Abstract
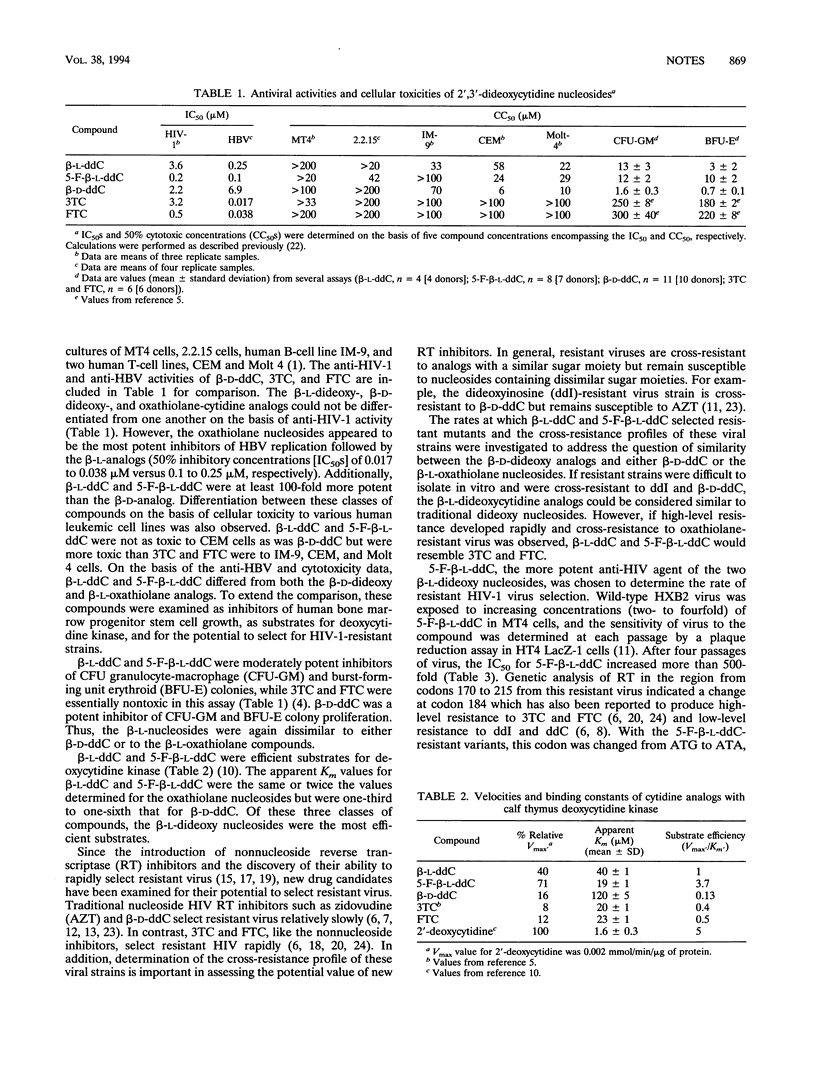
beta-L-2',3'-Dideoxycytidine (beta-L-ddC) and beta-L-5-fluoro-2',3'-dideoxycytidine (5-F-beta-L-ddC) were prepared and shown to have potent activity against human immunodeficiency virus type 1 (HIV-1) and hepatitis B virus (HBV). These compounds were compared with beta-D-2',3'-dideoxycytidine (beta-D-ddC) and two beta-L-oxathiolane nucleosides (beta-L-3'-thio-2',3'-dideoxycytidine and beta-L-5-fluoro-3'-thio-2',3'-dideoxycytidine) in terms of anti-HIV and anti-HBV activity, cytotoxicity, and development of HIV-1 resistance. Compared with beta-D-ddC, the beta-L-dideoxycytidine nucleosides had similar anti-HIV-1 activities, significantly greater anti-HBV activities, and decreased toxicities to a B-cell line, T-cell lines, and human bone marrow progenitor cells. HIV-1 strains resistant to beta-D-ddC were susceptible to the beta-L-ddC analogs. Compared with the beta-L-oxathiolane nucleosides, beta-L-ddC and 5-F-beta-L-ddC had similar anti-HIV-1 activities, decreased anti-HBV activities, and greater toxicities to B- and T-cell lines and bone marrow progenitor cells. There were similarities between the beta-L-ddC and beta-L-oxathiolane nucleosides in the rate of development and pattern of resistant HIV-1 selection. While the in vitro activity and cytotoxicity profiles of the beta-L-ddC nucleosides differed from those of the beta-D-ddC and beta-L-oxathiolane nucleosides, the data presented herein suggest that the sugar configuration of a dideoxynucleoside analog may play a major role in the rate of development and the pattern of HIV-1 resistance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averett D. R. Anti-HIV compound assessment by two novel high capacity assays. J Virol Methods. 1989 Mar;23(3):263–276. doi: 10.1016/0166-0934(89)90159-6. [DOI] [PubMed] [Google Scholar]
- Coates J. A., Cammack N., Jenkinson H. J., Mutton I. M., Pearson B. A., Storer R., Cameron J. M., Penn C. R. The separated enantiomers of 2'-deoxy-3'-thiacytidine (BCH 189) both inhibit human immunodeficiency virus replication in vitro. Antimicrob Agents Chemother. 1992 Jan;36(1):202–205. doi: 10.1128/aac.36.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doong S. L., Tsai C. H., Schinazi R. F., Liotta D. C., Cheng Y. C. Inhibition of the replication of hepatitis B virus in vitro by 2',3'-dideoxy-3'-thiacytidine and related analogues. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8495–8499. doi: 10.1073/pnas.88.19.8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornsife R. E., St Clair M. H., Huang A. T., Panella T. J., Koszalka G. W., Burns C. L., Averett D. R. Anti-human immunodeficiency virus synergism by zidovudine (3'-azidothymidine) and didanosine (dideoxyinosine) contrasts with their additive inhibition of normal human marrow progenitor cells. Antimicrob Agents Chemother. 1991 Feb;35(2):322–328. doi: 10.1128/aac.35.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., Davis M., Liotta D. C., Paff M., Frick L. W., Nelson D. J., Dornsife R. E., Wurster J. A., Wilson L. J., Fyfe J. A. The anti-hepatitis B virus activities, cytotoxicities, and anabolic profiles of the (-) and (+) enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992 Dec;36(12):2686–2692. doi: 10.1128/aac.36.12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q., Gu Z. X., Parniak M. A., Li X. G., Wainberg M. A. In vitro selection of variants of human immunodeficiency virus type 1 resistant to 3'-azido-3'-deoxythymidine and 2',3'-dideoxyinosine. J Virol. 1992 Jan;66(1):12–19. doi: 10.1128/jvi.66.1.12-19.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q., Gu Z., Parniak M. A., Cameron J., Cammack N., Boucher C., Wainberg M. A. The same mutation that encodes low-level human immunodeficiency virus type 1 resistance to 2',3'-dideoxyinosine and 2',3'-dideoxycytidine confers high-level resistance to the (-) enantiomer of 2',3'-dideoxy-3'-thiacytidine. Antimicrob Agents Chemother. 1993 Jun;37(6):1390–1392. doi: 10.1128/aac.37.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z., Gao Q., Li X., Parniak M. A., Wainberg M. A. Novel mutation in the human immunodeficiency virus type 1 reverse transcriptase gene that encodes cross-resistance to 2',3'-dideoxyinosine and 2',3'-dideoxycytidine. J Virol. 1992 Dec;66(12):7128–7135. doi: 10.1128/jvi.66.12.7128-7135.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R. W., Johnson L. C., Averett D. R. High-capacity in vitro assessment of anti-hepatitis B virus compound selectivity by a virion-specific polymerase chain reaction assay. Antimicrob Agents Chemother. 1993 Mar;37(3):441–447. doi: 10.1128/aac.37.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenitsky T. A., Tuttle J. V., Koszalka G. W., Chen I. S., Beacham L. M., 3rd, Rideout J. L., Elion G. B. Deoxycytidine kinase from calf thymus. Substrate and inhibitor specificity. J Biol Chem. 1976 Jul 10;251(13):4055–4061. [PubMed] [Google Scholar]
- Larder B. A., Chesebro B., Richman D. D. Susceptibilities of zidovudine-susceptible and -resistant human immunodeficiency virus isolates to antiviral agents determined by using a quantitative plaque reduction assay. Antimicrob Agents Chemother. 1990 Mar;34(3):436–441. doi: 10.1128/aac.34.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Coates K. E., Kemp S. D. Zidovudine-resistant human immunodeficiency virus selected by passage in cell culture. J Virol. 1991 Oct;65(10):5232–5236. doi: 10.1128/jvi.65.10.5232-5236.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Darby G., Richman D. D. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989 Mar 31;243(4899):1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- Mellors J. W., Dutschman G. E., Im G. J., Tramontano E., Winkler S. R., Cheng Y. C. In vitro selection and molecular characterization of human immunodeficiency virus-1 resistant to non-nucleoside inhibitors of reverse transcriptase. Mol Pharmacol. 1992 Mar;41(3):446–451. [PubMed] [Google Scholar]
- Mitsuya H., Broder S. Inhibition of the in vitro infectivity and cytopathic effect of human T-lymphotrophic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) by 2',3'-dideoxynucleosides. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1911–1915. doi: 10.1073/pnas.83.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunberg J. H., Schleif W. A., Boots E. J., O'Brien J. A., Quintero J. C., Hoffman J. M., Emini E. A., Goldman M. E. Viral resistance to human immunodeficiency virus type 1-specific pyridinone reverse transcriptase inhibitors. J Virol. 1991 Sep;65(9):4887–4892. doi: 10.1128/jvi.65.9.4887-4892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman D. D. HIV drug resistance. AIDS Res Hum Retroviruses. 1992 Jun;8(6):1065–1071. doi: 10.1089/aid.1992.8.1065. [DOI] [PubMed] [Google Scholar]
- Richman D., Shih C. K., Lowy I., Rose J., Prodanovich P., Goff S., Griffin J. Human immunodeficiency virus type 1 mutants resistant to nonnucleoside inhibitors of reverse transcriptase arise in tissue culture. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11241–11245. doi: 10.1073/pnas.88.24.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinazi R. F., Lloyd R. M., Jr, Nguyen M. H., Cannon D. L., McMillan A., Ilksoy N., Chu C. K., Liotta D. C., Bazmi H. Z., Mellors J. W. Characterization of human immunodeficiency viruses resistant to oxathiolane-cytosine nucleosides. Antimicrob Agents Chemother. 1993 Apr;37(4):875–881. doi: 10.1128/aac.37.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinazi R. F., McMillan A., Cannon D., Mathis R., Lloyd R. M., Peck A., Sommadossi J. P., St Clair M., Wilson J., Furman P. A. Selective inhibition of human immunodeficiency viruses by racemates and enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992 Nov;36(11):2423–2431. doi: 10.1128/aac.36.11.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T., Stonehuerner J. G., Biron K. K., Averett D. R. Ribonucleotide reductase induced by varicella zoster virus. Characterization, and potentiation of acyclovir by its inhibition. Biochem Pharmacol. 1987 Dec 15;36(24):4341–4346. doi: 10.1016/0006-2952(87)90682-4. [DOI] [PubMed] [Google Scholar]
- St Clair M. H., Martin J. L., Tudor-Williams G., Bach M. C., Vavro C. L., King D. M., Kellam P., Kemp S. D., Larder B. A. Resistance to ddI and sensitivity to AZT induced by a mutation in HIV-1 reverse transcriptase. Science. 1991 Sep 27;253(5027):1557–1559. doi: 10.1126/science.1716788. [DOI] [PubMed] [Google Scholar]
- Tisdale M., Kemp S. D., Parry N. R., Larder B. A. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3'-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5653–5656. doi: 10.1073/pnas.90.12.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]



