadily into adolescence (e.g. age 2-3 years in the macaque); conversely, DA concentrations peak in the parietal cortex at age 5 months and do not exhibit any change after birth in the occipital cortex (Brown & Goldman, 1977; Goldman-Rakic & Brown, 1982). Adult monkeys were not included in the above samples; however, other work indicates that DA concentrations in the PFC, caudate, putamen, and nucleus accumbens of squirrel monkeys decrease significantly between 3 and 9 years, with additional decreases between 9 and 18 years (Irwin et al., 1994). Human findings are mixed, with some data indicating age-related decreases in DA between ages 9 and 30 in the striatum and others finding no changes with development (Haycock et al., 2003; Kalaria et al., 1993).
Changes in DA tissue concentrations are related to innervation density; thus, in primates, DA innervation of the frontal cortex peaks during adolescence. Cross-sectional comparisons of developing rhesus monkeys indicate that the total number of DA varicosities in Brodmann’s areas 4 (i.e. primary motor cortex), 9 (i.e. dorsomedial cortex), and 46 (i.e. principal sulcus) increases from birth to 2-3 years and decreases afterwards (Rosenberg & Lewis, 1994; 1995). The axonal length of DA cells projecting to the cortex undergoes similar developmental changes, with adolescents exhibiting longer axons compared to infants, children, and adults. Although axonal length and varicosities peak during adolescence in all three areas, important differences occur between cortical layers. Adolescent-limited peaks in varicosities and axonal length in area 9 are found only in cortical layer III, whereas in area 46, varicosities also decrease between adolescence and adulthood in layer VI. Conversely, area 4 is characterized by an adolescent peak in axonal length in layers I/II and III, while the number of varicosities peak during adolescence in all three cortical layers (Rosenberg & Lewis, 1995). In contrast to these PFC regions, axonal length and varicosities peak at age 7 months in the rostral entorhinal cortex and then decline into adulthood (Erickson et al., 1998).
While region-wide DA innervation of the PFC appears to peak during adolescence in non-human primates, the density of connections from individual axons appears to undergo a more linear development. The density of catecholaminergic appositions on individual pyramidal cells in the PFC of rhesus monkeys increases gradually until 2 years and plateaus thereafter (Lambe, Krimer, & Goldman-Rakic, 2000). Conversely, the density of inputs to interneurons does not change with increasing age and is lower at all ages compared to inputs to pyramidal neurons. Similar to the work of Rosenberg and Lewis, these changes have been documented specifically in cortical layer III, although other layers were not specifically targeted.
A majority of DA terminals in layer III form synapses with pyramidal neurons, which have been implicated in the maintenance of working memory (Goldman-Rakic, 1998) and are the primary source of afferent projections to other cortical regions (Goldman-Rakic et al., 1989; Jones, 1984). DA modulates the excitability of pyramidal cells by lowering the voltage threshold required for cell firing (Henze et al., 2000); thus, it is possible that adolescence is characterized by increased excitability of the neural substrates responsible for cognitive functioning and cortico-cortical information processing. In addition, projections to layer III may originate from a different cell grouping as compared to deep cortical layers. In rodents, projections to layer III have been shown to stem from lateral A10/A9 cell groupings (originating from the VTA and substantia nigra), whereas deep layers appear to receive projections from the medial A10 (VTA) grouping (Berger, Gaspar, & Verney, 1991). No replications of this work are available.
Consistent with DA’s role in modulating excitatory cortical circuits, DA release is thought to aid cognitive performance by increasing neural signal-to-noise ratios (Chambers, Taylor, & Potenza, 2003; Sawaguchi, Matsumura, & Kubota, 1990; Thurley et al., 2008). These effects stand in contrast to that of other monoamines, such as serotonin, which is hypothesized to offset the role of DA by exerting an inhibitory effect (Chambers, Taylor, & Potenza, 2003). Thus, it is important to note that the development of serotonin tissue concentrations and cortical innervation during adolescence differ from those of DA. For example, concentrations of serotonin shift gradually toward posterior regions between birth and adolescence, which is due largely to developmental increases in the parietal and occipital cortex while levels in the premotor and prefrontal cortices remain stable with increasing age (Goldman-Rakic & Brown, 1982). In addition, serotonergic appositions on pyramidal cells and interneurons remain unchanged between childhood and adulthood (Lambe, Krimer, & Goldman-Rakic, 2000). Thus, while DA is found in higher concentrations compared to serotonin in the cerebral cortex during adulthood (Lewis et al., 1992), adolescence might be characterized by a temporally limited increase in the ratio of DA to serotonin, which may have functional implications with respect to the excitability of the PFC, perhaps biasing behaviors in the direction of “go” relative to “stop” signals.
Unfortunately, the adolescent group used in the Rosenberg and Lewis studies contained only male rhesus monkeys, whereas the younger and older groups contained both males and females. Thus, it is possible that their findings were due to sex effects rather than age. While there were no sex differences within the other age groups studied (i.e. childhood and adulthood), adolescence may be unique due to hormonal changes.
Unlike primates, there is little evidence that the rodent cortex undergoes adolescent-limited increases in DA innervation; rather, it appears that innervation increases steadily until approximately postnatal day (P) 60, which corresponds to the early stages of adulthood in the rat (Berger et al., 1985; Kalsbeek et al., 1988). This pattern has been replicated in both the PFC and ACC. Findings regarding DA concentrations in the rodent brain are mixed. Several reports indicate slight age-dependent increases in DA tissue concentrations in the cortex (Giorgi et al., 1987; Nomura, Naitoh, & Seqawa, 1976) and striatum and midbrain (Ungethüm et al., 1996). However, others suggest that the available synaptic storage pool is increased in adolescence relative to both childhood and adulthood (Andersen, Dumont, & Teicher, 1997; Stamford, 1989). In addition, two major developmental milestones appear to be met during this time. First, the topographical distribution of DA innervation in the cortex attains an adult pattern (Berger et al., 1985). Second, neurons innervating the cortex develop with two distinct fiber structure characteristics: thick fibers that are also present in subcortical regions such as the hippocampus and thin fibers with multiple varicosities that form dense connectivity with (presumably) pyramidal cells in middle cortical layers (Kalsbeek et al., 1988). These maturations are achieved by postnatal days 30 and 35, respectively, which correspond to the early to mid stages of peri-adolescence.
Overall, the above evidence suggests that the primate cortex continues to undergo changes in DA innervation into adulthood, with rates increasing towards adolescence and decreasing shortly thereafter. This pattern is particularly evident in cortical layer III, indicating a specific pattern of development in the areas subserving intra-cortical information processing. Conversely, rat cortex is characterized by slight increases in DA innervation until adulthood, and adolescence does not appear to be unique with respect to these changes. The reasons for these cross-species differences are unknown. However, there are clear differences in adult level prefrontal structure and function between primates and rodents, with the former exhibiting both increased cerebral differentiation and more complex cognitive functioning (Preuss & Kaas, 1999; Uylings, Groenewegen, & Kolb, 2003). These differences, while not directly related to DA innervation during adolescence, may contribute to the species differences mentioned above.
Receptor Density
Unlike the prenatal period, where DA cell proliferation precedes the origin of receptors, D1 and D2 densities appear to peak in the cortex prior to adolescent-limited increases in cortical innervation and DA axonal growth. Both D1 and D2 receptors peak between 2 and 4 postnatal months in the developing rhesus monkey, with concurrent development occurring across prefrontal, motor, somatosensory, and visual cortices (Lidow, Goldman-Rakic, & Rakic, 1991; Lidow & Rakic, 1992). After peaking, receptor densities decrease slowly until early adulthood (e.g. 60 months), and at all time points D1 receptors can be found at higher densities compared to D2 receptors. Few studies have systematically assessed D1 and D2 receptor changes in humans, but similar to work in non-human primates, they indicate decreases in D1 and D2 binding during adolescence. Seeman et al. (1987) demonstrated that D1 and D2 binding increases to age 4 and decreases throughout late childhood and adolescence. Sex differences were assessed, but none were found. Because the study relied on postmortem specimens, there were relatively few subjects between the ages of 5 and 20 years. As a result, conclusions regarding developmental changes in receptor density during adolescence were based on very few cases and should be considered tentative. Others have demonstrated decreases in D1 density between infancy and adulthood in the caudate and putamen but also based on very few subjects (Montague et al., 1999).
Although changes in D1 and D2 densities occur simultaneously across cortical areas in non-human primates, the motor and visual cortices appear to be unique in that they display differential receptor changes across cortical layers that result in the reorganization of the laminar distributions of D1 and D2 densities (Lidow & Rakic, 1992). In the motor cortex, D1 receptors are concentrated in layer V at birth but increase in layer III relative to other regions so that by 2-4 months of age, they are most densely concentrated there. Layers I, II, and III undergo the smallest rate of loss between 8 months and adulthood and as a result, they are the most densely concentrated areas in adults. D2 receptors are most dense in layers I and II at birth, but within the first month of life, they increase significantly in layer V so that this area contains the highest density of receptors from that point forward. The laminar distribution in the visual cortex undergoes less substantial change compared to the motor cortex, with D2 receptors shifting from layers II and III to V in the first month of life. In all regions, receptor affinity does not change after birth (Lidow, Goldman-Rakic, and Rakic, 1991), suggesting that the kinetic properties of DA receptors mature prenatally.
Whereas primate studies are relatively few, there is a large body of evidence assessing similar changes in the rodent. Although some variation across studies exists due to the fact that receptor concentrations are measured at different postnatal ages, most data indicate that in the striatum, D1 receptors increase in number between birth and PD 35 to 40 and decrease thereafter (Andersen et al., 1997; Tarazi, Tomasini, & Baldessarini, 1999; Teicher, Andersen, & Hostetter Jr., 1995). This finding is most reliably demonstrated in the caudate and putamen (Gelbard et al., 1989; Giorgi et al., 1987; Tarazi, Tomasini, & Baldessarini, 1999; Teicher, Andersen, & Hostetter Jr., 1995), although other studies suggest that D1 binding in the dorsal striatum increases to adult levels in periadolescence and then stabilizes, as opposed to peaking on approximately PD 35 (Leslie et al., 1991; Rao et al., 1991). These seemingly incoherent findings are likely due to differences in the age groups measured, as Rao et al. (1991) did not measure D1 density between PD 30 and 60 and Leslie et al. (1991) did not measure past PD 42. The developmental trajectory of D1 receptors in the nucleus accumbens is less clear. Some groups have found steady increases in D1 density into adolescence (Leslie et al., 1991), others have reported a periadolescent-limited peak in density (Tarazi, Tomasini, & Baldessarini, 1999), and yet others suggest a periadolescent-limited peak followed by decline and then another peak at PD 80 (Andersen et al., 1997; Teicher, Andersen, & Hostetter Jr., 1995). The reasons for these cross-study differences are unclear but may be related to different binding agents, as well as differences in the age groups measured (similar to those mentioned above). For example, only Andersen et al. (1997) and Teicher, Andersen, & Hostetter Jr. (1995) measured D1 density until day PD 80, whereas Leslie et al. (1991) measured until PD 42.
Interestingly, changes in D1 receptor density in the frontal cortex of rats do not demonstrate the periadolescent overproduction exhibited in subcortical structures. For example, some have found that similar to primates, D1 density peaks prior to periadolescence (e.g. PD 21) and decreases afterwards (Leslie, 1991). However, others have demonstrated that density increases steadily between birth, adolescence, and adulthood (Murrin & Zeng, 1990; Tarazi & Baldessarini, 2000), a pattern similar to the development of cortical innervation in the rodent. This overall developmental trajectory, characterized by subcortical peaks in D1 density occurring after cortical peaks, runs contrary to expectations given that the cortex is thought to be the last brain region to develop. This finding suggests that the cortical and subcortical circuits may develop independently. None of the above studies have demonstrated that D1 densities peak in the cortex prior to the striatum.
D2 receptor density follows a similar developmental pattern compared to its D1 counterpart. Specifically, it appears to peak in the caudate and putamen between PD 28 and PD 40 depending on the study (Gelbard et al., 1989; Tarazi, Tomasini, & Baldessarini, 1998b; Teicher, Andersen, & Hostetter Jr., 1995), though others have demonstrated non-significant increases during that time compared to adulthood (Rao, Molinoff, & Joyce, 1991). In addition, D2 density increases in the medial PFC until adulthood and does not exhibit a periadolescent-limited peak (Tarazi, Tomasini, & Baldessarini, 1998b). Contrary to observations in the striatum and cortex, similarities between D1 and D2 development have not been found in the nucleus accumbens (nAcc), where D2 receptors do not exhibit density increases in later adulthood as do D1 receptors (Andersen et al., 1997; Teicher, Andersen, & Hostetter Jr., 1995). In all studies directly comparing receptor subclasses, D1 binding has been found to be higher compared to D2 binding in the striatum and nAcc at all age groups studied (Andersen et al., 1997; Gelbard et al., 1989), and age interactions have been demonstrated whereby D1 density increased faster and dropped off more rapidly around periadolescence (Gelbard et al., 1989). Interestingly, a pronounced medial-to-lateral gradient in D2 density has been established in the striatum, with a specific increase in magnitude occurring during PD 40 (Teicher, Andersen, & Hostetter Jr., 1995). Similar findings have not been demonstrated within the D1 family.
Sex differences in D1 and D2 receptor development are evident. Andersen et al. (1997) compared the changes in D1 and D2 receptor densities in the striatum and nAcc of rats aged between PD 25 and 100. They demonstrated that only males exhibited periadolescent-limited increases of D1 and D2 receptors in the striatum, although males and females exhibited similar densities in adulthood. A similar pattern was exhibited by D2 receptors in the nAcc. Conversely, both males and females exhibited overproduction of D1 receptors in the nAcc during peri-adolescence, although males also exhibited a peak at PD 100 and had higher D1 density at all ages studied. This pattern is consistent with human MRI evidence that the striatum shrinks during adolescence in males but stays relatively the same size in females (Giedd et al., 1996). Interestingly, sex differences in receptor overproduction do not appear to be related to adolescent surges in gonadal hormones, as neither castration nor ovariectomy changed the developmental trajectory of D1 or D2 receptor density (Andersen et al., 2002). Other studies have indicated that the development of DA cells and receptors is similar in males and females (Lieb et al., 1996); however, rates of change during adolescence were not directly compared.
D3 and D4 receptors have been less extensively studied. Available evidence suggests that D4 and D2 receptors follow similar developmental trajectories. D4 density within the striatum increases until periadolescence and decreases thereafter; conversely, the forebrain undergoes steady increases in density until periadolescence that then plateau into adulthood (Tarazi, Tomasini, & Baldessarini, 1998b). Unfortunately, studies of D3 development bypass periadolescence and exclude measurements between PD 21 and PD 90 (Demotes-Mainard et al., 1996; Stanwood et al., 1997). Despite these limitations, evidence indicates D3 density in the striatum and nAcc increases between birth and PD 21 and increases again to PD 90. Whether a brief peak occurs during periadolescence is unknown.
Transporter Density
Findings regarding postnatal changes in DA transporters have been inconsistent. This is especially true in the SN and VTA, where different lines of evidence have indicated no developmental changes (Moll et al., 2000), steady increases between birth and adulthood (Galineau et al., 2004), and peaks in transporter density at PD 21 (Coulter, Happe, & Murrin, 1996). The reasons for these discrepancies are unclear, although different binding agents were used in each study and the age groups studied are not totally comparable. For example, neither the Moll et al. (2000) nor the Galineau et al. (2004) studies examined transporter levels at age PD 21, which is the age at which density peaked according to the evidence of Coulter, Happe, and Murrin (1996). Findings in subcortical regions are more coherent, with converging lines of evidence indicating that transporter density increases into periadolescence and plateaus thereafter in the striatum, nAcc, thalamus, and bed nucleus of the stria terminalis (Coulter, Happe, & Murrin, 1996; Galineau et al., 2004; Tarazi, Tomasini, & Baldessarini, 1998a; but see Moll et al., 2000 for contrary findings). Studies of developmental changes in the rat cortex are sparse, likely due to the relative dearth of DA transporters in this area. Coutler, Happe, and Murrin (1996) found that frontal transporter density increased rapidly between PD 21 and adulthood, while Moll et al. (2000) were unable to yield interpretable results.
Summary
D1 and D2 receptors in nonhuman primate and human cortex appear to peak in early childhood and decline thereafter, reaching relatively stable levels by early adulthood. D1 receptors are more densely concentrated at every developmental stage, and similar findings have been found in subcortical regions. Contrary to the results in primates, DA receptor densities in the rodent cortex appear to increase during adolescence, and D1 and D2 densities in the striatum peak during peri-adolescence.
Precursors and Metabolites
Evidence regarding changes in DA precursors and metabolites is inconsistent and may provide limited information about the development of DA signaling. This is especially true because metabolite levels are confounded by DA usage, which decreases with age (Leslie et al., 1991; Teicher et al., 1993), and regional innervation patterns (McGeer et al., 1967), which vary widely across developmental periods and species (Kalsbeek et al., 1988; Rosenberg & Lewis, 1994). For example, TH activity has been shown to decrease with age in human PFC (Weickert et al., 2006) and striatum (McGeer & McGeer, 1976), though others have shown brief increases in the striatum in early childhood (Haycock et al., 2003). Findings in other species have indicated the opposite to be true, as TH increases during childhood and adolescence in rat and rabbit midbrain, striatum, and cortex (McGeer et al., 1967; Ungethüm et al., 1996). Similar inconsistencies have been found with respect to AADC, where data demonstrate both decreases in the striatum after the age of 2 in humans (Haycock et al., 2003) and increases throughout the brain in pigs (Brust et al., 2004). HVA levels have been shown to decrease in the rat striatum (Ungethüm et al., 1996) and human cerebrospinal fluid (Seifert Jr., Foxx, & Butler, 1980), though others have indicated that it does not change with age in the human striatum (Haycock et al., 2003). COMT, on the other hand, is unique in that its activity has been shown to increase in both human PFC (Tunbridge et al., 2007) and porcine striatum (Brust et al., 2004) during adolescence. Overall, the factors underlying the discrepancies in these data are unknown but may relate to differences in the species and brain areas studied, as well as the underlying changes in regional DA innervation and usage, neither of which are typically measured simultaneously with presynaptic metabolites. At the very least, these data indicate that DA metabolites may vary with age.
Integration: Evidence for Increased Dopamine Availability in Adolescence Compared to Adulthood
Despite some conflicting results, the available evidence, summarized in Table 1, suggests that both primates and rodents exhibit increases in DA signaling during adolescence, though differences exist with respect to the regions and aspects of the DA system affected. Rodents do not exhibit peaks in cortical DA innervation during adolescence, as evidence suggests that innervation undergoes monotonic increases lasting until early adulthood (Kalsbeek et al., 1988). However, cortical DA availability, and synaptic DA availability in the striatum, peak during adolescence (Andersen, Dumont, & Teicher, 1997; Stamford, 1989). Similar to innervation patterns, receptor densities in the cortex increase throughout adolescence (Tarazi & Baldessarini, 2000). D1 and D2 densities appear to peak in subcortical structures such as the striatum and nAcc, as do the densities of D2-like D3 and D4 receptors (Andersen et al., 2002; Tarazi, Tomasini, & Baldessarini, 1998b). Thus, it appears that in rodents, adolescence is characterized by a subcortical increase in DA compared to childhood and adulthood. Conversely, DA availability in the cortex is lower compared to adulthood (but see Andersen, Dumont, & Teicher, 1997).
TABLE 1.
Summary of major changes in dopaminergic signaling in primates and rodents. Highlighted boxes indicate evidence suggesting heightened dopamine activity compared to adulthood as discussed in the text
| Cortical | Subcortical | References | ||
|---|---|---|---|---|
| Primates | Tissue Concentrations | Peaks during adolescence | NA | Brown & Goldman, 1977; Goldman-Rakic & Brown, 1982 |
| Dopaminergic Innervation |
Peaks in PFC layer III during adolescence |
NA |
Rosenberg & Lewis, 1994; 1995; Lambe et al., 2000 |
|
| D1-Type Density | Peaks in childhood. Elevated in adolescents compared to adults |
Peaks in childhood. Elevated in adolescents compared to adults |
Lidow et al., 1991; Lidow & Rakic, 1992, Montague et al., 1999; Seeman et al., 1987 | |
| D2-Like Density | Peaks in childhood. Elevated in adolescents compared to adults |
Peaks in childhood. Elevated in adolescents compared to adults |
Lidow et al., 1991; Lidow & Rakic, 1992, Seeman et al., 1987 | |
| Rodents | Tissue Concentrations | Monotonic increases between childhood and adulthood, but adolescent peaks in dopamine synthesis |
Monotonic increases between childhood and adulthood, but adolescent peaks in synaptic availability |
Andersen, Dumont, & Teicher, 1997; Giorgi et al., 1987; Nomura, Naitoh, & Seqawa, 1976, Stamford, 1989; Ungethüm et al., 1996 |
| Dopaminergic Innervation |
Monotonic increase between childhood and adulthood. |
NA | Berger et al., 1985; Kalsbeek et al., 1988 | |
| D1-Type Density | Monotonic increases between childhood and adulthood. |
Peaks during periadolescence |
Andersen et al., 1997; Gelbard et al., 1989; Giorgi et al., 1987; Rao et al., 1991; Tarazi et al., 1999; Tarazi & Baldessarini, 2000; Teicher et al., 1995 | |
| D2-Like Density | Monotonic increases between childhood and adulthood. |
Peaks during periadolescence |
Andersen et al., 1997; Gelbard et al., 1989; Rao et al., 1991; Tarazi et al., 1998b; Teicher et al., 1995 |
In primates, cortical and subcortical tissue concentrations of DA are increased during adolescence compared to childhood and adulthood (Goldman-Rakic & Brown, 1982; Irwin et al., 1994). In addition, DA innervation of the frontal cortex peaks during adolescence relative to childhood and adulthood, specifically in cortical layer III, which contains pyramidal cells responsible for cortico-cortical information processing (Rosenberg & Lewis, 1995). D1 and D2 receptor densities appear to be heightened during adolescence compared to adulthood in both cortical and subcortical regions, though peaks in receptor density occur in childhood (Lidow & Rakic, 1992; Seeman et al., 1987). Thus, cortical and subcortical regions undergo specific increases in DA concentrations and innervation during adolescence, with receptor levels decreasing from peaks achieved during childhood.
These patterns indicate that the DA system is patterning itself in a manner during adolescence that could support functional changes in behaviors that are DA-modulated. For example, the receptor evidence supports the notion that limbic and striatal DA systems may be in a state of overdrive during adolescence. Moreover, there is evidence in rodents of a cortical/subcortical distinction. This distinction is not as apparent in primates, but it could be the case that “overdose” thresholds differ between cortical and subcortical regions in primates based on autoreceptor and dopamine transporter characteristics. Accordingly, adolescent-limited increases in dopamine availability could account for the accelerated drive toward potential rewards that is a hallmark of adolescent behavior.
Of course, virtually all of this work relies on animal models. There are limited strategies for assessing DA system development in healthy human developmental samples. Postmortem samples have been the primary means through which changes in DA system characteristics have been inferred. Other potential strategies include the use of pharmacological probes to provoke reactions in targeted chemical systems and/or positron emission tomography (PET) to measure brain activation changes in the presence of radioactive ligands. Neither of these methods is easy to justify for non-clinical samples. Studies of clinical samples, such as children with attention-deficit-hyperactivity-disorder (ADHD) are potentially informative because of the use of psychostimulants to treat the disorder, however the assessment of psychostimulant effects in ADHD is complicated by the unknown influences of the disorder itself on observed patterns. Other conditions of interest, such as PKU, have been used as models to illustrate developmental aspects of PFC DA availability (Diamond, 1996; Luciana, Whitney, & Hanson, 2004), but this work is indirect.
Despite these logistical obstacles, several groups have used fMRI techniques that allow for assessment of changes in neural systems innervated by DA (which may reflect changes in DA signaling in these areas). For example, despite activating a similar network of neural structures, adolescents generate larger BOLD activation in the nAcc compared to adults and young children when presented with rewarding stimuli (Ernst et al., 2005; Galvan et al., 2006; May et al., 2004). In addition, either smaller (Esher et al., 2007) or less focally distributed (Galvan et al., 2006) activations in response to reward presentation have been demonstrated in the orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC), indicating that increased subcortical activity might be accompanied by less-developed prefrontal control. As mentioned previously, these data have led to theories of adolescent risk-taking whereby active ventral striatal responsivity in the context of poor prefrontal regulation accounts for an increased propensity to engage in risky activities (Casey, Getz, & Galvan, 2008). On the one hand, more focal activation in brain regions in response to task demands have been interpreted to reflect more efficient neural processing (see Mattay et al., 2003), whereas other data suggest that activation increases as a function of learning and practice (Karni et al., 1998). Furthermore, cross-sectional and longitudinal studies indicate that development is accompanied by more focal activation in brain areas necessary for task completion, further complicating interpretation (for review, see Casey et al., 2008).
While it is possible to speculate about how these fMRI findings might be related to neurotransmission, inferences regarding neurochemical development are impossible to make with any certainty. Increases in neurochemical availability could result in BOLD signal increases or decreases depending on the task employed, the region of interest, and the nature of the chemical system. Thus, the BOLD signal in and of itself is not necessarily informative regarding directional changes in neurochemical activity.
In the absence of more direct assessment strategies, we have been intrigued by the notion of using molecular genetic probes to index the underlying status of the DA system to study neurochemical development. We have hypothesized, based on the animal data, that adolescents have high functional levels of DA activity in the cortex (Wahlstrom et al., 2007; Wahlstrom et al., 2010). If true, then there might be age-related variation in the relationship between genes and cognition, when genes that code for specific aspects of DA transmission are examined. The COMT SNP is of interest, because it is thought to have some specificity to PFC function and acts on the DA system in the synapse, impacting how much released DA reaches postsynaptic receptors. If DA release changes in magnitude throughout development and if COMT activity does not change to parametrically match how much DA is released, then the optimal COMT genotype for the enhancement of prefrontally-guided cognition might change in concert with underlying changes in DA availability. This model will be expanded upon in the next section.
COMT: Functions and Associations with Human Cognition
As described above, the COMT enzyme, along with monoamine oxidase (MAO), represents the first step in the catabolism of excess synaptic DA with some specificity to the PFC due to a lack of DA transporter activity in this region (Giros et al., 1996; Gogos et al., 1998; Huotari et al., 2002; Lewis et al., 2001; Napolitano, Cesura, & Da Prada, 1995; Sesack et al., 1998; Weinshilboum et al., 1999). The gene that codes the COMT enzyme resides on the q11 region of Chromosome 22 (Grossman et al., 1992), and the val158/108met polymorphism results from a single nucleotide polymorphism (SNP) on exon 4 caused by a G-to-A base-pair mutation (Lachmann et al., 1996). This mutation results in the substitution of valine (Val) for methianine (Met) on codon 158 of the membrane-bound enzyme and codon 108 of the soluble enzyme (Lundström et al., 1991). Thus, there are two alleles, Met and Val. Functionally, the Met allele confers four times lower enzymatic activity compared to Val, which results in increased levels of synaptic DA (Lachmann et al., 1996). In humans, individuals that are homozygous Met exhibit higher levels of PFC DA compared to individuals homozygous for Val. Heterozygotes may exhibit intermediate levels of DA, as the alleles are codominant (Lotta et al., 1995; Weinshilboum et al., 1977).
COMT and Cognitive Functioning: Adult Studies
Due to DA’s role in regulating prefrontal cognition, the COMT polymorphism was identified early as a possible contributor to individual variation in functions dependent on this region. A wealth of evidence suggests that COMT is associated with performance on working memory (Goldberg et al., 2003) and executive set-shifting tasks such as the Wisconsin Card Sort (Malhotra et al., 2002), with the Met allele typically conferring relatively better performance. This effect has been demonstrated and replicated in healthy volunteers and schizophrenic patients (Bilder et al., 2002; Egan et al., 2001; Mattay et al., 2003; though for a recent meta-analysis that suggests the effect of COMT holds in healthy control samples only, see Barnett, Jones, Robbins, & Muller, 2007). Egan and colleagues (2001) have shown that COMT is related to changes in prefrontal physiology during the completion of cognitive tasks, as they demonstrated that Met/Met volunteers performed better on the Wisconsin Card Sort compared to Val/Met and Val/Val individuals while simultaneously generating smaller BOLD responses in the DLPFC, indicating more efficient neural processing. Better attentional function, as measured by the digit span forward task, has also been demonstrated in a sample of elderly Asian males who were free of cognitive impairment (Liu et al., 2008). Overall, it should be noted that the effect of COMT on cognitive variables is small, similar to other polymorphisms; for example, the effect size of COMT on Wisconsin Card Sort performance as estimated by Barnett and colleagues (2007) in healthy adult controls is Cohen’s d = 0.22. Moreover, sample sizes are often small in individual studies, which may account for inconsistencies in the literature, particularly developmental studies.
COMT and Cognition: Developmental Findings
The literature linking COMT to cognitive functioning in children and adolescents is relatively sparse; moreover, available findings are inconsistent. In a sample of 8 to 14 year-old children (mean age = 9), Diamond et al. (2004) demonstrated that the Met allele was associated with a test of inhibition previously shown to depend on prefrontal DA. Similarly, Barnett and colleagues (2007) found that being homozygous Met predicted optimal performance on measures of verbal IQ, working memory, and inhibition in a sample of 8 and 10 year-old children; however, COMT appeared to predict cognitive performance only in males, and heterozygotes outperformed both homozygote groups on a measure of sustained attention. COMT was not related to intelligence in a sample of healthy children in China (Zhang et al., 2007). Studies of children with ADHD have suggested that COMT is unrelated to performance on tests of executive functions such as working memory, set-shifting, planning, and response inhibition (Mills et al., 2004; Taerk et al., 2004), whereas others have found that being homozygous for the Met allele impairs sustained attention performance (Bellgrove et al., 2005).
The role of COMT in prefrontal cognition has been of considerable interest to those groups studying 22q11.2 deletion syndrome, which is caused by a hemizygous deletion in a small region of chromosome 22 that contains the COMT gene and results in debilitating cognitive impairments and a significant increase in the risk for schizophrenia (Bassett & Chow, 1999; Scambler, 2000). Individuals with 22q11.2 deletion are either hemizygous Met or hemizygous Val, and as a result, the effects of the Val158/Met polymorphism on behavior are presumably larger than in healthy controls. The Met allele has been linked to higher overall IQ and improved sustained attention in children with 22q11.2 (Shahi et al., 2006), though other work using samples of both children and young adults found no association between COMT and measures of IQ, verbal fluency, or response inhibition (Glaser et al., 2006). The reasons for the null findings in the latter study may have to do with the broad range of ages assessed (6 – 37 years) and the fact that the relationship between COMT and cognition changes with age in 22q11.2. Specifically, Golthelf et al. (2005) demonstrated that the Met allele predicts higher IQ in young adolescents (mean age 13 years) with 22q11.2; however, their cognitive abilities decline at a more rapid rate so that by 18 years, Val individuals exhibit higher IQs.
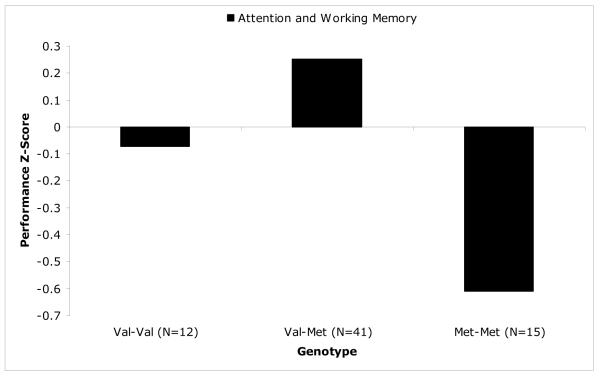
Noticeably missing from the developmental literature are studies assessing the link between COMT genotype and prefrontal cognition in adolescence, especially given the importance of this time as a period of normative developmental transitions and increased risk for psychopathology. We have associated the COMT genotype with working memory and attention in a sample of adolescents and young adults (Wahlstrom et al., 2007). We administered a battery of working memory, attention, motor coordination, and motor speed measures to children and adolescence ages 9-17 (n = 70) genotyped for COMT. Composites were created to represent the cognitive domains of working memory/attention and motor coordination/speed. These composites were created to maximize both theoretical coherence (e.g., similarity of function) and psychometric strength (e.g., high internal consistency) and to increase statistical power in reporting associations given the small sample size. We reported that the COMT Val158/Met genotype was significantly related to measures of working memory/attention, and motor coordination, all of which depend on prefrontal DA (Gllickstein, DeSteno, Hof, & Schmauss, 2005; Luciana & Collins, 1997; Yang et al., 2003). Conversely, genotype was not related to a measure of motor speed, indicating specificity in the modulation of frontal behaviors, which is consistent with other studies of children (Diamond et al., 2004). The critical finding in our study concerned the optimal genotype for cognitive performance in this adolescent sample. Instead of finding Met-Met superiority of cognitive function as would have been predicted from the adult literature, we found that Val-Met heterozygotes exhibited superior performance on all cognitive constructs compared to both homozygote groups (see Figure 1: Wahlstrom et al., 2007). Specifically, heterozygotes outperformed the Met-Met group (but not the Val-Val group) on the working memory/attention composite, and heterozygotes outperformed both homozygote groups on the motor composite. At first glance, this pattern might be seen as consistent with the overall degree of inconsistency of findings relating the COMT genotype to cognition in developmental samples. However, another proposal is that these data are reflective of underlying transitions in DA availability that occur during adolescence as compared to other points in the lifespan. The rationale for this proposal is as follows.
Figure 1.
The COMT/Cognition Relationship in 9-17 year-old Healthy Adolescents
Adapted from Wahlstrom et al. (2007), this figure illustrates working memory and attentional performance in healthy adolescents as a function of COMT genotype. Working memory and attention were assessed through spatial delayed response, digit span, and spatial span tasks and scored as a standardized composite. The heterozygote Val/Met group performed better than either homozygote group.
First, in the adult literature, the optimal COMT genotype for cognitive performance varies based on state characteristics. Mattay and colleagues (2003) recruited a sample that varied in its representation of the COMT genotype. All participants completed a short battery of executive function measures (the N-back task and the Wisconsin Card Sort Task) under baseline conditions. All participants then completed two fMRI scans, during which the N-back task was administered. The fMRI scans were distinct in that one scan took place under placebo conditions and the other took place after participants ingested a small dose of d-amphetamine, which non-specifically increases DA activity. Under placebo conditions, Met/Met individuals exhibited better working memory performance compared to Val/Val individuals, and this improved performance was associated with smaller BOLD activations in the DLPFC, which the authors interpreted as an indication of greater PFC efficiency. However, after the administration of d-amphetamine, which presumably raised dopamine levels in the PFC, the Val/Val group improved their working memory performance in the context of decreased BOLD activations, indicating that less neural processing was necessary to obtain improved behavioral performance. The opposite pattern was evident in the Met/Met group, which exhibited less efficient neural processing and poorer behavioral performance in the d-amphetamine, relative to the placebo, condition. Thus, under conditions of increased DA release, the Met-Met group performed worse, while the Val-Val group performed better. The results were interpreted by the study’s authors in accord with the inverted-U model that represents the DA-cognition association. That is, there is an optimal level of dopamine availability in relation to cognitive function (Williams & Goldman-Rakic, 1995). It was suggested that Met/Met individuals rested near the apex of this curve at baseline (as evidenced by better performance and more efficient neural processing under placebo conditions) and that Val/Val individuals rested near the lower left slope of the curve due to decreased DA levels. After increasing DA levels with d-amphetamine, Met/Met individuals’ neural processing suffered due to excess levels of DA, which pushed them to the right-most downslope of the inverted-U, while the Val/Val group showed improvements as they shifted from the lower left portion of the curve to a point close to its apex. Recently, animal models have been developed to illustrate similar relationships. Transgenic mice who over-express the Val polymorphism exhibit disrupted attentional set-shifting and show impairments in multiple forms of memory, including both working and recognition memory, as compared to mice with a null COMT mutation (Papaleo et al., 2008). Amphetamine administration improved the experimental animals’ memory deficits but impaired memory in control animals.
Based on these findings, one interpretation is that the relationship between COMT alleles and cognitive functioning changes in a similar manner during adolescence due to the underlying development of the DA system (Wahlstrom et al, 2007). Specifically, in accord with the evidence that DA levels increase in the PFC during adolescence, it may be that adolescents who have the Met/Met genotype may experience excessive levels of DA in prefrontal circuits, and hence, poorer cognitive functioning as compared to Val/Met and even Val/Val individuals.
Figure 2 illustrates this hypothesis. In adulthood, characterized by mature levels of DA, Met/Met individuals rest near the apex of the inverted-U shaped function that describes the relationship between DA and cognition. Conversely, Val/Met and Val/Val individuals, who exhibit lower levels of synaptic DA due to more efficient COMT enzymes, would rest toward the left-most downslope of the curve, which is characterized by less optimal cognitive functioning. In adolescence, when increased levels of DA are experienced due to developmental changes, the relationship between COMT allele and cognitive functioning may presumably shift as seen in adults administered d-amphetamine. Specifically, increases in DA may interact with the Met/Met genotype to cause excess levels of DA in the PFC, shifting these individuals to the upper right portion of the inverted U that is characterized by inefficient cognitive functioning. Val/Met and Val/Val individuals would benefit from these increases in DA transmission, which would shift them closer to the apex of the curve and result in improvements in cognitive functioning relative to Met/Met volunteers.
Figure 2.
Modeling the COMT-Cognition Relationship from Adolescence to Adulthood
Changes in the relationship between COMT genotype and cognitive performance as a function of age. In adolescence, Val homozygotes are conferred functional benefits due to developmental increases in dopaminergic availability. Conversely, the performance of Met homozygotes suffers due to excess dopamine (as a result of genotype and age-related increases in dopaminergic availability), which moves them to the right of the inverted U-shaped function.
In understanding this model, several points are worthy of emphasis. First, developmentally, it is expected that regardless of genotype, individuals will improve their levels of performance on cognitive tasks between adolescence and adulthood, as many factors in addition to the COMT genotype contribute to the development of executive function skills (e.g., structural brain development, increasing levels of experience, other components of the DA system, etc.) (Nagy, Westerberg, Klingberg, 2004). Second, it is assumed that all individuals, regardless of genotype, experience increases in functional DA availability during adolescence. And finally, although there may be changes in COMT enzyme activity throughout development (Turnbridge et al., 2007), these changes are stable within age groups. What is expected on the basis of this model is that there will be age by genotype interactions related to the differential impacts of individual COMT genotypes on cognition at each point in development and related to how the different genotypes respond in the course of development. That is, Met/Met individuals may make larger developmental gains between adolescence and adulthood, because they are moving from an inefficient area of the curve (due to excess DA) to the apex. Conversely, the developmental gains made by Val/Met and Val/Val individuals may be smaller, because as they move into adulthood, which is characterized by lower DA levels compared to adolescence, they shift down to the lower downslope of the curve as defined by their COMT genotype.
Ideally, longitudinal designs would be implemented to test this model, and our laboratory is in the midst of data collection toward this end. This framework is consistent with a growing body of literature that indicates the relationship between COMT and cognition changes as a function of development. As mentioned above, Golthelf and colleagues (2005) demonstrated in a longitudinal design that individuals with 22q11.2 deletion syndrome hemizygous for Met exhibited more rapid cognitive decline compared to those hemizygous for Val. Given the widespread impairments associated with 22q11.2 deletion, it is difficult to interpret these findings within the context of normative development, but their results do indicate that age modulates the effect of COMT genotype on cognition during the adolescent period.
Similar models have been advocated to explain age-related decrements in behaviors that are DA-modulated (Backman et al., 2006). In the context of healthy aging, several cognitive processes are known to decline, notably processing speed, working and episodic memory, and fluid reasoning skills (Verhaeghen & Salthouse, 1997). Similarly, there is evidence for age-related loss in functional dopamine availability, primarily observed in nigrostriatal regions due to the relative ease through which biomarkers of DA availability can be measured in the striatum during Positron Emission Tomography (PET) scans and SPECT imaging (Bäckman et al., 2006; Kaasinen et al., 2000; Suhara et al., 1991; Volkow et al., 1994; 2000; 2004). These studies, along with findings from human autopsy studies, indicate age-related declines in D1 and D2 receptor numbers, the DA transporter, and DA cells in the midbrain and striatum (Volkow et al., 1998). Consistent with our model, it has been suggested that these normative mechanisms may have greater functional consequences for individuals with lower levels of baseline DA availability (Backman et al., 2006), and indeed, individuals who carry the Val-Val COMT configuration appear to be more susceptible to these age-related changes. In a sample of adults ages 20-31 and 60-70 years, Nagal et al. (2008) demonstrated a COMT genotype by age interaction on Wisconsin Card Sorting Test (WCST) performance, whereby the functional consequence of the Val allele was increased in the oldest group. This finding would seem to reflect the interaction of the Val allele with age-related decreases in dopamine, which would theoretically shift Val homozygotes further down the inverted-U relating DA transmission and cognition. In contrast, Met-Met individuals, who have presumably higher levels of functionally available dopamine at the start, may be more invulnerability to age-related declines in the system. Accordingly, this model predicts individual differences in neuroplasticity on the basis of genotype, and indeed, we observe that adolescents who carry the Met-Met genotype have a more protracted rate of working memory development than individuals with the Val-Met and Val-Val genotypes (Wahlstrom et al., in press). Given that the aging work is the subject of another paper within this issue, we have not covered it in detail here but refer interested readers to the paper by Backman and colleagues.
Recently, some supportive evidence has emerged indicating that sex also modulates the effect of COMT on cognition, as elderly females appear to benefit from the Val allele when performing the Wisconsin Card Sort, whereas aging males show benefit from possessing the Met allele (Diamond, 2007). COMT activity is lower in females compared to males (Boudikova, Szumlanski, Maidak, & Weinshilboum, 1990); thus, females presumably exhibit higher DA levels in the PFC and would show no benefit from the Met allele, which pushes them into the excess range of the inverted-U and impairs their performance. Males, on the other hand, exhibit lower levels of baseline DA and do show improvement. How these sex effects interact with normative development is unclear, but these findings raise interesting questions about how hormonal state and perhaps pubertal development might impact some of these observed associations.
The evidence presented above suggests that the influence of COMT on cognition may depend on state factors such as age and individual differences, such as sex, both of which are known to impact the DA system that mediates the relationship between COMT and cognitive processes. Given that small samples and effect sizes characterize most COMT studies, results regarding its impact on cognitive performance in adults have been inconsistently replicated, and even meta-analyses have yielded inconsistent findings (Barnett et al., 2007; Barnett, Scoriels, & Munafò, 2008). Thus, the results discussed here are admittedly preliminary, and several replications focused on specific age groups (e.g., adolescents, elderly individuals, etc.) will be necessary. It is important to note within this context that the findings of Mattay and colleagues (2003), which stimulated the conceptualization of this framework, have not been replicated.
Finally, research utilizing the COMT Val158/Met polymorphism is complicated by the fact that the precise mechanisms by which it impacts DA signaling are debated. Although it is typically assumed that COMT’s relationship to prefrontal cognition is accounted for by generalized changes in DA in that region, others have proposed that Val and Met alleles differentially affect tonic vs. phasic aspects of striatal DA transmission. Specifically, the Met allele may be related to higher tonic levels of subcortical DA, and, by association, decreased subcortical phasic amplitude in response to neuronal firing and increased cortical D1 transmission (Bilder et al., 2004). Functionally, these changes may result in stability of neural networks underlying cognition, which comes at the cost of decreased flexibility and would thus affect various phenotypes differentially. Interestingly, fMRI evidence supports these claims, with Met individuals exhibiting a heightened transient activation during the maintenance phase of a working memory task and Val individuals a heightened sustained response throughout the maintenance period (DeFrias et al., 2009). With respect to the data presented in this review, the tonic/phasic theory would suggest that Met is related to improved working memory (due to improved cognitive stability during maintenance phases of the task); albeit by different mechanisms that those outlined in this review (which suggest they are related to prefrontal dopamine transmission).
Our group is one of many to suggest that the relationship between behavior and genetic polymorphisms is state dependent (Diamond et al., 2007; Mattay et al., 2003; Tunbridge, Harrison, & Weinberger, 2007). Presumably, state changes are occurring in the neurochemical systems that are mediating the relationships between these polymorphisms and behaviors, but the factors causing these changes are numerous and may include development (Wahlstrom et al., 2007), interactions between gender and stress (Diamond, 2007), or interactions between age and psychopathology (Getholf et al., 2005). Furthermore, a number of recent studies have received considerable interest because they demonstrate that early life experiences modify the risk for future psychopathology conferred by genetic polymorphisms (Arseneault et al., 2002; Caspi et al., 2002). The mechanisms underlying these findings are unknown, but it is possible that environmental influences result in changes within the neurochemical systems acted upon by the polymorphisms of interest, resulting in different risk potentials. Moreover, this model suggests that genotypic groups may vary in the quality of their life experiences based on how an individual’s “neurochemical tone” provokes him/her to select experiences. These selections, in turn, may impact the continued development of the neurochemical system. It is clear that further work is necessary to clarify how environmental influences interact with genetics and underlying neurochemical systems to impact current and future behavior.
Given that our findings suggest a role for development in modulating the influence of genetic polymorphisms on behavior, future studies attempting to link genes to behavioral phenotypes should consider age as an important variable in study design. The effect sizes of individual polymorphisms on behavior are often small (Barnett, Jones, Robbins, & Müller, 2007), and meta-analyses attempting to link COMT to cognition or psychopathology have sometimes resulted in null findings (Barnett et al., 2008; Munafó, Bowes, Clark, & Flint, 2005). Given these results, attempts to link individual genes to behavior may benefit from more homogeneous samples with respect to age, which could hypothetically increase the likelihood of detecting significant relationships. Similar consideration should be given to demographic variables such as gender, which have also been shown to modulate the underlying neurochemical systems acted on by candidate genes (Diamond, 2007).
Conclusions
Human adolescence is recognized as a period of rapid cognitive development, increased risk-taking and inconsistencies in behavioral regulation. These patterns have been attributed to structural aspects of brain development but may also be impacted by neurochemical changes that interact with these structural refinements. The dopamine system is intriguing in this respect, because it has been linked to reinforcement learning and to higher-level cognitive processes salient to cognitive control. Yet, there are few (if any) studies that have directly documented changes in DA transmission in human adolescents, most likely because of the invasiveness of the techniques necessary for this type of measurement and the related human subjects concerns. Although DA’s modulation of working memory and other behavioral processes is well-established, behavioral findings alone cannot directly assess the question of whether this system demonstrates unique properties during the adolescent period. The animal literature suggests that such properties may be evident and that the system should be a focus of study in humans. Although based primarily on findings in rodents and non-human primates, the available evidence indicates that the dopamine system, which includes factors such as cell proliferation, receptor density, and metabolite concentrations, undergoes widespread and dramatic changes. Furthermore, while the data are characterized by some cross-species differences and inconsistencies across research groups, the general trends point to a system that is overactive compared to both childhood and adulthood. The implications of these changes are not fully understood, but it is likely that specific developmental alterations of dopamine transmission play an important role in both normative developmental behaviors (i.e. preferences for novelty, increased interest in risky situations such as drugs and promiscuous sex) and the onset of psychiatric disorders. We have suggested a model through which the underlying state of dopaminergic tone can be inferred through developmental changes in gene-behavior associations.
Several limitations of this presentation should be mentioned. First, our primary goal is to provide a rationale for why neurochemistry should be directly assessed in human developmental samples. The behavioral evidence is compelling in suggesting that dopamine activity changes during this lifespan period. In reviewing the animal data pertinent to dopamine system development, it is important to recognize the limitations in generalizing the findings to humans. That said, the findings are compelling in suggesting that dopamine receptor densities change throughout adolescence and that there are cortical versus subcortical distinctions. Many researchers might find limbic/subcortical dopamine activity particularly compelling as a target of study, and we agree that dopamine overactivity in this circuitry may account for adolescent risk-taking and other problem behaviors (Wahlstrom et al., 2010). Yet, we have focused our molecular genetic presentation on the COMT enzyme and prefrontally-guided working memory functions. That decision was guided by our laboratory’s available data. It should be emphasized that this model is intended to use the COMT genotype as an exemplar that may apply to other polymorphisms. It is unlikely, for instance, that the COMT genotype modulates reward salience or other incentive-motivated behaviors given that the enzyme is most active in dorsal prefrontal regions (Beyer & Stekee, 2000; Carr & White, 1987). It is likely that there are other candidate genes that control for processes that are expressed more strongly in limbic and striatal regions and that could be examined for age-by-genotype interactions. For instance, the Taq1 A allele, associated with D2 dopamine receptor mechanisms (Neville et al., 2004), has been associated with novelty seeking as well as with reinforcement learning (Berman et al., 2002; Frank & Hutchinson, 2009). The D4 dopamine receptor and the dopamine transporter genes are also likely candidates for analysis, because individual differences in SNPs associated with these genes have been linked to variations in personality traits, externalizing behaviors, and higher-order cognition (DeYoung et al., 2006; Durston et al., 2008; Munafo et al., 2008). Moreover, as the aging and animal developmental literatures suggest, there are lifespan changes in dopamine receptor and transporter mechanisms that impact behavior. The primary point to be made here is that genotype-by-age interactions can be informative regarding the underlying state of this neurochemical system given that more direct assessments (via psychopharmacological probes, for example) are lacking. In our view and based on success in using pharmacological probes to index neurochemical reactivity in young adults (Luciana & Collins, 1997; Luciana et al., 2004; Vrshek-Schallhorn et al., 2006), this is a technology that could be applied safely to adolescent samples. However, such an approach requires justification in the context of the current scientific climate. Perhaps neuroimaging techniques could be combined with behavioral paradigms to index differences in brain activation across age groups and genotypes to provide a more compelling rationale for the use of pharmacological probes to assess the integrity of neurochemical systems in developmental groups.
Such studies would be informative in resolving several issues. First, the precise relationship between dopamine activity and risk-taking behaviors is unclear. In addition, there is little knowledge of how changes in one facet of the dopaminergic system impact others during adolescence, how these changes are influenced by other neurochemical systems, and how these changes are influenced by or otherwise interact with structural brain changes, particularly those associated with white matter. The factors that regulate these changes are also unknown. Many may be under strong genetic control, but it could also be that differences in the onset or length of adolescent-typical events (i.e. dating, school, parenthood) have an influence on when dopaminergic changes come online or stabilize to adult levels. Unlike changes in brain structure, those that relate to neurochemistry may be more readily malleable. Accordingly, if adolescent behavior is to continue to be described as poorly regulated, as disordered in some respect, or in terms of the increased mortality that accompanies it, it should be viewed as a scientific and ethical imperative to broaden the scope of inquiry into the reasons for such behavior in humans and how we might successfully intervene.
Acknowledgements
This work was supported by grant DA017843-05 awarded by the National Institute on Drug Abuse to Monica Luciana and by a seed grant from the Biomedical Genomics Center at the University of Minnesota to Tonya White. Dustin Wahlstrom was supported by T32 grant MH017069-26 from the National Institute on Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman J, Bayer SA. Development of the brain stem in the rat. V. Thymidine-radiographic study of the time of origin of neurons in the midbrain tegmentum. The Journal of Comparative Neurology. 1981;198:677–716. doi: 10.1002/cne.901980409. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. NeuroReport. 1997;8:1495–1498. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AP, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27:683–691. doi: 10.1016/s0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. British Medical Journal. 2002;325:1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato MR, Sweeney JA, Luna B. Cognitive processes in the development of TOL performance. Neuropsychologia. 2006;44(12):2259–2269. doi: 10.1016/j.neuropsychologia.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, et al. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. Neuroimage. 2007;35(2):501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li S, Farde L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neuroscience and Biobehavioral Reviews. 2006;30(6):791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Baird AA, Gruber SA, Fein DA, Mass LC, Steingard RJ, Renshaw PF, et al. Functional magnetic resonance imaging of facial affect recognition in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(2):195–199. doi: 10.1097/00004583-199902000-00019. [DOI] [PubMed] [Google Scholar]
- Bannon MJ, Roth RH. Pharmacology of mesocortical dopamine neurons. Pharmacological Reviews. 1983;35:53–68. [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cerebral Cortex. 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Heron J, Ring SM, Golding J, Goldman D, Xu K, Jones PB. Gender-Specific Effects of the Catechol-O-Methyltransferase Val108/158Met Polymorphism on Cognitive Function in Children. American Journal of Psychiatry. 2007;164:142–149. doi: 10.1176/ajp.2007.164.1.142. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Jones PB, Robbins TW, Müller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Molecular Psychiatry. 2007;12:502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Scoriels L, Munafó MR. Meta-analysis of the cognitive effects of the catechol-O-Methyltransferase gene Val158/108Met polymorphism. Biological Psychiatry. 2008;64(2):137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, et al. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed. 2002;15(78):456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Bassett AS, Chow EWC. 22q11 deletion syndrome: a genetic subtype of schizophrenia. Biological Psychiatry. 1999;46(7):882–891. doi: 10.1016/s0006-3223(99)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EWC. 22q11 deletion syndrome: a genetic subtype of schizophrenia. Biological Psychiatry. 1992;46(7):882–891. doi: 10.1016/s0006-3223(99)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJH. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Research. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Bell MA, Fox NA. The relations between frontal brain electrical activity and cognitive development during infancy. Child Development. 1992;63:1142–1163. [PubMed] [Google Scholar]
- Bellgrove MA, Domschke K, Hawi Z, Kirley A, Mullins C, Robertson IH, et al. The methionine allele of the COMT polymorphism impairs prefrontal cognition in children and adolescents with ADHD. Experimental Brain Research. 2005;163:352–360. doi: 10.1007/s00221-004-2180-y. [DOI] [PubMed] [Google Scholar]
- Ben Bashat D, Ben Sira L, Graif M, Pianka P, Hendler T, Cohen Y, et al. Normal white matter development from infancy to adulthood: comparing diffusion tensor and high b value diffusion weighted MR images. J Magn Reson Imaging. 2005;21(5):503–511. doi: 10.1002/jmri.20281. [DOI] [PubMed] [Google Scholar]
- Berger B, Alvarez A, Goldman-Rakic PS. Neurochemical development of the hippocampal region in the fetal rhesus monkey. I. Early appearance of peptides, calcium binding proteins, DARPP-32, and the monoamine innervation in the entorhinal cortex during the first half of gestation (E47 to E90) Hippocampus. 1993;3:293–318. doi: 10.1002/hipo.450030305. [DOI] [PubMed] [Google Scholar]
- Berger B, Verney C, Febvret A, Vigny A, Helle K. Postnatal ontogenesis of the dopaminergic innervation in the rat anterior cingulate cortex (area 24). Immunocytochemical and catecholamine fluorescence histochemical analysis. Developmental Brain Research. 1985;21:31–47. doi: 10.1016/0165-3806(85)90021-5. [DOI] [PubMed] [Google Scholar]
- Berger B, Gaspar B, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends in Neuroscience. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Berger B, Trottier S, Verney C, Gaspar P, Alvarez C. Regional and laminar distribution of the dopamine and serotonin innervation in the Macaque cerebral cortex: a radioautographic study. Journal of Computational Neurology. 1988;273:99–119. doi: 10.1002/cne.902730109. [DOI] [PubMed] [Google Scholar]
- Berman S, Ozkaragoz T, Young RM, Noble EP. D2 dopamine receptor gene polymorphism discriminates two kinds of novelty seeking. Personality and Individual Differences. 2002;33(6):867–882. [Google Scholar]
- Berns G, Moore S, Capra C. Adolescent engagement in dangerous behaviors is associated with increased white matter maturity of frontal cortex. PLoS ONE. 2009;4:e6773. doi: 10.1371/journal.pone.0006773. Published online, August 26, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer CE, Steketee JD. Intra-medial prefrontal cortex injection of quinpirole, but not SKF 38393, blocks the acute motor-stimulant response to cocaine in the rat. Psychopharmacology (Berlin) 2000;151(23):211–218. doi: 10.1007/s002139900345. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Czobor P, Malhotra AK, Kennedy JL, Ni X, Goldman RS, et al. Neurocognitive correlates of the COMT Val158Met polymorphism in chronic schizophrenia. Biological Psychiatry. 2002;52:701–707. doi: 10.1016/s0006-3223(02)01416-6. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29(11):1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends in Neuroscience. 2007;30(5):194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WM, Barker PB, et al. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34(2):733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudikova B, Szumlanski C, Maidak B, Weinshilboum R. Human liver catechol-O-methyltransferase pharmacogenetics. Clinical Pharmacology and Therapeutics. 1990;48:381–389. doi: 10.1038/clpt.1990.166. [DOI] [PubMed] [Google Scholar]
- Boyson SJ, McGonigle P, Molinoff PB. Quantitative radiographic localization of the D1 and D2 subtypes of dopamine receptors in the rat brain. The Journal of Neuroscience. 1986;6:3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes K, Xu X, Chen W, Zhou K, Neale B, Lowe N, et al. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1, and 16 other genes. Molecular Psychiatry. 2006;11:934–953. doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]
- Brown RM, Goldman PS. Catecholamines in neocortex of rhesus monkeys: regional distribution and ontogenetic development. Brain Research. 1977;124:576–580. doi: 10.1016/0006-8993(77)90960-x. [DOI] [PubMed] [Google Scholar]
- Brust P, Walter B, Hinz R, Füchtner F, Müller M, Steinbach J, Bauer R. Developmental changes in the activities of aromatic amino acid decarboxylase and catechol-O-methyl transferase in the porcine brain: A positron emission tomography study. Neuroscience Letters. 2004;364:159–163. doi: 10.1016/j.neulet.2004.04.041. [DOI] [PubMed] [Google Scholar]
- Butcher SP, Fairbrother IS, Kelly JS, Arbuthnott GW. Effects of selective monoamine oxidase inhibitors on the in vivo release and metabolism of dopamine in the rat striatum. Journal of Neurochemistry. 1990;55:981–998. doi: 10.1111/j.1471-4159.1990.tb04587.x. [DOI] [PubMed] [Google Scholar]
- Caille I, Dumartin B, Le Moine C, Beguerut J, Bloch B. Ontogeny of the D1 dopamine receptor in the rat striatonigral system: an immunohistochemical study. European Journal of Neuroscience. 1995;7(4):712–722. doi: 10.1111/j.1460-9568.1995.tb00675.x. [DOI] [PubMed] [Google Scholar]
- Carr GD, White NM. Effects of systemic and intracranial amphetamine injections on behavior in the open field: a detailed analysis. Pharmacology Biochemistry and Behavior. 1987;27(1):113–122. doi: 10.1016/0091-3057(87)90485-0. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt T, Mill J, Martin J, Craig I, et al. Evidence that the cycle of violence in maltreated children depends on genotype. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Cauffman E, Shulman E, Steinberg L, Claus E, Banich M, Graham S, Woolard J. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Developmental Psychology. doi: 10.1037/a0016128. in press. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period for addiction vulnerability. American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelune GJ, Baer RA. Developmental norms for the Wisconsin card sorting test. Journal of Clinical and Experimental Neuropsychology. 1986;8(3):219–228. doi: 10.1080/01688638608401314. [DOI] [PubMed] [Google Scholar]
- Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Progress in Brain Research. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- Conklin HH, Luciana M, Hooper CJ, Yarger RS. Working memory performance in typically developing children and adolescents: Behavioral evidence of protracted frontal lobe development. Developmental Neuropsychology. 2007;31(1):103–128. doi: 10.1207/s15326942dn3101_6. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Happe HK, Murrin LC. Postnatal development of the dopamine transporter: a quantitative autoradiographic study. Developmental Brain Research. 1996;92:172–181. doi: 10.1016/0165-3806(96)00004-1. [DOI] [PubMed] [Google Scholar]
- Crone EA, van der Molen MW. Developmental changes in decision making: Performance on a gambling task previously shown to depend on the ventromedial prefrontal cortex. Developmental Neuropsychology. 2004;25(3):251–279. doi: 10.1207/s15326942dn2503_2. [DOI] [PubMed] [Google Scholar]
- De Frias CM, Marklund P, Eriksson E, Larsson A, Öman L, Annerbrink K, et al. Influence of COMT gene polymorphism on fMRI-assessed sustained and transient activity during a working memory task. Journal of Cognitive Neuroscience. 2009 doi: 10.1162/jocn.2009.21318. doi:10.1162/jocn.2009.21318. [DOI] [PubMed] [Google Scholar]
- De Luca CR, Wood SJ, Anderson V, Buchanan J, Proffitt TM, Mahoney K, Pantelis C. Normative data from the CANTAB. I: Development of executive function over the lifespan. Journal of Clinical and Experimental Neuropsychology. 2003;25(2):242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- Demotes-Mainard J, Henry C, Jeantet Y, Arsaut J, Arnauld E. Postnatal ontogeny of dopamine D3 receptors in the mouse brain: autoradiographic evidence for a transient cortical expression. Developmental Brain Research. 1996;94:166–174. doi: 10.1016/0165-3806(96)00041-7. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Peterson JB, Séguin JR, Mejia JM, Pihl RO, Beitchman JH, Jain U, Tremblay RE, Kennedy JL, Palmour RM. The dopamine D4 receptor gene and moderation of the association between externalizing behavior and IQ. Archives of General Psychiatry. 2006;63:1410–1416. doi: 10.1001/archpsyc.63.12.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. The development and neural bases of memory functions as indexed by the AB and delayed response tasks in human infants and infant monkeys. In: Diamond A, editor. The development and neural basis of higher cognitive functions. Vol. 608. New York Academy of Sciences Press; New York: 1990a. pp. 267–317. [DOI] [PubMed] [Google Scholar]
- Diamond A. Developmental time course in human infants and infant monkeys and the neural basis of inhibitory control in reaching. In: Diamond A, editor. The development and neural basis of higher cognitive functions. Vol. 608. New York Academy of Sciences Press; New York: 1990b. pp. 637–676. [DOI] [PubMed] [Google Scholar]
- Diamond A. Evidence for the importance of dopamine for prefrontal cortex functions early in life. Philosophical Transactions of the Royal Society of London—Series B: Biological Sciences. 1996;351(1346):1483–1493. doi: 10.1098/rstb.1996.0134. [DOI] [PubMed] [Google Scholar]
- Diamond A. Consequences of variations in genes that affect dopamine in prefrontal cortex. Cerebral Cortex. 2007;17:i161–i170. doi: 10.1093/cercor/bhm082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Briand L, Fossella J, Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. American Journal of Psychiatry. 2004;161(1):125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear L. Motivational systems in adolescence: Possible implications for age differences in substance abuse and other risk-taking behaviors. Brain & Cognition. 2010 doi: 10.1016/j.bandc.2009.08.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Fossella JA, Mulder MJ, Casey BJ, Ziermans TB, Vessaz MN, Van Engeland H. Dopamine transporter genotype conveys familial risk of attention deficit/hyperactivity disorder through striatal activation. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(1):61–67. doi: 10.1097/chi.0b013e31815a5f17. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences of United States of America. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cerebral Cortex. 2007;17(12):2760–2768. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson SL, Akil M, Levey AI, Lewis DA. Postnatal development of tyrosine hydroxylase- and dopamine transporter-immunoreactive axons in monkey rostral entorhinal cortex. Cerebral Cortex. 1998;8:415–427. doi: 10.1093/cercor/8.5.415. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec SP, McClure EB, Monk CS, Leibenluft, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Esher N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choices selection in adults and adolescents: Development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Martin LN, Delgado MR. Reward-related processing in the human brain: developmental considerations. Developmental Psychopathoogy. 2008;20(4):1191–211. doi: 10.1017/S0954579408000576. [DOI] [PubMed] [Google Scholar]
- Feldman RS, Meyer JS, Quenzer LF. Principles of Neuropsychopharmacology. Sinauer Associates, Inc.; Sunderland, MA: 1997. [Google Scholar]
- Fiszman ML, Zuddas A, Masana MI, Barker JL, di Porzio U. Dopamine synthesis precedes dopamine uptake in embryonic rat mesencephalic neurons. Journal of Neurochemistry. 1991;56:392–299. doi: 10.1111/j.1471-4159.1991.tb08164.x. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Hutchinson K. Genetic contributions to avoidance-based decisions: Striatal D2 receptor polymorphisms. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.04.048. in press. doi: 10,1016/j.neuroscience.2009,04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galineau L, Kodas E, Guilloteau D, Vilar M, Chalon S. Ontogeny of the dopamine and serotonin transporters in the rat brain: an autoradiographic study. Neuroscience Letters. 2004;363:266–271. doi: 10.1016/j.neulet.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard HA, Teicher MH, Faedda G, Baldessarini RJ. Postnatal development of dopamine D1 and D2 receptor sites in rat striatum. Brain Research. 1989;49:123–130. doi: 10.1016/0165-3806(89)90065-5. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PI, et al. Quantitative magnetic resonance imaging of human brain development: Ages 4-18. Cerebral Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giorgi O, De Montis G, Porceddu ML, Mele S, Calderini G, Toffano G, Biggio G. Developmental and age-related changes in D1-dopamine receptors and dopamine content in the rat striatum. Developmental Brain Research. 1987;35:283–290. doi: 10.1016/0165-3806(87)90053-8. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifferences to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Glaser B, Debbane M, Hinard C, Morris MA, Dahoun SP, Antonarakis SE, Eliez S. No evidence for an effect of COMT Val158Met genotype on executive function in patients with 22q11 deletion syndrome. American Journal of Psychiatry. 2006;163(3):537–539. doi: 10.1176/appi.ajp.163.3.537. [DOI] [PubMed] [Google Scholar]
- Glickstein SB, DeSteno DA, Hof PR, Schmauss C. Mice lacking dopamine D2 and D3 receptors exhibit differential activation of prefrontal cortical neurons during tasks requiring attention. Cerebral Cortex. 2005;15:1016–1024. doi: 10.1093/cercor/bhh202. [DOI] [PubMed] [Google Scholar]
- Gogos J, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. Mapping gray matter development: Implications for typical development and vulnerability to psychopathology. Brain & Cognition. 2010 doi: 10.1016/j.bandc.2009.08.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, et al. Executive subprocesses in working memory: relationship to catechol-o-methyltransferase Val158Met genotype and schizophrenia. Archives of General Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of the prefrontal cortex and the regulation of behavior by representational knowledge. In: Plum F, Mountcastle V, editors. Handbook of Physiology. Vol. 5. American Physiological Society; Bethesda, MD: 1987. p. 373. [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annual Review of Neuroscience. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. Postnatal development of monoamine content and synthesis in the cerebral cortex of rhesus monkeys. Developmental Brain Research. 1982;4:339–349. doi: 10.1016/0165-3806(82)90146-8. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosso MF, de Geus EJ, Polderman TJ, Boomsma DI, Heutink P, Posthuma D. Catechol O-methyl transferase and dopamine D2 receptor gene polymorphisms: evidence of positive heterosis and gene-gene interaction on working memory functioning. European Journal of Human Genetics. 2008;16(9):1075–1082. doi: 10.1038/ejhg.2008.57. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Eliez S, Thompson T, Hinard T, Penniman L, Feinstein C, et al. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nature Neuroscience. 2005;8:1500–1502. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology. 1994;11:245–256. doi: 10.1038/sj.npp.1380111. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Sankar A, Halphen C, Kramer LA, Brandt ME, Juranek J, et al. Development and organization of the human brain tissue compartments across the lifespan using diffusion tensor imaging. Neuroreport. 2007;18(16):1735–1739. doi: 10.1097/WNR.0b013e3282f0d40c. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. Journal of Neurochemistry. 2003;87:574–585. doi: 10.1046/j.1471-4159.2003.02017.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Henze DA, Gonzales-Burgos GR, Urban NN, Lewis DA, Barrionuevo G. Dopamine increases excitability of pyramidal neurons in primate prefrontal cortex. Journal of Neurophysiology. 2000;84:2799–2809. doi: 10.1152/jn.2000.84.6.2799. [DOI] [PubMed] [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, Yarger RS. Adolescents’ performance on the Iowa Gambling Task: Implications for the development of decision making and ventromedial prefrontal cortex. Developmental Psychology. 2004;40:1148–1158. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Hu Z, Cooper M, Crockett DP, Zhou R. Differentiation of the midbrain dopaminergic pathways during mouse development. The Journal of Comparative Neurology. 2004;476:301–311. doi: 10.1002/cne.20230. [DOI] [PubMed] [Google Scholar]
- Huotari M, Gogos J, Karayiorgou M, Koponen O, Forsberg M, Raasmaja A, Hyttinen J, Mannisto P. Brain catecholamine metabolism in catechol-O-methyltransferase (COMT)-deficient mice. The European Journal of Neuroscience. 2002;15:246–256. doi: 10.1046/j.0953-816x.2001.01856.x. [DOI] [PubMed] [Google Scholar]
- Irwin I, DeLanney LE, McNeil T, Chan P, Forno LS, Murphy GM, Jr., et al. Aging and the nigrostriatal dopamine system: a non-human primate study. Neurodegeneration. 1994;3:251–265. [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114(Pt 5):2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Jones EG. Laminar distribution of cortical efferent cells. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol. 1. Plenum Press; New York: 1984. pp. 521–553. [Google Scholar]
- Jung AB, Bennett JP., Jr. Development of striatal dopaminergic function. I. Pre- and postnatal development of mRNAs and binding sites for striatal D1 (D1a) and D2 (D2a) receptors. Developmental Brain Research. 1996;94:109–120. doi: 10.1016/0165-3806(96)00033-8. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, Olsson H, et al. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiology of Aging. 2000;21(5):683–688. doi: 10.1016/s0197-4580(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Fiedler C, Hunsaker C, III, Sparks DL. Synaptic neurochemistry of human striatum during development: changes in sudden infant death syndrome. Journal of Neurochemistry. 1993;60:2098–2105. doi: 10.1111/j.1471-4159.1993.tb03494.x. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Matthijssen MAH, Uylings HBM. Morphometric analysis of prefrontal and cortical development following neonatal lesioning of the dopaminergic mesocortical projection. Experimental Brain Research. 1989;78:279–289. doi: 10.1007/BF00228899. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HBM. Development of the dopaminergic innervation in the prefrontal cortex of the rat. The Journal of Comparative Neurology. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Kehr W, Lindqvist M, Carlsson A. Distribution of dopamine in the rat cerebral cortex. Journal of Neurological Transmission. 1976;38:173–180. doi: 10.1007/BF01249437. [DOI] [PubMed] [Google Scholar]
- Kerr A, Zelazo PD. Development of “hot” executive function: The children’s gambling task. Brain and Cognition. 2004;55:148–157. doi: 10.1016/S0278-2626(03)00275-6. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Life-time prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of Geeral. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Anderson CM, Ely TD, Nishita JK. Absence of synthesis-modulating nerve terminal autoreceptors on mesoamygdaloid and other mesolimbic neuronal populations. The Journal of Neuroscience. 1987;7:3961–3975. doi: 10.1523/JNEUROSCI.07-12-03961.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, et al. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25(2):493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski C, Weinshilboum R. Human catechol O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Krimer LS, Goldman-Rakic PS. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkeys. The Journal of Neuroscience. 2000;20:8780–8787. doi: 10.1523/JNEUROSCI.20-23-08780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankford KL, DeMollo FG, Klein WL. D1-type dopamine receptors inhibit growth cone motility in cultured retina neurons: evidence that neurotransmitters act as morphogenic growth regulators in the developing nervous system. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:4567–4571. doi: 10.1073/pnas.85.12.4567-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain & Cognition. 2010 doi: 10.1016/j.bandc.2009.10.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie CA, Robertson MW, Cutler AJ, Bennett JP., Jr. Postnatal development of D1 dopamine receptors in the medial prefrontal cortex, striatum, and nucleus accumbens of normal and neonatal 6-hydroxydopamine treated rats: A quantitative autoradiographic analysis. Developmental Brain Research. 1991;62:109–114. doi: 10.1016/0165-3806(91)90195-o. [DOI] [PubMed] [Google Scholar]
- Levin HS, Culhane KA, Hartman J, Evankovich K, Mattson AJ, Harward H, Ringholz G, Ewing-Cobbs, Fletcher JM. Developmental changes in performance on tests of purported frontal lobe functioning. Developmental Neuropsychology. 1991;7(3):377–395. [Google Scholar]
- Levitt P, Rakic P. The time of genesis, embryonic origin and differentiation of the brain stem monoamine neurons in the rhesus monkey. Developmental Brain Research. 1982;4:35–57. doi: 10.1016/0165-3806(82)90095-5. [DOI] [PubMed] [Google Scholar]
- Lewis D, Hayes T, Lund J, Oeth K. Dopamine and the neural circuitry of primate prefrontal cortex: implications for schizophrenia research. Neuropsychopharmacology. 1992;6:127–134. [PubMed] [Google Scholar]
- Lewis DA, Melchiztky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: Regional, laminar and ultrastructural localization. Journal of Comparative Neurology. 2001;432:119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- Li TQ, Noseworthy MD. Mapping the development of white matter tracts with diffusion tensor imaging. Dev Sci. 2002;5(3):293–300. [Google Scholar]
- Lidow MS. D1- and D2 dopaminergic receptors in the developing cerebral cortex of macaque monkey: A film autoradiographic study. Neuroscience. 1995;65:439–452. doi: 10.1016/0306-4522(94)00475-k. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Rakic P. Synchronized overproduction of neurotransmitter receptors in diverse regions of the primate cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:10218–10221. doi: 10.1073/pnas.88.22.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS, Rakic P. Scheduling of monoaminergic neurotransmitter receptor expression in the primate neocortex during postnatal development. Cerebral Cortex. 1992;2:401–416. doi: 10.1093/cercor/2.5.401. [DOI] [PubMed] [Google Scholar]
- Lieb K, Andersen C, Lazarov N, Zienecker R, Urban I, Reisert I, Pilgrim C. Pre- and postnatal development of dopaminergic neuron numbers in the male and female mouse midbrain. Developmental Brain Research. 1996;94:37–43. doi: 10.1016/0165-3806(96)00063-6. [DOI] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16(4):553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Liu M, Hong CJ, Liou Y, Tsai Y, Hsieh C, Tsai J. Association study of a functional catechol-O-methyltransferase polymorphism and executive function in elderly males without dementia. Neuroscience Letters. 2008;436(2):193–5. doi: 10.1016/j.neulet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melén K, Julkunen I, Taskinen J. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: A revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF. Dopaminergic modulation of working memory for spatial but not object cues in normal humans. Journal of Cognitive Neuroscience. 1997;9:330–347. doi: 10.1162/jocn.1997.9.3.330. [DOI] [PubMed] [Google Scholar]
- Luciana M, Collins PF, Olson EA, Schissel AM. Tower of London performance in healthy adolescents: The development of planning skills and associations with self-reported inattention and impulsivity. Developmental Neuropsychology. 2009;34(4):461–475. doi: 10.1080/87565640902964540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005;76:697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Luciana M, Hanson KL, Whitley CB. A preliminary report on dopamine system reactivity in PKU: Acute effects of haloperidol on neuropsychological, physiological and neuroendocrine functions. Psychopharmacology. 2004;175(1):18–25. doi: 10.1007/s00213-004-1775-0. [DOI] [PubMed] [Google Scholar]
- Luciana M, Nelson CA. Assessment of neuropsychological function in children using the Cambridge Neuropsychological Testing Automated Battery (CANTAB): Performance in 4 to 12 year-olds. Developmental Neuropsychology. 2002;22(3):595–623. doi: 10.1207/S15326942DN2203_3. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Woods RP, Rex DE, Jancke L, et al. Mapping cortical gray matter in the young adult brain: effects of gender. Neuroimage. 2005;26(2):493–501. doi: 10.1016/j.neuroimage.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain & Cognition. 2010 doi: 10.1016/j.bandc.2009.08.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström K, Salminen M, Jalanko A, Savolainen R, Ulmanen I. Cloning and characterization of human placental catechol-o-methyltransferase cDNA. DNA and Cell Biology. 1991;10:181–189. doi: 10.1089/dna.1991.10.181. [DOI] [PubMed] [Google Scholar]
- Madras BK, Fahey MA, Canfield DR, Spealman RD. D1 and D2 dopamine receptors in caudate-putamen of nonhuman primates (Macaca fascicularis) Journal of Neurochemistry. 1988;51:934–943. doi: 10.1111/j.1471-4159.1988.tb01830.x. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. American Journal of Psychiatry. 2002;159:652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(10):6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, Carter CS. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biological Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- McGeer EG, Gibson S, Wada JA, McGeer PL. Distribution of tyrosine hydroxylase activity in adult and developing brain. Canadian Journal of Biochemistry. 1967;45:1943–1952. doi: 10.1139/o67-227. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Enzymes associated with the metabolism of catecholamines, acetylcholine, and GABA in human controls and patients with Parkinson’s disease and Huntington’s chorea. Journal of Neurochemistry. 1976;26:65–76. [PubMed] [Google Scholar]
- Meador-Woodruff JH, Mansour A, Grandy DK, Damask SP, Civelli O, Watson SJ., Jr. Distribution of D5 dopamine receptor mRNA in rat brain. Neuroscience Letters. 1992;145:209–212. doi: 10.1016/0304-3940(92)90024-2. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Mansour A, Healy DJ, Kuehn R, Zhou QY, Bunzow JR, et al. Comparison of the distributions of D1 and D2 dopamine receptor mRNAs in rat brain. Neuropsychopharmacology. 1991;5(4):231–242. [PubMed] [Google Scholar]
- Meng SZ, Ozawa Y, Itoh M, Takashima S. Developmental and age-related changes of dopamine transporter, and dopamine D1 and D2 receptors in human basal ganglia. Brain Research. 1999;843:136–144. doi: 10.1016/s0006-8993(99)01933-2. [DOI] [PubMed] [Google Scholar]
- Mills S, Langley K, Van den Bree M, Street E, Turic D, Owen MJ, et al. No evidence of association between Catechol-O-Methyltransferase (COMT) Val158Met genotype and performance on neuropsychological tasks in children with ADHD: a case-control study. BMC Psychiatry. 2004;4:15. doi: 10.1186/1471-244X-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Rüther E, Huether G. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Developmental Brain Research. 2000;119:251–257. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- Montague DM, Lawler CP, Mailman RB, Gilmore JH. Developmental regulation of the dopamine D1 receptor in human caudate and putamen. Neuropsychopharmacology. 1999;21:641–649. doi: 10.1016/S0893-133X(99)00062-7. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: Anatomy and physiology of the dopamine systems. Annual Review of Neuroscience. 1978;1:129–169. doi: 10.1146/annurev.ne.01.030178.001021. [DOI] [PubMed] [Google Scholar]
- Muetzel RL, Collins PF, Mueller BA, A MS, Lim KO, Luciana M. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. Neuroimage. 2008;39(4):1918–1925. doi: 10.1016/j.neuroimage.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafó MR, Bowes L, Clark TG, Flint J. Lack of association of the COMT (Val158/108Met) gene and schizophrenia: a meta-analysis of case-control studies. Molecular Psychiatry. 2005;10:765–770. doi: 10.1038/sj.mp.4001664. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Yalcin B, Willis-Owen SA, Flint J. Association of the dopamine D4 receptor (DRD4) gene and approach-related personality traits: Meta-analysis and new data. Biological Psychiatry. 2008;63(2):197–206. doi: 10.1016/j.biopsych.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Zeng W. Ontogeny of dopamine D1 receptors in rat forebrain: a quantitative autoradiographic study. Developmental Brain Research. 1990;57:7–13. doi: 10.1016/0165-3806(90)90178-2. [DOI] [PubMed] [Google Scholar]
- Nagal IE, Chicherio C, Li S, von Oertzen T, Sander T, Villringer A, et al. Human aging modifies genetic effects on executive functioning and working memory. Frontiers in Human Neuroscience. 2008;2(1) doi: 10.3389/neuro.09.001.2008. doi: 10.3389/neuro.09.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Napolitano A, Cesura AM, Da Prada M. The role of monoamine oxidase and catechol O-methyltransferase in dopaminergic neurotransmission. Journal of Neural Transmission. 1995;45:35–45. [PubMed] [Google Scholar]
- Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum Mutat. 2004;23:540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- Noble EP. D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. American Journal of Medical Genetics. 2003;116B(1):103–125. doi: 10.1002/ajmg.b.10005. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Naitoh F, Segawa T. Regional changes in monoamine content and uptake of the rat brain during postnatal development. Brain Research. 1976;101:305–315. doi: 10.1016/0006-8993(76)90271-7. [DOI] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Research. 1987;434(2):117–65. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain Research Cognitive Brain Research. 2003;18(1):48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in adolescents: a diffusion tensor imaging study. Journal of Cognitive Neuroscience. 2008 doi: 10.1162/jocn.2009.21107. Posted on-line 3 September 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman WH. Sex differences in early childhood, adolescence, and adulthood on cognitive tasks that rely on orbital prefrontal cortex. Brain and Cognition. 2004;55:134–147. doi: 10.1016/S0278-2626(03)00279-3. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, Chen J. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. Journal of Neuroscience. 2008;28(35):8709–23. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez H, Ismahan G, Parvez S, Youdim MBH. Developmental changes in the activity catechol-O-methyltransferase in rat and rabbit fetuses. Journal of Neural Transmission. 1979;44(12):65–75. doi: 10.1007/BF01252702. [DOI] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: Myelin or axon? Brain & Cognition. 2010 doi: 10.1016/j.bandc.2009.06.002. in press. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54(3):255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J, Brandeis L, Goldstein M. Appearance of tyrosine hydroxylase immunoreactivity in human embryo. Developmental Neuroscience. 1980;3:140–150. doi: 10.1159/000112387. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Kaas JH. Human brain evolution. In: Zigmund MJ, Bloom FE, Landis SC, Roberts JL, Squire LR, editors. Fundamentals of Neuroscience. Academic Press; San Diego, CA: 1999. pp. 1283–1311. [Google Scholar]
- Qiu D, Tan LH, Zhou K, Khong PL. Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage. 2008;41(2):223–232. doi: 10.1016/j.neuroimage.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Rao PA, Molinoff PB, Joyce JN. Ontogeny of dopamine D1 and D2 receptor subtypes in rat basal ganglia: a quantitative autoradiographic study. Developmental Brain Research. 1991;60:161–177. doi: 10.1016/0165-3806(91)90045-k. [DOI] [PubMed] [Google Scholar]
- Reuter M, Peters K, Schroeter K, Koebke W, Lenardon D, Bloch B, Hennig J. The influence of the dopaminergic system on cognitive functioning: A molecular genetic approach. Behavioural Brain Research. 2005;164:93–99. doi: 10.1016/j.bbr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Reuter M, Schmitz A, Corr P, Hennig J. Molecular genetics support Gray’s personality theory: the interaction of COMT and DRD2 polymorphisms predict the behavioural approach system. The International Journal of Neuropsychopharmacology. 2006;9(2):155–166. doi: 10.1017/S1461145705005419. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van der Molen MW. Mental resources, processing speed, and inhibitory control: a developmental perspective. Biological Psychology. 1997;45(13):241–261. doi: 10.1016/s0301-0511(96)05230-1. [DOI] [PubMed] [Google Scholar]
- Roffman JL, Weiss AP, Deckersbach T, Freudenreich O, Henderson DC, Wong DH, Halsted CH, Goff DC. Interactive effects of COMT Val108/158Met and MTHFR C677T on executive function in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(6):990–5. doi: 10.1002/ajmg.b.30684. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: A tyrosine hydroxylase immunohistochemical study. Biological Psychiatry. 1994;36:272–277. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: A tyrosine hydroxylase immunohistochemical analysis. The Journal of Comparative Neurology. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Sabol Z, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Human Genetics. 1998;103(3):273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Sales N, Martres MP, Bouthenet ML, Schwartz JC. Ontogeny of dopaminergic D-2 receptors in the rat nervous system: characterization and detailed autoradiographic mapping with [125]iodosulpride. Neuroscience. 1989;28:673–700. doi: 10.1016/0306-4522(89)90014-6. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Matsumura M, Kubota K. Dopamine enhances the neuronal activity related to a delayed response task in monkey prefrontal cortex. Journal of Neurophysiology. 1990;63:1401–1412. doi: 10.1152/jn.1990.63.6.1401. [DOI] [PubMed] [Google Scholar]
- Scambler PJ. The 22q11 syndromes. Human Molecular Genetics. 2000;9(16):2421–2426. doi: 10.1093/hmg/9.16.2421. [DOI] [PubMed] [Google Scholar]
- Schambra UB, Duncan GE, Breese GR, Fornaretto MG, Caron MG, Fremeau RT., Jr. Ontogeny of D1A and D2 dopamine receptor subtypes in rat brain using in situ hybridization and receptor binding. Neuroscience. 1994;62:65–85. doi: 10.1016/0306-4522(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: A diffusion tensor MRI study. Human Brain Mapping. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Yuan WY. White matter development during adolescence as shown by diffusion MRI. Brain & Cognition. 2010 doi: 10.1016/j.bandc.2009.06.005. in press. [DOI] [PubMed] [Google Scholar]
- Schneider JF, Il’yasov KA, Hennig J, Martin E. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology. 2004;46(4):258–266. doi: 10.1007/s00234-003-1154-2. [DOI] [PubMed] [Google Scholar]
- Schultz W, Preuschoff K, Camerer C, Hsu M, Fiorillo CD, Tobler PN, et al. Explicit neural signals reflecting reward uncertainty. Philos Trans R Soc Lond B Biol Sci. 2008;363:3801–3811. doi: 10.1098/rstb.2008.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. Selective impairment on a delayed radial arm task following local administration of a selective D1, but not a D2, antagonist into the prefrontal cortex. Abstracts of the Society for Neuroscience. 1995;21:1942. [Google Scholar]
- Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, et al. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Lundgren KH, Tray TMD, Guthrie KM, Isackson PJ, Gall CM. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophins-3 mRNAs. The Journal of Comparative Neurology. 1994;342:321–334. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- Sesak SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. The Journal of Neuroscience. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashi V, Keshavan MS, Howard TD, Berry MN, Baseshore MJ, Lewandowski E, Kwapil TR. Cognitive correlates of a functional COMT polymorphism in children with 22q11.2 deletion syndrome. Clinical Genetics. 2006;69(3):234–238. doi: 10.1111/j.1399-0004.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shifman S, Bronstein M, Sternfeld M, Pisante-Shalom A, Lev-Lehman E, Weizman A, et al. A highly significant association between a COMT haplotype and schizophrenia. American Journal of Human Genetics. 2002;71:1296–1302. doi: 10.1086/344514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H, Scatton B, LeMoal M. Dopaminergic A10 neurons are involved in cognitive functions. Nature. 1980;286:150–151. doi: 10.1038/286150a0. [DOI] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26(4):1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Snook L, Plewes C, Beaulieu C. Voxel based versus region of interest analysis in diffusion tensor imaging of neurodevelopment. Neuroimage. 2007;34(1):243–252. doi: 10.1016/j.neuroimage.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical changes across the lifespan. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spear LP. Neurodevelopment during adolescence. In: Cicchetti D, Walker EF, editors. Neurodevelopmental Mechanisms in Psychopathology. Cambridge University Press; Cambridge: 2003. pp. 62–83. [Google Scholar]
- Stamford JA. Development and aging of the rat nigrostriatal system dopamine system studied with fast cyclic voltammetry. Journal of Neurochemistry. 1989;52:1582–1589. doi: 10.1111/j.1471-4159.1989.tb09212.x. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, McElligot S, Lu L, McGonigle P. Ontogeny of dopamine D3 receptors in the nucleus accumbens of the rat. Neuroscience Letters. 1997;223:13–16. doi: 10.1016/s0304-3940(97)13396-1. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Cauffman E, Woolard J, Graham S, Banich M. Are adolescents less mature than adults? Minors’ access to abortion, the juvenile death penalty, and the alleged APA“flip-flop”. American Psychologist. 2009;64(7):583–594. doi: 10.1037/a0014763. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Development. 2009;80:28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ. Effects of dopamine-related gene-gene interactions on working memory component processes. European Journal of Neuroscience. 2009;29:1056–1063. doi: 10.1111/j.1460-9568.2009.06647.x. [DOI] [PubMed] [Google Scholar]
- Suhara T, Fukuda H, Inoue O, Itoh T, Suzuki K, Yamasaki T, Tateno Y. Age-related changes in human D1 dopamine receptors measured by positive emission tomography. Psychopharmacology. 1991;103(1):41–45. doi: 10.1007/BF02244071. [DOI] [PubMed] [Google Scholar]
- Taerk E, Grizenko N, Ben Amor L, Lageix P, Mbekou V, Deguzman R, et al. Catechol-O-methyltransferase (COMT) Val108/158 Met polymorphism does not modulate executive function in children with ADHD. BMC Medical Genetics. 2004;5:30. doi: 10.1186/1471-2350-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HY, Chen Q, Sust S, Buckholtz JW, Meyers JD, Egan MF, et al. Epistasis between catechol-O-methyltransferase and type II metabotropic glutamate receptor 3 genes on working memory brain function. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(30):12536–12541. doi: 10.1073/pnas.0610125104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam SY, Roth RH. Mesoprefrontal dopaminergic neurons: Can tyrosine availability influence their function? Biochemical Pharmacology. 1997;53(4):441–453. doi: 10.1016/s0006-2952(96)00774-5. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss A. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D1, D2, and D4 receptors in rat forebrain. International Journal of Developmental Neuroscience. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine and serotonin transporters in rat caudate-putamen and nucleus accumbens septi. Neuroscience Letters. 1998a;254:21–24. doi: 10.1016/s0304-3940(98)00644-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D4-like receptors in rat forebrain regions: comparisons with D2-like receptors. Developmental Brain Research. 1998b;110:227–233. doi: 10.1016/s0165-3806(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Developmental Neuroscience. 1999;21:43–49. doi: 10.1159/000017365. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Developmental Brain Research. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Barber NI, Gelbard HA, Gallitano AL, Campbell A, Marsh E, Baldessarini RJ. Developmental differences in acute nigrostriatal and mesocorticolimbic system response to haloperidol. Neuropsychopharmacology. 1993;9:147–156. doi: 10.1038/npp.1993.53. [DOI] [PubMed] [Google Scholar]
- Teitelman G, Baker H, Joh TH, Reis DJ. Appearance of catecholamine-synthesizing enzymes during development of rat sympathetic nervous system: Possible role of tissue environment. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:509–513. doi: 10.1073/pnas.76.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biological Psychiatry. 2001;49(4):309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Sowell ER, Gogtay N, Giedd JN, Vidal CN, Hayashi KM, et al. Structural MRI and brain development. Int Rev Neurobiol. 2005;67:285–323. doi: 10.1016/S0074-7742(05)67009-2. [DOI] [PubMed] [Google Scholar]
- Thurley K, Senn W, Luscher HR. Dopamine increases the gain of the input-output of the rat prefrontal pyramidal neurons. Journal of Neurophysiology. 2008;99:2985–2997. doi: 10.1152/jn.01098.2007. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Meyer M, Barde Y. Neurotrophins are required for nerve growth during development. Nature Neuroscience. 2001;4:29–37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. COMT inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. The Journal of Neuroscience. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biological Psychiatry. 2007;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Weickert CS, Kleinman JE, Herman MM, Chen J, Kolachana BS, et al. Catechol-o-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cerebral Cortex. 2007;17(5):1206–1212. doi: 10.1093/cercor/bhl032. [DOI] [PubMed] [Google Scholar]
- Ungethüm U, Chen Y, Gross J, Bjelke B, Bolme P, Eneroth P, et al. Effects of perinatal asphyxia on the mesocortical/mesolimbic dopamine system of neonatal and 4-week-old male rats. Experimental Brain Research. 1996;112:403–410. doi: 10.1007/BF00227946. [DOI] [PubMed] [Google Scholar]
- Unis AS, Roberson MD, Robinette R, Ha J, Dora DM. Ontogeny of human brain dopamine receptors. I. Differential expression of [3H]-SCH23390 and [3H]-YM09151-2 specific binding. Developmental Brain Research. 1998;106:109–117. doi: 10.1016/s0165-3806(97)00202-2. [DOI] [PubMed] [Google Scholar]
- Uylings HBM, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behavioural Brain Resesarch. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SARB, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2009 doi: 10.1093/cercor/bhp078. Epub ahead of print: doi:10.1093. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, Uhl GR. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Verhaegen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood: Estimates of linear and nonlinear age effects and structural models. Psychological Bulletin. 1997;122:231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- Verney C, Milosevic A, Alvarez C, Berger B. Immunocytochemical evidence of well-developed dopaminergic and noradrenergic innervations in the frontal cerebral cortex of human fetuses at midgestation. Journal of Comparative Neurology. 1993;336:331–344. doi: 10.1002/cne.903360303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Logan J, Schlyer D, MacGregor R, et al. Decreased dopamine transporters with age in healthy human subjects. Annals of Neurology. 1994;36(2):237–239. doi: 10.1002/ana.410360218. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Logan J, Fowler JS, Wang GJ, Gur RC, Wong C, et al. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. American Journal of Psychiatry. 2000;157:175–180. doi: 10.1176/ajp.157.1.75. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Ding YS, Gur RC, Gatley J, et al. Parellel loss of presynaptic and postsynaptic dopamine markers in normal aging. Annals of Neurology. 2004;44(1):143–147. doi: 10.1002/ana.410440125. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. The Journal of Neuroscience. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P, Jorritsma-Byham B, Van Dijk C, Buijs RM. The dopaminergic innervation of the ventral striatum in the rat: A light- and electron-microscopical study with antibodies against dopamine. The Journal of Comparative Neurology. 1986;251:84–99. doi: 10.1002/cne.902510106. [DOI] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Wahlstrom D, Benolkin K, White T, Luciana M. Affective bias and response modulation following tyrosine depletion in healthy adults. Neuropsychopharmacology. 2006;31:2523–2536. doi: 10.1038/sj.npp.1301172. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, Collins PF, White T, Luciana M. Developmental Changes in Dopamine Neurotransmission in Adolescence: Behavioral Implications and Issues in Assessment. Brain & Cognition. 2010 doi: 10.1016/j.bandc.2009.10.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Hooper CJ, Vrshek-Schallhorn S, Oetting WS, Brott MJ, Luciana M. Association of the Catechol-O-methyltransferase (COMT) gene to prefrontally-mediated cognitions in adolescents. Biological Psychiatry. 2007;61(5):626–632. doi: 10.1016/j.biopsych.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Webster MJ, Gondipalli P, Rothmond D, Fatula RJ, Herman MM, et al. Postnatal alterations in dopaminergic markers in the human prefrontal cortex. Neuroscience. 2006;144:1109–1119. doi: 10.1016/j.neuroscience.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: Catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annual Review of Pharmacology and Toxicology. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Raymond FA. Inheritance of low erythrocyte catechol-methyltransferase activity in man. American Journal of Human Genetics. 1977;29:125–135. [PMC free article] [PubMed] [Google Scholar]
- Welsh MC, Pennington BF, Groisser DB. A normative-developmental study of executive function: a window on prefrontal function in children. Developmental Neuropsychology. 1991;7(2):131–149. [Google Scholar]
- Westerink BHC. Sequence and significant of dopamine metabolism in the rat brain. Neurochemistry International. 1985;7:221–227. doi: 10.1016/0197-0186(85)90108-1. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Blockade of dopamine D1 receptors enhances memory fields of prefrontal neurons in primate cerebral cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Yang YK, Chiu NT, Chen CC, Chen M, Yeh TL, Lee IH. Correlation between fine motor activity and striatal dopamine D2 receptor density in patients with schizophrenia and healthy controls. Psychiatry Research: Neuroimaging. 2003;123:191–197. doi: 10.1016/s0925-4927(03)00066-0. [DOI] [PubMed] [Google Scholar]
- Zappia M, Manna I, Serra P, Cittadella R, Andreoli V, La Russa A, et al. Increased risk for Alzheimer disease with the interaction of MPO and A2M polymorphisms. Archives of Neurology. 2004;61:341–344. doi: 10.1001/archneur.61.3.341. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Rakic P. Synaptogenesis in monkey somatosensory cortex. Cerebral Cortex. 1991;1:510–523. doi: 10.1093/cercor/1.6.510. [DOI] [PubMed] [Google Scholar]
- Zhang K, Zheng Z, Gao X, Li J, Zhang F. Possible relationship between the COMT gene ValMet polymorphism and psychometric IQ in girls of the Qinba region in China. Neuropsychobiology. 2007;56:98–103. doi: 10.1159/000112950. [DOI] [PubMed] [Google Scholar]
- Zhang L, Thomas KM, Davidson MC, Casey BJ, Heier LA, Ulug AM. MR quantitation of volume and diffusion changes in the developing brain. AJNR Am J Neuroradiol. 2005;26(1):45–49. [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Grimsby J, Chen K, Shih JC. Promoter organization and activity of human monoamine oxidase (MAO) A and B genes. The Journal of Neuroscience. 1992;12:4437–4446. doi: 10.1523/JNEUROSCI.12-11-04437.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]