Abstract
Adenovirus is an important respiratory pathogen. Adenovirus fiber from most serotypes co-opts the Coxsackie-Adenovirus Receptor (CAR) to bind and enter cells. However, CAR is a cell adhesion molecule localized on the basolateral membrane of polarized epithelia. Separation from the lumen of the airways by tight junctions renders airway epithelia resistant to inhaled adenovirus infection. Although a role for CAR in viral spread and egress has been established, the mechanism of initial respiratory infection remains controversial. CAR exists in several protein isoforms including two transmembrane isoforms that differ only at the carboxy-terminus (CAREx7 and CAREx8). We found low-level expression of the CAREx8 isoform in well-differentiated human airway epithelia. Surprisingly, in contrast to CAREx7, CAREx8 localizes to the apical membrane of epithelia where it augments adenovirus infection. Interestingly, despite sharing a similar class of PDZ-binding domain with CAREx7, CAREx8 differentially interacts with PICK1, PSD-95, and MAGI-1b. MAGI-1b appears to stoichiometrically regulate the degradation of CAREx8 providing a potential mechanism for the apical localization of CAREx8 in airway epithelial. In summary, apical localization of CAREx8 may be responsible for initiation of respiratory adenoviral infections and this localization appears to be regulated by interactions with PDZ-domain containing proteins.
Introduction
The Coxsackievirus and Adenovirus Receptor (CAR) plays a vital role in cell adhesion and viral infection [1]–[3]. The importance of CAR within epithelial junctions, where it behaves as an adhesion protein interacting with and potentially modulating the trafficking of key PDZ domain containing molecules, is becoming evident [4]–[7]. In contrast, how adenovirus initiates infection of the airway epithelium and whether CAR plays a role in initial adenoviral attachment and infection, when it is sequestered on the basolateral side of airway epithelia, remains unclear [8].
Alternative splicing plays an important role in eukaryotes. During pre-mRNA splicing, the spliceosome cleaves intron sequences, and joins exons together, forming an mRNA. Regulation of the spliceosome can result in alternative splicing of mRNA, determining which exons are present or absent in template mRNA. Alternative splicing not only regulates protein expression, but also allows multiple proteins to be expressed from the same gene resulting in significant proteomic diversity [9]. Alternatively spliced proteins may maintain similar activity, differing only in localization or interactions, or may vary widely in activity or regulation. It is estimated that alternative splicing occurs in 70–80% of human genes, but is more common in regulatory genes, and tissues with diverse cell types [10].
CAR is encoded by a highly conserved, alternatively spliced gene with five described transcripts. Three alternative transcripts encode CAR, lacking the transmembrane domain, yielding a soluble extracellular domain (splicing between exons 4/7, 3/7, 2/7) [11]. In experimental murine models, soluble CAR is able to inhibit viral infection but also results in toxicity [12]–[16]. Although the mechanism of toxicity is unknown, soluble CAR may be predicted to alter CAR-CAR interactions and thus epithelial cell adhesion [17].
Human CAR was first described by Bergelson et al as a 7 exon protein [1]. In contrast with other species, mouse CAR (mCAR) was initially cloned as a protein composed of 8 exons [18] and was named mCAR1. The 7 exon mouse form was subsequently identified and termed mCAR2. A detailed analysis of protein expression and localization in mice has revealed differential tissue-dependent expression and localization for the exon 7 and exon 8 isoforms [19], [20]. This suggests that the interactions and potentially the functional importance of these two isoforms may be distinct. Furthermore, considering the emerging importance of signal transduction originating from microdomains within the cell membrane, these two isoforms would be predicted to differentially regulate cellular biology.
The splicing event to create the 8th exon form occurs within the 7th exon. Thus, these two isoforms contain identical extracellular and transmembrane domains, which predicts identical adenovirus binding and serotype preference. The majority of the cytoplasmic domain is identical except for the last 26 (CAREx7) or 13 (CAREx8) amino acids. Although comprised of distinct sequences, the last 4 amino acids of both isoforms encode class I PSD95/DlgA/ZO-1 (PDZ) binding domain sequences (-X-(S/T)-X-Ф, where X = any amino acid and Ф = any hydrophobic amino acid). Interacting PDZ-domain-containing proteins for human CAREx7 include MAGI-1b, PICK1, PSD-95, MUPP-1, LNX1, and ZO-1 [5]–[7], [21]. Furthermore, murine CAREx7 and CAREx8 interact with LNX2 and this interaction appears to be modulated by both the PDZ binding domain of each isoform as well as an upstream sequence common to both isoforms [22].
In contrast with the murine isoform, the protein for human CAREx8 has never been described. We used a computational approach to identify human exon 8 encoding CAR, and investigated the functional significance of this isoform in primary human airway epithelia.
Results
Human CAR exon 8 containing isoform
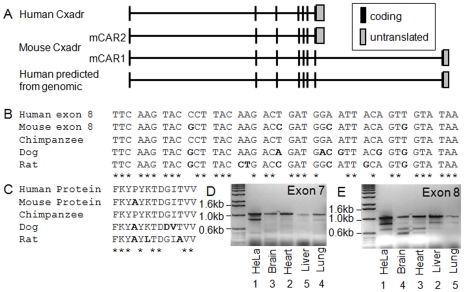
Mouse CAR was originally cloned as an 8 exon protein. We hypothesized that human CAR may exist as an 8 exon protein as well (Figure 1A). We first determined whether the human genome contained homologous sequence for exon 8, by performing a BLAT search against the mouse sequence (www.genome.ucsc.edu). Similar sequences were identified for human, chimpanzee, dog and rat (Figure 1B). The predicted amino acid sequence for human and chimpanzee is identical. This sequence differs from mouse by one amino acid. Comparison of hCAR P343 to mCAR A343 using Conseq software identified this amino acid as an exposed or buried residue respectively with a non-structural role. Conservation could not be determined due to insufficient data. The score assigned by Conseq was validated using PolyPhen (http://www.bork.embl-heidelberg.de/polyPhen/), which assigns scores, derived from likelihood matrices, as “benign”, “possibly damaging”, “probably damaging”, or “unknown”. The change P343A was designated as “benign.” Although an intuitive difference between these two amino acids exists, based on these analyses, additional studies to determine whether protein function was affected were not pursued. Primers were designed for RT-PCR to evaluate the presence of hCAREx8 in several human cell lines including HeLa, 293, A549, Caco-2, and primary human airway epithelia. Transcripts for both hCAREx7 and hCAREx8 were detected in all cell lines and primary cells examined (data not shown). A panel of human RNA was subsequently screened for the full-length hCAREx7 (Figure 1D) or hCAREx8 (Figure 1E), and yielded full length as well as smaller bands for both transcripts. Semi-quantitative analysis of the bands for hCAREx7 suggested that heart>brain∼lung>liver. In contrast, hCAREx8 transcripts showed a rank order of liver∼heart>brain>lung. These data suggest that as in mice, human splice variant expression varies between organs.
Figure 1. Human CAR is an 8 exon alternatively spliced protein.
Panel A shows a schematic diagram of the published and predicted human and mouse exon arrangement. Panel B shows the alignment of the mouse CAR exon 8 with the predicted exon 8 from other species. Panel C and D show representative RT-PCR for human CAR Exon 8 or 7, respectively, in cells (HeLa) or tissues.
Localization and function of hCAREx8 is similar to hCAREx7 in non-polarized cells
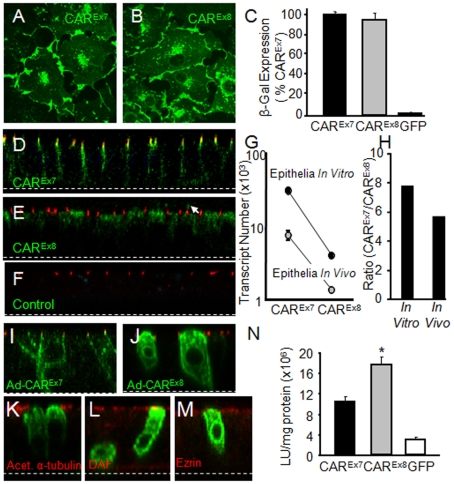
COS-7 cells were transfected with the cDNA for hCAREx7 (Figure 2A) or hCAREx8 (Figure 2B). Immuno-localization using the FLAG antibody showed a similar distribution for both with predominant junctional staining in addition to some perinuclear staining. CAR-negative CHO cells transiently expressing hCAREx7, hCAREx8, or GFP were infected with recombinant adenovirus containing the bacterial LacZ gene (Figure 2C). As previously described, CHO cells and CHO cells expressing GFP are refractory to adenovirus-mediated gene transfer [23]. In contrast, cells expressing hCAREx7 or hCAREx8 showed robust and similar levels of adenovirus-mediated gene transfer proving that both forms of CAR function as adenoviral receptors.
Figure 2. Human CAREx8 localization and adenovirus-mediated gene transfer is similar to hCAREx7 in cell monolayers but distinct in polarized cells.
COS-7 cells transfected with hCAREx7 (A) or hCAREx8 (B) show a similar distribution. Panel C shows that CHO cells transfected with hCAREx7 or hCAREx8 mediate similar adenovirus gene transfer. Immunocytochemistry for endogenous hCAREx7 (D) or hCAREx8 (E) (green) reveals distinct localization in polarized primary human airway epithelia greater than 2 weeks of age. hCAREx7 localizes to the basolateral membrane and shows co-localization with the basolateral portion of ZO-1 (red, D, E, F). hCAREx8 localizes diffusely in the upper region of the cytoplasm with some apical staining (see arrowhead). Panel F shows ZO-1 (red) and a lack of staining with control rabbit pre-immune serum (green). Panel G shows the abundance of CAREx7 or CAREx8 transcripts in primary cultures (in vitro) or from lung tissue (in vivo). Panel H shows that the relative enrichment of CAREx7 to CAREx8 transcripts is similar in vitro and in vivo. Localization of hCAREx7 (I) or hCAREx8 (J, K, L, M) after over-expression in primary cultures and co-stained (red) for ZO-1 (I, J), acetylated α-tubulin (K), CD55/decay accelerating factor (DAF, L), or ezrin (M). Panel N shows that expression of exogenous hCAREx8 in polarized human airway epithelia mediates two-fold greater adenovirus gene transfer than hCAREx7 in comparison to control GFP transduced cells. *p<0.01. Confocal microscopy (60x oil immersion).
Localization of hCAREx8 is distinct from hCAREx7 in polarized cells
We hypothesized that hCAREx8 localization may differ from hCAREx7 in polarized cells. Primary well-differentiated human airway epithelia, greater than two weeks of age, were stained for endogenous hCAREx7 (Figure 2D, green), hCAREx8 (Figure 2E, green) with antibodies raised to peptides composed of the last 13 amino acids of either hCAREx7 or hCAREx8, or pre-immune serum control (Figure 2F, green), and were co-stained for the tight junction protein ZO-1 (red). Distinct patterns of localization were observed for these two isoforms. As previously shown, hCAREx7 localizes to the tight and adherens junctions of airway epithelia [23]. In contrast, hCAREx8 localizes primarily to the upper region of the cytoplasm and apical surface above ZO-1 (Figure 2E, arrow), without ZO-1 tight junction overlap. In contrast to ZO-1, co-localization was observed when co-stained with the apical protein ezrin (Figure S1). To determine the relative abundance of the two isoforms in polarized airway epithelia (in vitro) or lung tissue (in vivo), RNA was extracted from human airway epithelia greater than two weeks of age or total lung tissue, respectively, and subjected to quantitative RT-PCR (see Figure S2 for primer specificity). Despite donor variability, hCAREx8 levels were consistently markedly lower than hCAREx7 both in cultures and tissues (Figure 2G, Figure S2) with a similar ratio of hCAREx7 to hCAREx8 both in vitro and in vivo (Figure 2H). To confirm the distinct localization of these isoforms, airway epithelia, greater than two weeks of age, were transduced from the basolateral side with adenovirus containing the cDNA for hCAREx7 or hCAREx8, and subjected to immunocytochemistry 36 hours later. Recombinant expression levels are markedly higher than endogenous levels, thus confocal microscopy settings are set at a level that does not detect endogenous. Nevertheless, the localization of recombinant hCAR was similar to that seen with endogenous isoforms. Whereas the majority of hCAREx7 localized to the basolateral membrane (Figure 2I), hCAREx8 was largely diffusely distributed throughout the cell but was also present at the apical membrane where it appeared above ZO-1 (Figure 2J), below the cilia, marked by acetylated α-tubulin (Figure 2K), and overlapped at the same level with apical membrane markers decay accelerating factor (DAF, Figure 2L) and ezrin (Figure 2M) [24]–[26]. We hypothesized that endogenous hCAREx8 may be responsible for the inefficient, albeit detectable, level of adenovirus infection from the apical surface of airway epithelia. To determine if augmenting expression would augment adenovirus infection, dissociated airway epithelia were transduced with hCAREx7, hCAREx8 or GFP and seeded on semi-permeable filters. Cultures were allowed to polarize and form an epithelium over 1 week. When the resistance of all cultures was above 300 mΩ•cm−2, cells were infected from the apical surface with adenovirus containing the LacZ gene (Figure 2N). Human airway epithelia expressing GFP showed baseline low level adenovirus-mediated gene transfer. Epithelia expressing hCAREx8 showed approximately 5-fold greater gene transfer than epithelia expressing GFP (Figure 2N) or mock transduced (Figure S3) and close to a 100% increase in gene transfer compared to epithelia expressing hCAREx7 (Figure 2N) This increase in infection is similar to previously published results for glycophosphatidylinositol-linked hCAR which is missing the transmembrane and cytoplasmic domains and localizes explicitly to the apical surface of polarized airway epithelia [27]. This suggests that hCAREx8 explains the low-level baseline apical adenovirus infection and that there may be a maximal amount of infection possible through apically localized receptor. These data also raise the question why hCAREx8 does not localize to the basolateral surface.
hCAREx8 interacts with PSD-95 but not PICK1 via the PDZ binding domain
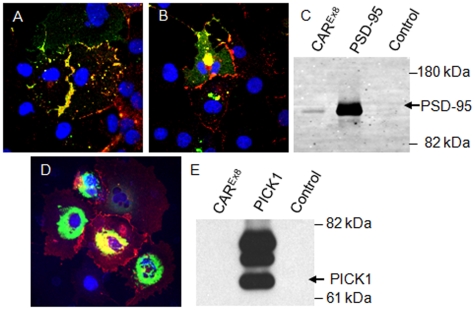
PDZ-interactions may modulate localization as well as function. It is known that both the sequence of the binding domain and the upstream sequences affect PDZ domain interactions. The mechanism by which the upstream sequences affect the specificity of interaction remains unclear. Although hCAREx7 and hCAREx8 have type 1 PDZ binding domains (X(S/T)XΦ) at the C-terminus (hCAREx7 –GSIV; hCAREx8 –ITVV), these and the upstream sequences are distinct. Thus we hypothesized that PDZ-interactions may be responsible for altered localization in human airway epithelia. We have previously shown that hCAREx7 interacts with PICK1 and PSD-95 via a PDZ binding domain specific interaction such that co-localization by immunocytochemistry reveals hCAREx7 is able to pull these proteins out of the cytoplasm and co-localize at the junctions between cells. We further hypothesized that hCAREx8 may interact with some hCAREx7 partners. COS-7 cells were co-transfected with hCAREx8 and PSD-95-GFP and immunocytochemistry was performed to determine localization. hCAREx8 localized to the junctions as observed in Figure 2B. In the absence of hCAREx8, PSD-95-GFP localizes diffusely within the cytoplasm [6]. In the presence of hCAREx8, the localization of PSD-95-GFP was altered to co-localize with hCAREx8 at the junctions of cells (Figure 3A). Localization of a PDZ mutant form of hCAREx8 (hCAREx8-PDZ) was similar to full length hCAREx8 (Figure 3B) and was unable to alter the diffuse localization of PSD-95-GFP upon co-transfection. Whereas an interaction with full length hCAREx8 was evident by co-immunoprecipitation (Figure 3C), no interaction was observed by coimmunoprecipitation with hCAREx8-PDZ, indicating that this interaction requires the ITVV PDZ binding domain sequence. Next, hCAREx8 and PICK1-GFP were co-transfected into COS-7 cells. Immunocytochemistry for hCAREx8 revealed the lack of interaction between PICK1-GFP and CAREx8 at the junctions where the majority of hCAREx8 was localized (Figure 3D). In some cells there was co-localization in the perinuclear region. To determine if there was a physical interaction, each protein was immunoprecipitated and evaluated by Western blot (Figure 3E). No interaction was detected. Taken together, these data suggest that either there is no interaction or the interaction is too weak to pull PICK1-GFP to the intercellular junctions. Co-localization of hCAREx8 and PICK1-GFP in the perinuclear region may represent an artifact of high protein expression or alternatively PICK1-related retention of CAREx8.
Figure 3. hCAREx8 co-localizes and interacts with PSD-95 but not PICK1.
Panel A shows co-localization (yellow) of hCAREx8 (red) and PSD-95-GFP (green). In contrast, in panel B, hCAREx8-PDZ does not co-localize at the junctions of cells. hCAREx8-PDZ localizes to the junctions between cells whereas PSD-95-GFP fluorescence remains diffuse. Panel C shows immunoprecipitation of PSD-95-GFP with the hCAR specific extracellular domain monoclonal antibody RmcB, GFP antibody, but not a control antibody (MopC). Panels D and E shows the lack of co-localization and immunoprecipitation between hCAREx8 (junctional) and PICK1-GFP (perinuclear). Confocal microscopy (60x oil immersion).
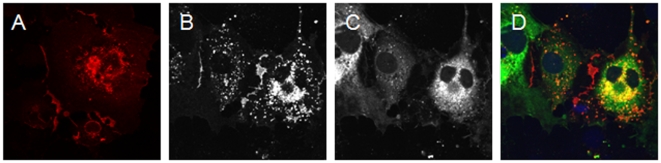
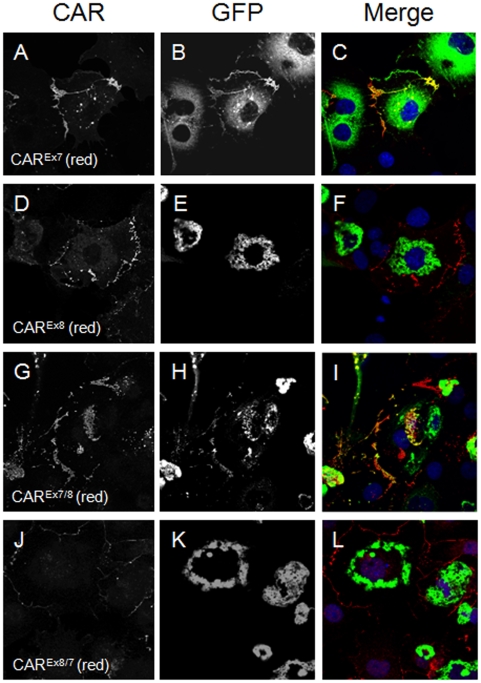
hCAREx8 interaction with MAGI-1b-GFP results in hCAREx8 degradation
hCAREx8 differentially interacts with hCAREx7 PDZ-mediated interacting partners despite having a similar class of PDZ binding domain. We have previously shown an interaction between hCAREx7 and MAGI-1b-GFP (Figure 4A-C). To investigate the interaction between hCAREx8 and MAGI-1b-GFP, COS-7 cells were co-transfected and evaluated by immunocytochemistry. Surprisingly, little to no hCAREx8 staining (Figure 4D) was present in cells expressing MAGI-1b-GFP (Figure 4E). Most of the small amount of hCAREx8 present within MAGI-1b-GFP positive cells appeared within vesicular structures and not the junctions (Figure 4F). The presence, localization and amount of hCAREx8 appeared to be dependent on the amount of MAGI-1b-GFP. A small percentage of cells appeared to express hCAREx8 alone and the expression was robust in comparison to neighboring MAGI-1b-GFP expressing cells (Figure 4G-I).This was not the case for PSD-95-GFP, PICK1-GFP, or GFP (Figure 4G-I). hCAREx8 was detectable by Western blot, presumably due to the relatively few cells transfected with hCAREx8 but not MAGI-1b-GFP. No interaction between hCAREx8 and MAGI-1b-GFP was observed by co-immunoprecipitation even when cells were treated with proteosomal inhibitors (data not shown). These data suggest that a transient interaction between MAGI-1b and hCAREx8 results in the disappearance of hCAREx8.
Figure 4. Co-expression of hCAREx8 and MAGI-1b-GFP results in the loss of hCAREx8.
In contrast to the co-localization of hCAREx7 (A, red) and MAGI-1b-GFP (B, green) as shown in panel C (yellow), co-expression of hCAREx8 (D, G, red) and MAGI-1b-GFP (E, H, green) results in decreased levels of hCAREx8 (F) unless MAGI-1b-GFP is absent from the cell (I). Co-expression of hCAREx8 (J, red) with GFP (K, green) results in abundant hCAREx8 expression at the junctions of the cells and diffuse GFP expression (L). Confocal microscopy (60x oil immersion).
Quantitation of the stoichiometric hCAREx8 interaction with MAGI-1b-GFP
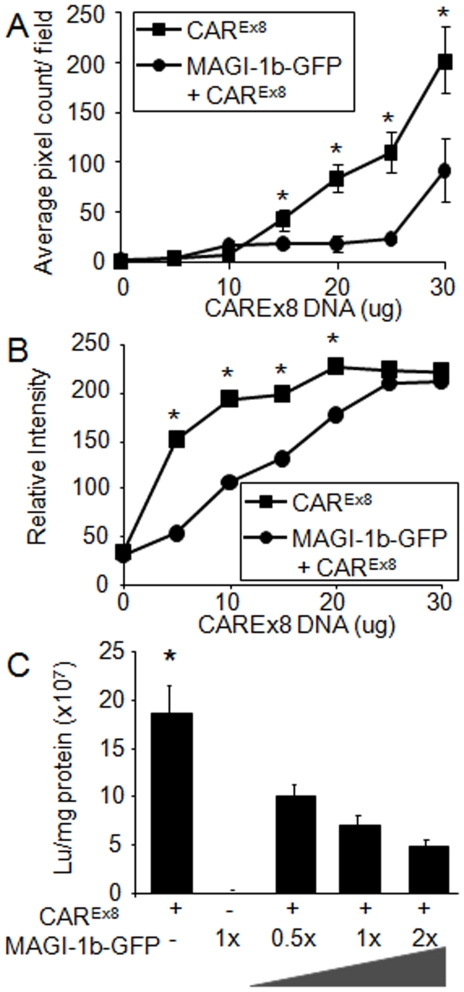
Since some MAGI-1b-GFP expressing cells also expressed some, albeit mislocalized, hCAREx8, we hypothesized that there would be a stoichiometric relationship between these proteins. COS-7 cells were electroporated with a dose response of hCAREx8 (0-30 µg plasmid DNA) in the presence of constant (15 µg plasmid) MAGI-1b-GFP or GFP. hCAREx8 expression was evaluated by immunocytochemistry (red fluorescence) using fluorescence microscopy and Western blot. Fluorescence intensity was calculated as average pixel count per field of view (n = 6, Figure 5A). Increasing the amount of hCAREx8 plasmid, in the presence of constant GFP plasmid, resulted in a relatively linear increase in hCAR fluorescence (Figure 5A). In contrast, when hCAREx8 was co-transfected with a constant amount of MAGI-1b-GFP, the dose response curve was shifted significantly to the right, indicating that hCAREx8 expression was suppressed in the presence of MAGI-1b-GFP relative to co-expression with GFP. Western blot using the hCAREx8 specific antibody was quantitated by chemiluminescence imaging, relative to β-actin protein expression (Figure 5B), and revealed a similar relationship. Although a rapid increase in hCAREx8 protein in the presence of GFP was observed, the sensitivity of detection was apparent by the plateau of the curve. The dose response curve for hCAREx8, when co-transfected with MAGI-1b-GFP, was again shifted significantly to the right. The observed difference in hCAREx8 protein was not due to transfection or transcription since quantitative PCR for plasmid and RT-PCR for hCAREx8 RNA were similar (data not shown). Moreover, no effect was observed on GFP or MAGI-1b-GFP fluorescence upon increasing hCAREx8 (data not shown).
Figure 5. Co-expression of hCAREx8 with MAGI-1b-GFP results in less immunofluorescence, protein, and adenovirus-mediated gene transfer.
COS-7 cells were transfected with 0 to 30 µg of hCAREx8 +/− 15 µg MAGI-1b-GFP and evaluated for hCAREx8 specific immunofluorescence (A) or CAREx8 specific protein by Western blot (B). In the presence of MAGI-1b-GFP, the hCAREx8 expression curve is shifted to the right suggesting a loss of hCAREx8 protein. Panel C shows CHO cells transfected with varying amounts of hCAREx8 and MAGI-1b-GFP, and evaluated for Ad-β-galactosidase gene transfer. Co-expression of MAGI-1b-GFP resulted in a decrease of adenovirus-mediated gene transfer in a dose response relationship. *p<0.03.
MAGI-1b-GFP interaction with CAREx8 decreases adenovirus infection
To determine a physiological response to the loss of hCAREx8 in the presence of MAGI-1b-GFP, CHO-K1 cells were transfected with hCAREx8 or MAGI-1b-GFP, or co-transfected with hCAREx8, at a constant dose, with MAGI-1b-GFP at increasing doses. All transfections were balanced with GFP plasmid to maintain equal amounts of DNA. Transfected cells were transduced with Ad-β-gal 48h later. β-galactosidase expression was determined 24 hr post-transduction (Figure 5C). Similar to Figure 2C, transfection of CHO-K1 cells with hCAREx8 renders them susceptible to adenovirus infection while transfection with MAGI-1b-GFP does not. Co-transfection of hCAREx8 with a dose response of MAGI-1b-GFP resulted in a dose-related reduction of susceptibility to Ad-βgal-mediated gene expression, indicating that there was a reduction of cell surface hCAREx8 available as a receptor. Taken together with the previous data, we concluded that in contrast to hCAREx7, co-expression of hCAREx8 with MAGI-1b-GFP results in the disappearance of hCAREx8 and may explain the absence of hCAREx8 in the adherens junctions of airway epithelia.
The hCAREx8-MAGI-1b-GFP interaction requires the hCAREx8 PDZ binding domain
The interaction between the hCAREx7 isoform with MAGI-1b-GFP requires the CAREx7 PDZ binding domain (-GSIV). To determine the requirement of the hCAREx8 PDZ binding domain, a stop codon was added to the cDNA of hCAREx8 by site-directed mutagenesis resulting in a protein missing the last 4 amino acids (-ITVV). Similar to wild-type hCAREx8 (Figure 2B), COS-7 cells transfected with hCAREx8-PDZ showed robust junctional localization with some protein within intracellular vesicles (Figure 6A). Co-transfection of hCAREx8-PDZ with MAGI-1b-GFP resulted in co-expression of both proteins and did not alter the junctional localization of hCAREx8-PDZ (Figure 6B) or show diffuse localization of MAGI-1b-GFP (Figure 6C). These data indicate that the PDZ binding domain is required for the disappearance of hCAREx8. Thus, an interaction between hCAREx8 and MAGI-1b is required, raising the question of how this interaction differs from hCAREx7 and MAGI-1b.
Figure 6. MAGI-1b-GFP-mediated loss of hCAREx8 requires the PDZ-binding domain (ITVV) of hCAREx8.
COS-7 cells were transfected with hCAREx8-PDZ (A, red) or co-transfected with hCAREx8-PDZ (B, red) and MAGI-1b-GFP (C, green). Panel D shows the lack of co-localization of hCAREx8-PDZ (junctions) and MAGI-1b-GFP (cytoplasmic/diffuse). Confocal microscopy (60x oil immersion).
The upstream sequence plays a role in PDZ interactions
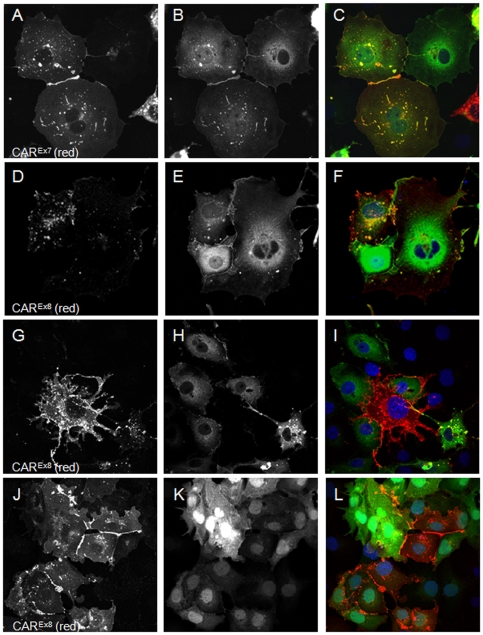
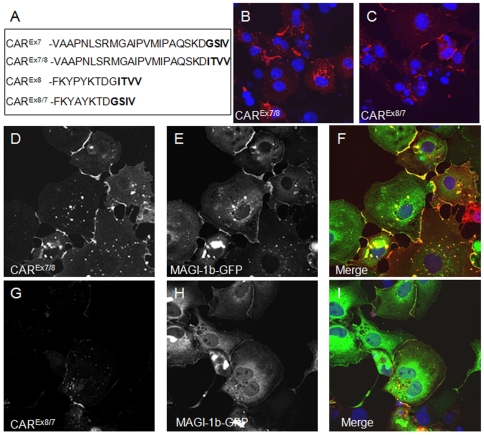
We have previously shown a PDZ dependent interaction between hCAREx7 and PICK1-GFP [6]. Figure 3D demonstrates that there is no co-localization, and Figure 3E, shows there is no co-immunoprecipitation when hCAREx8 and PICK1-GFP are co-expressed. hCAREx7 and hCAREx8 differ by only the terminal 26 or 13 amino acids respectively. Since both hCAREx7 and hCAREx8 have type I PDZ binding domains we asked whether the PICK1 interaction was dependent on the terminal 4 amino acid sequence or the unique upstream sequences. To further define the role these sequences play in the PDZ domain-PDZ binding domain interaction, the hCAREx7 PDZ binding domain was swapped with the hCAREx8 PDZ binding domain (i.e. 22aa of hCAREx7 followed by ITVV, hCAREx7/8) or the hCAREx8 was swapped with hCAREx7 (i.e. 9aa of hCAREx8 followed by GSIV, hCAREx8/7). hCAREx7, hCAREx8, hCAREx7/8, or hCAREx8/7 were each co-transfected with PICK1-GFP. As previously shown, co-transfection of COS-7 cells with hCAREx7 and PICK1-GFP results in accumulation of hCAREx7 at the junctions of cells (Figure 7A) and hCAREx7 is able to pull PICK1-GFP (Figure 7B) from a perinuclear localization to the junctions of cells (Figure 7C). In contrast, as also demonstrated in Figure 3D, co-transfection of COS-7 cells with hCAREx8 and PICK1-GFP results in accumulation of hCAREx8 at the junctions of cells (Figure 7D). However, PICK1-GFP (Figure 7E) remains in a perinuclear localization (Figure 7F). Transfection of either hCAREx7/8 (Figure 7G) or hCAREx8/7 (Figure 7H) alone resulted in junctional localization. Interestingly, co-transfection of COS-7 cells with hCAREx7/8 and PICK1-GFP results in the co-localization of hCAREx7/8 (Figure 7I) and PICK1-GFP (Figure 7J) at the junctions of cells (Figure 7K). This suggests that the sequence ITVV is able to interact with PICK1-GFP but not in the context of the upstream hCAREx8 unique sequence. Finally, co-transfection of COS-7 cells with hCAREx8/7 and PICK1-GFP results in accumulation of hCAREx8/7 at the junctions of cells (Figure 8L), while PICK1-GFP (Figure 8M) remains in a perinuclear localization in a manner similar to wild type hCAREx8 (Figure 8N). These data indicate that the upstream sequence plays a significant role in the specificity of the PDZ domain-PDZ binding domain interaction and thus interactions must be defined experimentally.
Figure 7. The result of the MAGI-1b-GFP interaction with hCAR requires both the PDZ binding domain and the upstream isoform specific amino acids.
The PDZ binding domain of hCAREx7 and hCAREx8 were swapped by PCR as shown in panel A. Both constructs contained identical upstream sequences. The localization was determined in transfected COS-7 cells either alone, hCAREx7/8 (B), hCAREx8/7 (C) or upon co-transfection with MAGI-1b-GFP (D-I). Panels D and E show junctional expression of hCAREx7/8 and MAGI-1b-GFP, respectively, and co-localization in panel F. Panel G shows minor expression of hCAREx8/7 in the presence of robust MAGI-1b-GFP expression in panel H. Some co-localization is observed in panel I. Confocal microscopy (60x oil immersion).
Figure 8. The interaction between the hCAR PDZ binding domain and PICK1 depends on both the PDZ binding domain and the upstream isoform specific amino acids.
The PDZ binding domain of hCAREx7 and hCAREx8 were swapped by PCR as shown in Figure 7, panel A. The localization was determined in transfected COS-7 cells. hCAREx7 (A) transfected with PICK1-GFP (B) results in co-localization at the junctions (C, yellow). hCAREx8 (D) transfected with PICK1-GFP (E) results in no co-localization at the junctions (F). hCAREx7/8 (G) transfected with PICK1-GFP (H) results some co-localization at the junctions (I, yellow). hCAREx8/7 (J) transfected with PICK1-GFP (K) results in no co-localization at the junctions (L). Confocal microscopy (60x oil immersion).
hCAREx8 PDZ binding domain and upstream nine amino acids define the MAGI-1 interaction
The above data indicates that sequence immediately upstream of the PDZ binding domain may define the PDZ interaction. The difference between the respective interactions of hCAREx7 or hCAREx8 with MAGI-1b-GFP may also be due to interactions with the upstream 22 or 9 amino acids, the 4 terminal PDZ binding domain amino acids or a combination of both. Co-transfection of hCAREx7/8 with MAGI-1b-GFP resulted in the relocation and co-localization of MAGI-1b-GFP with hCAREx7/8 at the junctions of cells (Figure 8A-C). Interestingly, co-transfection of hCAREx8/7 with MAGI-1b-GFP revealed an intermediate phenotype (Figure 8D-F). Although there were depressed levels of hCAREx8/7 within the population of transfected cells (Figure 8D), some cells showed low levels of hCAREx8/7 expression at the junctions and low levels of co-localization with MAGI-1b-GFP at these junctions. Taken together with the results in Figure 7, these data suggest that although the PDZ binding domain is required for the interaction, both the exact sequence of the binding domain and upstream sequences may modulate the interactions of PDZ domain containing proteins.
Discussion
Adenoviruses are among the most studied viruses from the viral biology, cell biology and pathogenesis perspective. However, the failure of these viruses in applications for airway gene therapy and the identification of a basolaterally localized receptor, hCAR, made it clear that apical infection is inefficient. We and others found that once an epithelial cell is infected, progeny viruses can easily infect neighboring cells via the basolateral route, and moreover, disruption of cell adhesion by fiber allows virus escape into the lumen of the airway and back into the environment [4]. The question remained: is the initial infection random, requiring damage or does it use the adenovirus fiber-knob or another receptor?
Human transcripts containing the predicted exon 8 have been described [2], [28] but protein presence never verified. To better understand the function of hCAR we sought to investigate the presence, localization and interactions of human CAREx8 and their relevance in the human airway epithelium.
Proteins exhibit regulation at multiple levels. Here we show that not only is the transmembrane form of human CAR regulated by splicing, but also the exon 8 specific isoform is subsequently regulated at the protein level by the PDZ domain containing protein MAGI-1b. Furthermore, hCAREx8 localizes to the apical compartment of human airway epithelia where it could serve as the receptor initiating adenovirus infection.
RNA splicing is an important event that significantly increases proteomic diversity and may impart cell specificity. Despite the importance of hCAR in viral infection, cell adhesion, and development, the genetic and splicing regulation of the gene for hCAR, CXADR, have not been well studied. It is known that expression of hCAR is upregulated by histone deacetylase (HDAC) inhibitors, a finding with significant implications for oncolytic adenoviral cancer therapy [29]–[31]. Whether HDAC inhibitors affect the splicing of hCAR is also currently unknown.
Several transcripts have been described for hCAR. However, the importance of each splice variant is unclear. Secreted splice variants that interact with the extracellular domain of the transmembrane form of hCAR could clearly alter homophilic transmembrane hCAR interactions and hence modulate junctional remodeling. These variants could also play a physiological role modulating interactions between hCAR and its ligand, AMICA1/JAML, found on transmigrating lymphocytes and dendritic cells [32].
Considering the importance of junctional organization, interactions, and signaling, alternative transmembrane forms could play equally important roles in junctional remodeling and responses to ligation with soluble isoforms or ligands on other cell types. Two transmembrane isoforms are known for CAR. Although several interactions have been discovered for both the mouse and human CAREx7 isoform, only one interaction has been described for the alternative, less prevalent mouse CAREx8 isoform. Both mouse CAREx7 and CAREx8 interact with Ligand-of-Numb Protein-X2 (LNX2), an intracellular scaffolding protein that may play a role in Notch signaling [22]. Interestingly, both isoforms interact with LNX2 through two different regions within the intracellular domain of mCAR; each unique C-terminal PDZ binding domain and a region in common just upstream of the splice junction.
Our previous work revealed that interaction with hCAREx7 results in junctional localization of MAGI-1b [6]. In contrast, this work shows that the interaction with hCAREx8 is unique to other MAGI-1b interactions since it results in the loss of hCAREx8. Epithelia express MAGI-1b where it localizes to the basolateral junctions. hCAREx7 also localizes to the basolateral junctional adhesion complex. The data presented herein is consistent with MAGI-1b interacting with hCAREx7 at the junctions between epithelial cells. It is also consistent with the unexpected apical localization of hCAREx8. If hCAREx8 were to go to the basolateral junctions, it would be predicted to interact with MAGI-1b and be degraded. Thus the only place it could exist in the cell is in an apical compartment devoid of MAGI-1b. Alternatively, there may be a specific mechanism for the apical localization of hCAREx8 that remains to be discovered. Apical localization is consistent with elevated adenovirus infection after expression of hCAREx8. Thus this isoform of hCAR would be able to interact with adenovirus on the luminal air-exposed surface and mediate the initial infection of an epithelium. It is notable that the increase of infection is similar to the extracellular domain of hCAR conjugated to a glycophosphatidyl-inositol (GPI) tail, which explicitly is apically localized in airway epithelia [27], [33]. In vitro, the C-terminus of hCAR is not required for adenovirus infection [27], however, considering the distinct localization and interactions between these isoforms, we cannot predict whether infection is identical in vivo. Furthermore, it is interesting to speculate that mutations altering the splicing or transcript abundance of the hCAREx8 isoform may be responsible for viral susceptibility.
PDZ-based regulation has been described for other membrane proteins [34], [35]. For example several PDZ-domain containing proteins are known to interact with the cystic fibrosis transmembrane conductance regulator (CFTR) [36], [37]. In contrast to hCAREx7, CFTR traffics to the apical membrane of airway epithelia where it behaves as a chloride channel. Interactions between the PDZ-binding domain of CFTR and the PDZ domain of Na+/H+ exchanger-3 regulatory factors 1 and 2 (NHERF1 and NHERF2) act to stabilize CFTR at the cell surface [38]. CFTR PDZ interactions with the CFTR-associated ligand (CAL) target CFTR for lysosomal degradation [39]. Cushing et al have recently shown that the delicate balance of interactions regulating cell surface maintenance and lysosomal degradation upon cycling is due to the relative affinity of the PDZ interactions [37]. The stronger interaction between CFTR and NHERF1/2 would be predicted to out compete the weaker interaction with CAL resulting in the relatively long CFTR half-life observed. This type of regulation differs from our data in at least two ways. One isoform (CAREx7) appears to be dominant in directing the localization of the PDZ-domain containing proteins while MAGI-1b is dominant in the interaction with hCAREx8 resulting in the loss of this protein. To our knowledge, all descriptions of PDZ-directed degradation have been during mature protein cycling. In contrast, we have observed immediate loss of hCAREx8 implying regulation during early protein synthesis or quality control stages.
The question remains why differential compartmentalization of these hCAR isoforms would exist. The signals transduced by these isoforms, despite having the same extracellular ligands, may differ. Differential localization of these two isoforms could result in the cell being able to discern whether the signal is from the apical or basolateral compartment and mount the appropriate response. For example, both isoforms would recognize AMICA1/JAML present on the surface of neutrophils and dendritic cells. hCAREx7 may be a gatekeeper for neutrophil transmigration but hCAREx8 may either tether neutrophils to the apical surface or sense how many neutrophils are present. Alternatively, these two isoforms may play a role in dendritic cell surveillance and maintaining the seal around dendritic cell filopodia.
These data also provide support for the importance of sequences upstream of the PDZ binding domain in dictating target PDZ domains and subsequent activity. Both the GSIV (Ex7) and ITVV (Ex8) sequences bind all three targets investigated, MAGI-1b, PICK1 and PSD95. However, the presence of the upstream 9 unique amino acids from hCAREx8 alters the resulting interaction such that PICK1 does not interact, PSD-95 does interact to localize at cellular junctions, and MAGI-1b results in degradation of the hCAREx8 protein. In contrast, the 22 amino acids from hCAREx7 allow a stable interaction between these three proteins and the ITVV PDZ-sequence from hCAREx8 resulting in co-localization at the junctions of cells. The exact mechanism requires further investigation and will lead to a greater understanding of this important class of protein interaction domains.
In summary, human airway epithelia express several isoforms of hCAR. Importantly, human CAREx8 localizes to the apical surface where it may play a key role in the initiation of adenoviral, and potentially coxsackievirus, pulmonary infection. We propose a model for the regulation of this localization based upon isoform-specific PDZ binding domain interactions with MAGI-1b. Although PDZ based interactions are known to be key regulators of membrane microdomain structure, stability and signaling, we have shown that this interaction may also regulate protein expression. Furthermore, PDZ based interactions are influenced by several factors, including PDZ binding domain and upstream sequence context. Affinity of interaction between the PDZ domain and PDZ binding domain may be higher or lower depending on surrounding sequences resulting in the specificity of interaction. Surprisingly, either interacting partner can be dominant in dictating the result of the interaction (i.e. junctional trafficking versus degradation). Further elucidation of these mechanisms may provide a novel target for either down regulation of the adenovirus receptor to limit viral infection or alternatively up regulation for the purpose of adenoviral-based therapies.
Materials and Methods
Ethics statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the Institutional Review Board of the University of Iowa (IRB ID No. 9507432). Primary human airway epithelia were isolated from discarded and deidentified trachea and bronchi of donor lungs. This study used discarded lung tissue, thus the IRB deemed consent was not needed.
Materials and constructs
FLAG M2 antibody (Ab) was purchased from Sigma (F3165, St. Louis, MO), mouse anti-CD55 (DAF) was from BD Pharmingen (555691, San Jose, CA), mouse anti-Ezrin was from Santa Cruz (SC-58758, Santa Cruz, CA), mouse anti-acetylated α-tubulin was from Sigma (St. Louis, MO), Alexa-488 and -568 conjugated goat anti-mouse or anti-rabbit Abs, mouse and rabbit anti-GFP were from Molecular Probes (Eugene, OR). RmcB Ab (CRL-2379, ATCC, Manassas, VA) was produced by the University of Iowa Hybridoma Core. Rabbit anti-hCAR 1605 was produced in rabbits immunized with a GST fusion to the intracellular c-terminus (aa 261–365) as previously described [40]. Rabbit anti-hCAREx7 5490 and Rabbit anti-hCAREx8 5678 were produced in rabbits immunized with peptides of 13 c-terminal amino acids (CVMIPAQSKDGSIV and FKYPYKTDGITVVC respectively). COS-7 cells were from ATCC (Manassas, VA), and maintained under standard culture conditions (D-MEM with 10% FCS, penicillin and streptomycin). CHO-K1 cells were from BD Biosciences (Franklin Lakes, NJ) and maintained under standard culture conditions (D-MEM with 10% FCS, supplemented with tetracycline L-glutamine, penicillin and streptomycin). Ad serotype 5 containing the β-galactosidase (Ad-βGal), eGFP, RFP (peGFP-N1, pDSRed1, Clontech, Palo Alto, CA), or hCAR gene have previously been described [41], [42]. The University of Iowa Gene Transfer Vector Core produced all viruses. Several cDNAs were kind gifts from the following investigators: hCAR was from Ronald Crystal; peGFP-MAGI-1b was from Irina Dobrosotskaya; PSD-95-GFP was from David Bredt. The cDNA for PICK1-GFP has previously been described [43] and MAGI-1b-CMV-myc was subcloned and contained aa 642-1287.
Web programs
We performed a BLAT search with the mouse sequence (www.genome.ucsc.edu) to determine the human sequence for exon 8. Comparison of hCAR P343 to mCAR A343 using Conseq software identified this amino acid as an exposed or buried residue respectively with a non-structural role. Conservation could not be determined due to insufficient data. The score assigned by Conseq was validated using PolyPhen (http://www.bork.embl-heidelberg.de/polyPhen/).
Cloning of hCAREx8 and site directed mutagenesis
hCAREx8 was cloned from RNA isolated from primary human airway epithelia (Qiagen, Valencia, CA; hCAREx8F: 5′GCGAATTCGCCACCATGGCGCTCCTGCTCTGCTTCG and hCAREx8R: 5′GTGGATCCTTATACAACTGTAATTCCATC). The fragment was digested with EcoRI and BamHI and cloned into pcDNA3.1(-) or an Ad5-CMV shuttle plasmid. DNA sequencing and Western blot confirmed the expected protein. hCAREx8 was modified with two FLAG tags (DYKDDDDK) added between amino acid 22 and 23, similar to previously described modifications in hCAREx7. Site-directed mutagenesis was performed to generate hCAREx7/8 according to manufacturer's standard protocol (Stratagene, Cedar Creek, TX) with the following primer: GSIV-ITVV, 5′-CCAGCACAGAGCAAGGATATCACTGTAGTATAGGGATCCGAGCTC.
Site-directed mutagenesis was performed to generate CAREx8/7 according to manufacturer's standard protocol (Stratagene, Cedar Creek, TX) with the following primer: ITVV-GSIV, 5′-CCTTACAAGACTGATGGAGGTTCAATTGTATAAGGATCAAGGGTG
Site-directed mutagenesis was performed to generate hCAREx8-PDZ according to manufacturer's standard protocol (Stratagene, Cedar Creek, TX) with the following primer: ITVV-*TVV, 5′-CCTTACAAGACTGATGGATAAACAGTTGTATAAGGATCAAGGGTGG.
Human airway epithelia
Primary human airway epithelia were isolated from trachea and bronchi of donor lungs and seeded onto collagen coated, semi-permeable membranes (Millipore, Bedford, MA) and grown at the air-liquid interface as previously described [44], [45]. Approximately two weeks after seeding, cultures were well-differentiated and attained a measurable transepithelial resistance. To augment endogenous hCAR expression, epithelia were transduced with adenovirus carrying hCAREx7 or hCAREx8 from the basolateral surface as previously described [23], [41].
Amaxa transfection
Primary airway epithelial cells seeded on plastic were trypsinized, washed and cells electroporated with 2.5 µg of plasmids encoding hCAREx7, hCAREx8 or eGFP using the Amaxa Nucleofector I (Amaxa Inc, Walkersville, MD) according to manufacturer's standard protocol for primary mammalian epithelial cells (VPI-1005, program T-20). Approximately 3×105 cells were seeded onto collagen coated, semi-permeable membranes as described above. Epithelia were infected with Ad-β-gal (MOI 10 pfu/cell) for 1 hr at 37°C, washed twice and lysed 48 hr post infection. β-galactosidase expression per mg protein was determined as previously described [42].
Adenovirus infection
Chinese hamster ovary cells were plated in 24 well dishes and transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. Twenty-four hours after transfection cells were infected with Ad serotype 5 containing the β-galactosidase gene (Ad-βGal) (MOI 100) for 1 hr at 37°C. 48 hours later cells were lysed and β-galactosidase expression per mg protein was determined as previously described [42].
Co-transfection in COS-7 cells
COS-7 cells were electroporated by standard methodologies. Briefly, 10 million cells were mixed with 20 µg of plasmid DNA for single transfection, 15 µg of each DNA for double transfections, or 10 µg of each for triple transfections, in 400 µl of cytomix (120mM KCl, 0.15mM CaCl2, 10mM K2HPO4, 10mM KH2PO4, 25mM HEPES, 2mM EGTA, 5mM MgCl2, 2mM ATP and glutathione) and put in an electroporation cuvette (Bio-Rad Laboratories, Hercules, CA) for 30 minutes on ice. After electroporation, cells were seeded onto 10cm dishes for immunoprecipitation (IP) and collagen coated glass chamber slides for immunofluorescence studies 2 days later.
Immunostaining
COS-7 cells grown on collagen coated chamber slides or airway epithelia were washed once with PBS, fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 2% BSA in SuperBlock (Pierce, Rockford, IL). Cells were incubated with primary Ab, washed extensively and incubated with goat anti-mouse Alexa-568 secondary Ab. After washing, slides were coverslipped with Vectashield mounting media (Vector Laboratories, Inc, Burlingame, CA). Images were acquired with a BioRad MRC-1024 Laser Scanning Confocal Microscope (Hercules, CA) mounted on a Nikon E600 microscope (Melville, NY) using a 60X oil immersion lens. Fluorescence imaging was performed on an Olympus IX71 X-Cite 120 fluorescence microscope (Center Valley, PA) followed by quantitation using Image J.
Quantitative real time PCR
RNA was isolated from primary airway cultures greater than 2 weeks old or primary lung tissue using TRIzol with the Pure Link RNA kit (Invitrogen). cDNA was synthesized using RT2 EZ First Strand Kit (SA Biosciences). Primers and probes for the seventh or eighth exon of CXADR were designed with Primer Express software (Applied Biosystems). The sequences for hCAREx7 were: hCAREx7-F, 5′-TGCCAGAAGCTACATCGGCAGTAA; hCAREx7-R, 5′-ATAGACCCATCCTTGCTCTGTGCT; hCAREx7 probe, 5′-AAGTCGAATGGGTGCGATTCCTGTGA (5′ FAM, 3′ TAMRA-SP); PCR product 141bp. The sequences for the hCAREx8 were: hCAREx8-F, 5′-AGGGAAGATGTGCCACCTCCAAA; hCAREx8-R, 5′-CAACTGTAATTCCATCAGTCTTGTAAG; hCAREx8 probe, 5′-ACTGCCAGAAGCTACATCGGCAGTAA (5′ FAM, 3′ TAMRA-SP); 165bp. Samples were run using TaqMan Fast Universal PCR Master Mix (Applied Biosystems) on a 7500 Fast Real-Time PCR System (Applied Biosystems). Transcript number was quantitated by plasmid standard curve. Abundance relative to 18s (Invitrogen, 115HM-02) or hGAPDH (Invitrogen, 100H-02) as the reference gene confirmed the presence of significantly more hCAREx7 transcripts than hCAREx8.
Immunoprecipitation and Western blot
Cells from two 100mm plates were placed on ice, washed once with ice cold PBS, and lysed with lysis buffer (50mM Tris-HCl, pH 7.5, 137mM NaCl, 1% Triton X-100, 5mM EDTA, 1mM EGTA, protease inhibitors (10 µg/ml) leupeptin, aprotinin, pepstatin, and 1mM phenylmethylsulfonyl fluoride) by rocking at 4°C. Cells were scraped, sonicated 5 times and spun in a microcentrifuge at full speed for 10 minutes. For co-immunoprecipitation, supernatant was incubated with the indicated Ab with rotation at 4°C overnight. Protein A or G conjugated sepharose (Amersham Biosciences, Uppsla Sweden) was added for 1-2 hours followed by a wash with lysis buffer, 10% lysis buffer in TBS (50mM Tris-HCl, pH 7.5, 137mM NaCl), and TBS. Beads were suspended in loading buffer (4% sodium dodecyl sulfate, 100mM dithiothreitol, 20% glycerol, 65mM Tris, pH 6.8, 0.005% bromophenol blue) and proteins were separated by SDS-poly acrylamide gel electrophoresis. Gels were transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA), blocked with 5% BSA, washed, probed with primary Ab as indicated, followed by washing and incubation with protein A or G conjugated HRP (Pierce, Rockford, IL). Bands were detected with ECL reagents (Pierce, Rockford, IL) and imaged on the EpiChemi3 Darkroom (UVP Inc, Upland, CA).
Supporting Information
Endogenously expressed CAREx8 (A, D, G) in polarized human airway epithelia localizes above ZO-1 (B, C) and co-localizes with the apical protein ezrin (E, F, G). Sections are shown in X-Y (A-F) or X-Z (G) axes. Confocal microscopy (60x oil immersion).
(9.08 MB TIF)
Quantitative RT-PCR primers for CAREx7 or CAREx8 are specific. Primary airway were either mock transduced or transduced with adenovirus carrying the gene for CAREx7 or CAREx8. RNA was isolated 36 hours later and subjected to isoform specific quantitative RT-PCR. Under mock conditions there was more endogenous CAREx7 than CAREx8. Epithelia transduced with CAREx7 or CAREx8 showed increased trascript levels but did not increase transcript levels of the other isoform.
(2.91 MB TIF)
Expression of exogenous CAREx8 in polarized human airway epithelia mediates five-fold greater Ad-β-Gal gene transfer than endogenous expression (mock transduced cells followed by Ad-β-Gal). *p<0.0001 Ad-CAREx8 vs. Mock/Ad-β-Gal or Mock/no virus. p = 0.03 Mock/Ad-β-Gal vs. Mock/no virus.
(2.50 MB TIF)
Acknowledgments
We thank Miriam Estin and Ashley Small for assistance with manuscript preparation, and Michael Welsh for discussions.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a PPG grant from the NIH (HL51670-11). The Gene Transfer Vector Core is supported by the Roy J. Carver Charitable Trust, the NHLBI, CFF, and NIDDK. The In Vitro Cell Models Core is supported by the National Heart, Lung and Blood Institute and NIDDK [DK54759]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, et al. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 2.Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci U S A. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Honda T, Saitoh H, Masuko M, Katagiri-Abe T, Tominaga K, et al. The coxsackievirus-adenovirus receptor protein as a cell adhesion molecule in the developing mouse brain. Brain Res Mol Brain Res. 2000;77:19–28. doi: 10.1016/s0169-328x(00)00036-x. [DOI] [PubMed] [Google Scholar]
- 4.Walters R, Freimuth P, Moninger T, Ganske I, Zabner J, et al. Adenovirus Fiber Disrupts CAR-Mediated Intercellular Adhesion Allowing Virus Escape. Cell. 2002;110:789–799. doi: 10.1016/s0092-8674(02)00912-1. [DOI] [PubMed] [Google Scholar]
- 5.Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, et al. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci U S A. 2001;98:15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Excoffon KJ, Hruska-Hageman A, Klotz M, Traver GL, Zabner J. A role for the PDZ-binding domain of the coxsackie B virus and adenovirus receptor (CAR) in cell adhesion and growth. J Cell Sci. 2004;117:4401–4409. doi: 10.1242/jcs.01300. [DOI] [PubMed] [Google Scholar]
- 7.Coyne CB, Voelker T, Pichla SL, Bergelson JM. The coxsackievirus and adenovirus receptor interacts with the multi-PDZ domain protein-1 (MUPP-1) within the tight junction. J Biol Chem. 2004;279:48079–48084. doi: 10.1074/jbc.M409061200. [DOI] [PubMed] [Google Scholar]
- 8.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh MJ. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J Clin Invest. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.House AE, Lynch KW. Regulation of alternative splicing: more than just the ABCs. J Biol Chem. 2008;283:1217–1221. doi: 10.1074/jbc.R700031200. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Dov C, Hartmann B, Lundgren J, Valcarcel J. Genome-wide analysis of alternative pre-mRNA splicing. J Biol Chem. 2008;283:1229–1233. doi: 10.1074/jbc.R700033200. [DOI] [PubMed] [Google Scholar]
- 11.Thoelen I, Magnusson C, Tagerud S, Polacek C, Lindberg M, et al. Identification of alternative splice products encoded by the human coxsackie-adenovirus receptor gene. Biochem Biophys Res Commun. 2001;287:216–222. doi: 10.1006/bbrc.2001.5535. [DOI] [PubMed] [Google Scholar]
- 12.Bernal RM, Sharma S, Gardner BK, Douglas JT, Bergelson JM, et al. Soluble coxsackievirus adenovirus receptor is a putative inhibitor of adenoviral gene transfer in the tumor milieu. Clin Cancer Res. 2002;8:1915–1923. [PubMed] [Google Scholar]
- 13.Dorner A, Xiong D, Couch K, Yajima T, Knowlton KU. Alternatively spliced soluble coxsackie-adenovirus receptors inhibit coxsackievirus infection. J Biol Chem. 2004;279:18497–18503. doi: 10.1074/jbc.M311754200. [DOI] [PubMed] [Google Scholar]
- 14.Yanagawa B, Spiller OB, Proctor DG, Choy J, Luo H, et al. Soluble recombinant coxsackievirus and adenovirus receptor abrogates coxsackievirus b3-mediated pancreatitis and myocarditis in mice. J Infect Dis. 2004;189:1431–1439. doi: 10.1086/382598. [DOI] [PubMed] [Google Scholar]
- 15.Goodfellow IG, Evans DJ, Blom AM, Kerrigan D, Miners JS, et al. Inhibition of coxsackie B virus infection by soluble forms of its receptors: binding affinities, altered particle formation, and competition with cellular receptors. J Virol. 2005;79:12016–12024. doi: 10.1128/JVI.79.18.12016-12024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorner A, Grunert HP, Lindig V, Chandrasekharan K, Fechner H, et al. Treatment of coxsackievirus-B3-infected BALB/c mice with the soluble coxsackie adenovirus receptor CAR4/7 aggravates cardiac injury. J Mol Med. 2006;84:842–851. doi: 10.1007/s00109-006-0076-y. [DOI] [PubMed] [Google Scholar]
- 17.Reimer D, Steppan I, Wiedemair A, Concin N, Hofstetter G, et al. Soluble isoforms but not the transmembrane form of coxsackie-adenovirus receptor are of clinical relevance in epithelial ovarian cancer. Int J Cancer. 2007;120:2568–2575. doi: 10.1002/ijc.22580. [DOI] [PubMed] [Google Scholar]
- 18.Bergelson JM, Krithivas A, Celi L, Droguett G, Horwitz MS, et al. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol. 1998;72:415–419. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw CA, Holland PC, Sinnreich M, Allen C, Sollerbrant K, et al. Isoform-specific expression of the Coxsackie and adenovirus receptor (CAR) in neuromuscular junction and cardiac intercalated discs. BMC Cell Biol. 2004;5:42. doi: 10.1186/1471-2121-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raschperger E, Thyberg J, Pettersson S, Philipson L, Fuxe J, et al. The coxsackie- and adenovirus receptor (CAR) is an in vivo marker for epithelial tight junctions, with a potential role in regulating permeability and tissue homeostasis. Exp Cell Res. 2006;312:1566–1580. doi: 10.1016/j.yexcr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Sollerbrant K, Raschperger E, Mirza M, Engstrom U, Philipson L, et al. The Coxsackievirus and adenovirus receptor (CAR) forms a complex with the PDZ domain-containing protein ligand-of-numb protein-X (LNX). J Biol Chem. 2003;278:7439–7444. doi: 10.1074/jbc.M205927200. [DOI] [PubMed] [Google Scholar]
- 22.Mirza M, Raschperger E, Philipson L, Pettersson RF, Sollerbrant K. The cell surface protein coxsackie- and adenovirus receptor (CAR) directly associates with the Ligand-of-Numb Protein-X2 (LNX2). Exp Cell Res. 2005;309:110–120. doi: 10.1016/j.yexcr.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 23.Excoffon KJ, Traver GL, Zabner J. The role of the extracellular domain in the biology of the coxsackievirus and adenovirus receptor. Am J Respir Cell Mol Biol. 2005;32:498–503. doi: 10.1165/rcmb.2005-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreda SM, Mall M, Mengos A, Rochelle L, Yankaskas J, et al. Characterization of wild-type and deltaF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol Biol Cell. 2005;16:2154–2167. doi: 10.1091/mbc.E04-11-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coyne CB, Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. 2006;124:119–131. doi: 10.1016/j.cell.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 26.Arcasoy SM, Latoche J, Gondor M, Watkins SC, Henderson RA, et al. MUC1 and other sialoglycoconjugates inhibit adenovirus-mediated gene transfer to epithelial cells. Am J Respir Cell Mol Biol. 1997;17:422–435. doi: 10.1165/ajrcmb.17.4.2714. [DOI] [PubMed] [Google Scholar]
- 27.Walters RW, van't Hof W, Yi SM, Schroth MK, Zabner J, et al. Apical localization of the coxsackie-adenovirus receptor by glycosyl- phosphatidylinositol modification is sufficient for adenovirus-mediated gene transfer through the apical surface of human airway epithelia. J Virol. 2001;75:7703–7711. doi: 10.1128/JVI.75.16.7703-7711.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fechner H, Haack A, Wang H, Wang X, Eizema K, et al. Expression of coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 1999;6:1520–1535. doi: 10.1038/sj.gt.3301030. [DOI] [PubMed] [Google Scholar]
- 29.Kitazono M, Goldsmith ME, Aikou T, Bates S, Fojo T. Enhanced adenovirus transgene expression in malignant cells treated with the histone deacetylase inhibitor FR901228. Cancer Res. 2001;61:6328–6330. [PubMed] [Google Scholar]
- 30.Pong RC, Roark R, Ou JY, Fan J, Stanfield J, et al. Mechanism of increased coxsackie and adenovirus receptor gene expression and adenovirus uptake by phytoestrogen and histone deacetylase inhibitor in human bladder cancer cells and the potential clinical application. Cancer Res. 2006;66:8822–8828. doi: 10.1158/0008-5472.CAN-05-4672. [DOI] [PubMed] [Google Scholar]
- 31.Segura-Pacheco B, Avalos B, Rangel E, Velazquez D, Cabrera G. HDAC inhibitor valproic acid upregulates CAR in vitro and in vivo. Genet Vaccines Ther. 2007;5:10. doi: 10.1186/1479-0556-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zen K, Liu Y, McCall IC, Wu T, Lee W, et al. Neutrophil migration across tight junctions is mediated by adhesive interactions between epithelial coxsackie and adenovirus receptor and a junctional adhesion molecule-like protein on neutrophils. Mol Biol Cell. 2005;16:2694–2703. doi: 10.1091/mbc.E05-01-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van't Hof W, Crystal RG. Manipulation of the cytoplasmic and transmembrane domains alters the cell surface levels of the coxsackie-adenovirus receptor and changes the efficiency of adenovirus infection. Hum Gene Ther. 2001;12:25–34. doi: 10.1089/104303401450933. [DOI] [PubMed] [Google Scholar]
- 34.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 35.Lamprecht G, Seidler U. The emerging role of PDZ adapter proteins for regulation of intestinal ion transport. Am J Physiol Gastrointest Liver Physiol. 2006;291:G766–777. doi: 10.1152/ajpgi.00135.2006. [DOI] [PubMed] [Google Scholar]
- 36.Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol. 2006;7:426–436. doi: 10.1038/nrm1949. [DOI] [PubMed] [Google Scholar]
- 37.Cushing PR, Fellows A, Villone D, Boisguerin P, Madden DR. The relative binding affinities of PDZ partners for CFTR: a biochemical basis for efficient endocytic recycling. Biochemistry. 2008;47:10084–10098. doi: 10.1021/bi8003928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall RA, Ostedgaard LS, Premont RT, Blitzer JT, Rahman N, et al. A C-terminal motif found in the beta2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc Natl Acad Sci U S A. 1998;95:8496–8501. doi: 10.1073/pnas.95.15.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng J, Wang H, Guggino WB. Modulation of mature cystic fibrosis transmembrane regulator protein by the PDZ domain protein CAL. J Biol Chem. 2004;279:1892–1898. doi: 10.1074/jbc.M308640200. [DOI] [PubMed] [Google Scholar]
- 40.Excoffon KJ, Gansemer N, Traver G, Zabner J. Functional effects of coxsackievirus and adenovirus receptor glycosylation on homophilic adhesion and adenoviral infection. J Virol. 2007;81:5573–5578. doi: 10.1128/JVI.02562-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walters RW, Grunst T, Bergelson JM, Finberg RW, Welsh MJ, et al. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274:10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 42.Ashbourne Excoffon KJ, Moninger T, Zabner J. The coxsackie B virus and adenovirus receptor resides in a distinct membrane microdomain. J Virol. 2003;77:2559–2567. doi: 10.1128/JVI.77.4.2559-2567.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hruska-Hageman AM, Wemmie JA, Price MP, Welsh MJ. Interaction of the synaptic protein PICK1 (protein interacting with C kinase 1) with the non-voltage gated sodium channels BNC1 (brain Na+ channel 1) and ASIC (acid-sensing ion channel). Biochem J 361(Pt. 2002;3):443–450. doi: 10.1042/0264-6021:3610443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zabner J, Wadsworth SC, Smith AE, Welsh MJ. Adenovirus-mediated generation of cAMP-stimulated Cl- transport in cystic fibrosis airway epithelia in vitro: effect of promoter and administration method. Gene Ther. 1996;3:458–465. [PubMed] [Google Scholar]
- 45.Karp PH, Moninger T, Weber SP, Nesselhauf TS, Launspach J, et al. An in vitro model of differentiated human airway epithelia: methods and evaluation of primary cultures. In: Wise C, editor. Epithelial Cell Culture Protocols. Totowa, , NJ: Humana Press, Inc; 2002. pp. 115–137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Endogenously expressed CAREx8 (A, D, G) in polarized human airway epithelia localizes above ZO-1 (B, C) and co-localizes with the apical protein ezrin (E, F, G). Sections are shown in X-Y (A-F) or X-Z (G) axes. Confocal microscopy (60x oil immersion).
(9.08 MB TIF)
Quantitative RT-PCR primers for CAREx7 or CAREx8 are specific. Primary airway were either mock transduced or transduced with adenovirus carrying the gene for CAREx7 or CAREx8. RNA was isolated 36 hours later and subjected to isoform specific quantitative RT-PCR. Under mock conditions there was more endogenous CAREx7 than CAREx8. Epithelia transduced with CAREx7 or CAREx8 showed increased trascript levels but did not increase transcript levels of the other isoform.
(2.91 MB TIF)
Expression of exogenous CAREx8 in polarized human airway epithelia mediates five-fold greater Ad-β-Gal gene transfer than endogenous expression (mock transduced cells followed by Ad-β-Gal). *p<0.0001 Ad-CAREx8 vs. Mock/Ad-β-Gal or Mock/no virus. p = 0.03 Mock/Ad-β-Gal vs. Mock/no virus.
(2.50 MB TIF)