Abstract
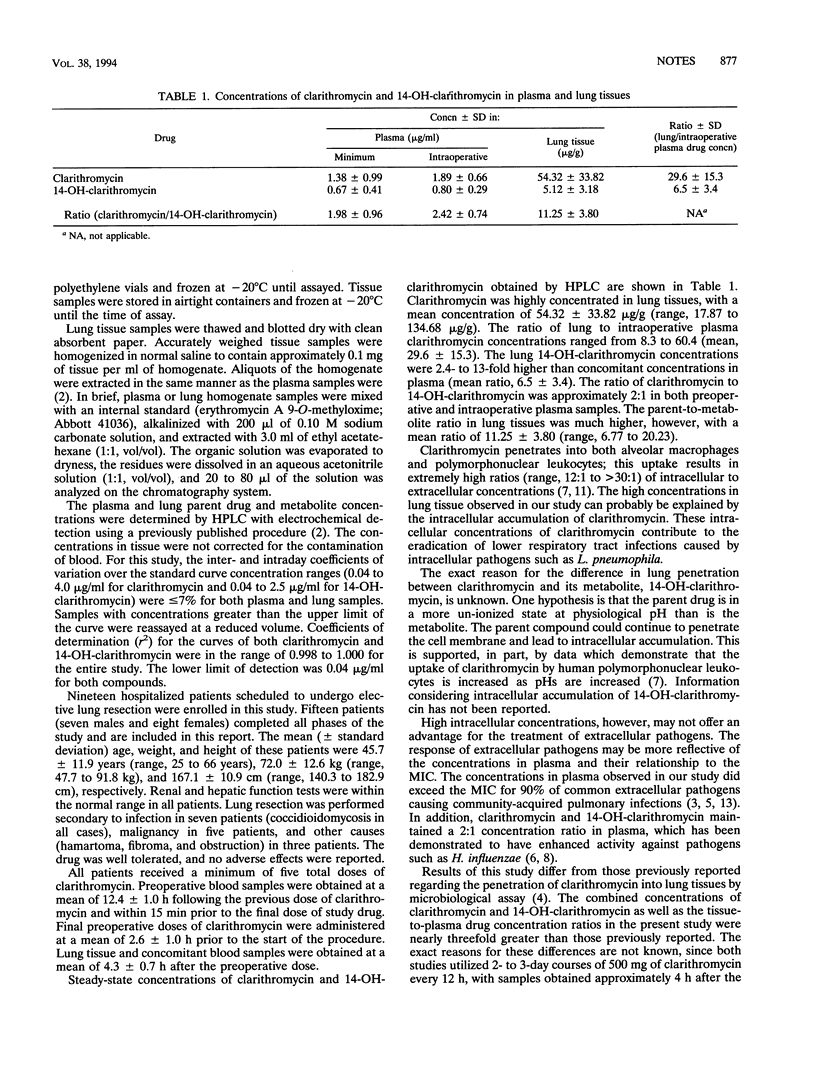
The concentrations of clarithromycin and its active principal metabolite, 14-(R)-hydroxy-clarithromycin, were determined in lung tissue obtained during lung resection and compared with concomitant concentrations in plasma. Concentrations of the parent and metabolite were determined by high-performance liquid chromatography. The 15 patients studied were given 500 mg orally every 12 h for a minimum of five doses to achieve steady-state concentrations. The mean concentrations of clarithromycin and 14-(R)-hydroxy-clarithromycin in plasma just prior to the final dose were 1.38 and 0.67 micrograms/ml, respectively, and those 4 h after the final dose (at the time of lung resection) were 1.89 and 0.80 microgram/mL, respectively. The concentrations of the parent and metabolite in lung tissue at the time of lung resection averaged 54.3 and 5.12 micrograms/g, respectively, with a mean calculated ratio of concentrations of the parent to metabolite being 11.3 in lung tissue and 2.4 in plasma. Clarithromycin and its active metabolite are extensively distributed into human lung tissue.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chin N. X., Neu N. M., Labthavikul P., Saha G., Neu H. C. Activity of A-56268 compared with that of erythromycin and other oral agents against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1987 Mar;31(3):463–466. doi: 10.1128/aac.31.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S. Y., Sennello L. T., Sonders R. C. Simultaneous determination of clarithromycin and 14(R)-hydroxyclarithromycin in plasma and urine using high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1991 Nov 15;571(1-2):199–208. doi: 10.1016/0378-4347(91)80446-j. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Bailer R., Swanson R., Hanson C. W., McDonald E., Ramer N., Hardy D., Shipkowitz N., Bower R. R., Gade E. In vitro and in vivo evaluation of A-56268 (TE-031), a new macrolide. Antimicrob Agents Chemother. 1986 Dec;30(6):865–873. doi: 10.1128/aac.30.6.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraschini F., Scaglione F., Pintucci G., Maccarinelli G., Dugnani S., Demartini G. The diffusion of clarithromycin and roxithromycin into nasal mucosa, tonsil and lung in humans. J Antimicrob Chemother. 1991 Feb;27 (Suppl A):61–65. doi: 10.1093/jac/27.suppl_a.61. [DOI] [PubMed] [Google Scholar]
- Hardy D. J., Hensey D. M., Beyer J. M., Vojtko C., McDonald E. J., Fernandes P. B. Comparative in vitro activities of new 14-, 15-, and 16-membered macrolides. Antimicrob Agents Chemother. 1988 Nov;32(11):1710–1719. doi: 10.1128/aac.32.11.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D. J., Swanson R. N., Rode R. A., Marsh K., Shipkowitz N. L., Clement J. J. Enhancement of the in vitro and in vivo activities of clarithromycin against Haemophilus influenzae by 14-hydroxy-clarithromycin, its major metabolite in humans. Antimicrob Agents Chemother. 1990 Jul;34(7):1407–1413. doi: 10.1128/aac.34.7.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro M., Koga H., Kohno S., Hayashi T., Yamaguchi K., Hirota M. Penetration of macrolides into human polymorphonuclear leucocytes. J Antimicrob Chemother. 1989 Nov;24(5):719–729. doi: 10.1093/jac/24.5.719. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. H., Maher L. A., Howell A. W. Activity of clarithromycin and its principal human metabolite against Haemophilus influenzae. Antimicrob Agents Chemother. 1991 Jul;35(7):1524–1526. doi: 10.1128/aac.35.7.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirst H. A., Sides G. D. New directions for macrolide antibiotics: pharmacokinetics and clinical efficacy. Antimicrob Agents Chemother. 1989 Sep;33(9):1419–1422. doi: 10.1128/aac.33.9.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirst H. A., Sides G. D. New directions for macrolide antibiotics: structural modifications and in vitro activity. Antimicrob Agents Chemother. 1989 Sep;33(9):1413–1418. doi: 10.1128/aac.33.9.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno S., Koga H., Yamaguchi K., Masaki M., Inoue Y., Dotsu Y., Masuyama Y., Hayashi T., Hirota M., Saito A. A new macrolide, TE-031 (A-56268), in treatment of experimental Legionnaires' disease. J Antimicrob Chemother. 1989 Sep;24(3):397–405. doi: 10.1093/jac/24.3.397. [DOI] [PubMed] [Google Scholar]
- Periti P., Mazzei T., Mini E., Novelli A. Clinical pharmacokinetic properties of the macrolide antibiotics. Effects of age and various pathophysiological states (Part I). Clin Pharmacokinet. 1989 Apr;16(4):193–214. doi: 10.2165/00003088-198916040-00001. [DOI] [PubMed] [Google Scholar]
- Piscitelli S. C., Danziger L. H., Rodvold K. A. Clarithromycin and azithromycin: new macrolide antibiotics. Clin Pharm. 1992 Feb;11(2):137–152. [PubMed] [Google Scholar]



