Abstract
Membrane uptake of long chain fatty acids (FA) is the first step in cellular fatty acid utilization and a point of metabolic regulation. CD36 facilitates a major fraction of fatty acid uptake by key tissues. This review highlights the contribution of CD36 to pathophysiology in rodents and humans. Novel concepts regarding regulation of CD36-facilitated uptake are discussed, i.e. the role of membrane rafts/caveolae, CD36 recycling between intracellular depots and the membrane, and chemical modifications of that impact its turnover and recruitment. Importantly, CD36 membrane levels and turnover are abnormal in diabetes, resulting in dysfunctional FA utilization. Also, variants in the CD36 gene were recently shown to influence susceptibility for metabolic syndrome, greatly increasing the risk of diabetes and heart disease.
Keywords: Fatty acid uptake, CD36, translocation, ubiquitination
Cellular FA uptake: an overview
Nutrient supply and cellular energy demands are constantly changing, so the molecular relationships governing cellular responses to nutrient availability are exceedingly important. To allow optimal utilization of available substrates and to best fit their energy needs, cells reprogram to change protein localization, turnover and/or gene expression [1].
The first regulatory step in nutrient homeostasis is cellular uptake at the plasma membrane. This is often highly regulated, involving specific membrane receptors [2] with a downstream network of interacting proteins that function in signal transduction or intracellular nutrient traffic. Long chain fatty acids (FA) are common dietary nutrients, a major energy source for most cells and precursors for synthesis of cellular lipids with structural or signaling functions [3, 4]. FA also regulate gene expression via transcription factors including the PPARs [5–7], nutrient sensors that control a variety of metabolic genes [8, 9], and FoxO1 [6, 10], a member of the forkhead family important for adaptation to nutrient shortage [11]. Most tissues, except for liver and adipose tissue, possess little capacity for de novo FA synthesis and depend on FA uptake for their needs, emphasizing the physiological importance of cellular FA uptake.
Long chain FA can diffuse rapidly across phospholipid bilayers [12], but there is now overwhelming evidence that their uptake by a wide variety of mammalian cells is facilitated by integral or membrane associated proteins [13–15]. The FA transport proteins (FATP1–6), present at the plasma membrane or in intracellular organelles, have fatty acyl-CoA ligase activity and appear to function in coupling FA uptake to the first reaction of FA utilization [15, 16]. The plasma membrane associated FA-binding protein (FABPpm) [17] that is identical to the mitochondrial aspartate aminotransferase, an enzyme that functions in maintaining the cytoplasmic/mitochondrial NADH/NAD ratios, may couple FA uptake to cellular redox shuttles that are crucial for oxidative metabolism. Consistent with this, upregulation of FABPpm in muscle occurs with contraction and AMP kinase activation [13]. Several G protein coupled receptors, specifically GPR40–43, have been recently shown to recognize short (GPR41 and GPR43), medium and long chain FA (GPR40) [18] and mediate some signaling regulatory effects of these nutrients. GPR40 is expressed in pancreatic islets signals and increases intracellular calcium and insulin secretion in response to FA. An additional receptor, GPR120, abundant in the intestine, modulates FA-induced glucagon-like peptide 1 secretion [19]. However, whether these GPRs play any role in modulating FA uptake and utilization has not yet been examined.
CD36 as a FA translocase (FAT)
In the past several years, the membrane protein CD36 has been extensively studied for its role in facilitating FA uptake and oxidation in rodents and humans and implicated in the pathophysiology associated with dysfunctional FA metabolism [13, 20–22]. This review highlights recent advances in our understanding of CD36 function in FA uptake and utilization.
CD36 is a multifunctional scavenger receptor
CD36 is an 88kDa membrane protein originally referred to as FAT (FA Translocase). Topology of CD36 predicts two transmembrane segments, a large extracellular domain with one hydrophobic sequence that may loop back into the membrane bilayer and two short cytoplasmic tails (Figure 1a). CD36’s role in FA uptake was documented by its covalent labeling with reactive esters of FA myristate and oleate (SSM, sulfo-N-succinimidyl myristate and SSO, sulfo-N-succinimidyl oleate), compounds that inhibited FA transport in isolated adipocytes [23]. Purification of FAT and isolation of its corresponding cDNA identified it as the rat adipose homolog of human CD36, a protein that had been isolated from platelets as a thrombospondin and collagen receptor [24] (Figure 1). CD36 is expressed in a variety of cells including and not limited to monocytes, platelets, macrophages, microvascular endothelial cells, adipocytes, muscle cells, enterocytes, and hepatocytes, to name a few [23]. It can function in a range of processes unrelated to FA uptake [24], e.g. binding of native and oxidized lipids/lipoproteins for apoptosis [25], phagocytosis [26], growth hormone releasing peptides [27] and toll like receptors [28]. As Febbraio noted recently, it would seem that “CD36 is shorthand for Can Do 36 things” [29]. How one protein can be involved in multiple and seemingly unrelated functions is unclear. It remains to be determined whether many of these functions, including that of FA uptake, involve a common underlying mechanism. CD36-dependent signaling via Src kinases that associate with the C-terminal cytoplasmic tail of CD36 [30, 31] may be involved in some of these functions.
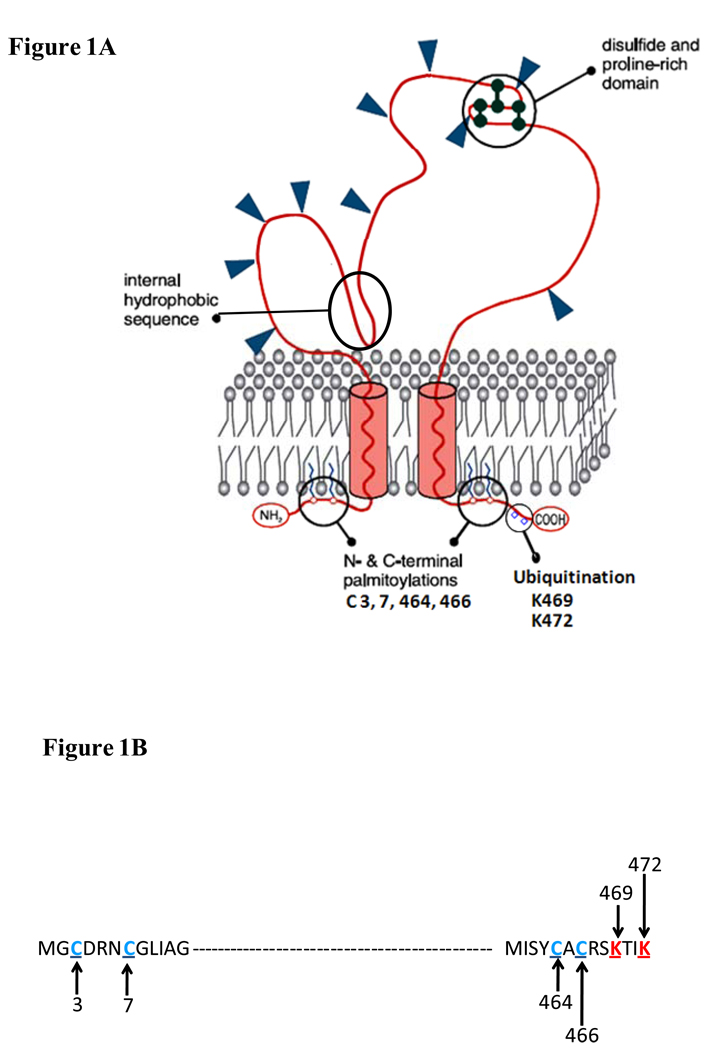
Figure 1. Predicted topography of CD36 in the plasma membrane.
(a) CD36 is a heavily glycosylated protein of 471 amino acids. Glysosylation accounts for the difference between the apparent (88Kd) and predicted (~53Kd) molecular weight of the protein. CD36 has a hairpin configuration in the plasma membrane. The extracellular domain has multiple N-linked glycosylation sites, a proline rich domain and a hydrophobic stretch that may associate with the membrane. The protein has two short cytoplasmic domains that are required for CD36 signaling after ligand binding. (b) Ubiquitination sites (in red) in the C-terminus and palmitoylation sites (in blue) in both the C- and N-tails are highlighted. The ubiquitination sites, which are sensitive to FA and insulin, may regulate CD36 sorting to the plasma membrane and CD36 turnover. Palmitoylation might help recruit CD36 to lipid rafts and could influence interaction with Src kinases. Regulation of CD36 palmitoylation is still unknown.
CD36 deficiency and overexpression influence FA uptake and contribute to pathophysiology
Evidence for a physiologically important role of CD36 in FA uptake was obtained with the generation of animal models of CD36 overexpression or deletion. CD36 overexpression in muscle of mice enhances FA oxidation during contraction and decreases plasma lipids, while CD36 deletion impairs FA uptake by key metabolic tissues, and increases plasma FA and triglyceride (TG) [23]. Uptake of the palmitic acid analog BMIPP (b-methyl-p-iodophenylpendadecanoic acid) is significantly reduced in muscle, heart and adipocytes of CD36 deficient mice. In humans, total CD36 deficiency is relatively common (3–5%) in persons of African [20] and Asian descents [32]. An almost complete lack of myocardial BMIPP uptake is observed in CD36 deficient humans using single-photon emission computed tomography [33]. The reduced myocardial FA uptake with CD36 deficiency observed in both mice and humans is compensated for by a several fold increase in glucose utilization to meet the heart’s energy demands. The impact of the altered metabolic profile on heart function remains incompletely understood. Two studies in mice using the perfused working heart reached different conclusions regarding susceptibility of CD36 deficient hearts to failure after ischemia [34, 35]. The difference likely reflected omission [34] versus inclusion [35] of insulin in the perfusion buffer, added to enhance glucose utilization after the ischemic episode and likely improving outcome. This implies that CD36 deficiency impacts heart function under conditions where glucose utilization is reduced such as with fasting, endurance exercise and insulin resistance. In humans, there are reports that CD36 deficiency contributes to certain forms of cardiomyopathy [24]. On the other hand, in older mice, increased heart CD36 levels leads to more intramyocardial lipids and is implicated in age-associated cardiomyopathy [36]. These apparently contradictory observations, while supporting the notion that appropriate functioning of CD36 is important for optimal heart function, highlight the need for more studies that examine the molecular relationships involved.
The role of CD36 in intestinal lipid processing was documented by several studies in mice [14, 37] and humans [38]. CD36 is abundant in the small intestine, decreasing from the proximal to distal ends [39]. Enterocytes of the proximal but not distal intestine of CD36 deficient mice exhibit a defect in uptake of FA and cholesterol [39]. As a result, more lipid appears to be absorbed in distal segments. CD36 deficiency in mice does not alter net intestinal FA absorption but does affect absorption of very long chain FA (VLCFA). It was speculated that inhibiting intestinal CD36 could be useful for preventing VLCFA accumulation and the associated neuropathology observed in X-linked adrenoleukodystrophy [40]. The proximal intestine, where CD36 is normally expressed at high levels, is active in chylomicron production; therefore, CD36 deficiency significantly reduces lymphatic chylomicron secretion and increases output of smaller lipoprotein particles [41]. The slower fat absorption in CD36 null mice is associated with a decrease in spontaneous fat intake [42]. Interestingly, CD36 function also impacts fat intake directly through its role in fat perception and preference since the protein expressed on taste bud cells transduces taste perception after binding dietary FA [30, 42, 43]. In addition to impairing chylomicron production, CD36 deficiency is associated with significantly delayed clearance of TG-rich particles in plasma. This likely is a consequence of slow tissue removal of FA released from particle TG by endothelial lipoprotein lipase since FA feedback inhibits lipase activity [37, 44]. In CD36 deficient humans, plasma lipids, apolipoprotein B-48 and small low density intestinal lipoproteins are increased in the postprandial state [38] suggesting that the findings regarding intestinal CD36 function in rodents also apply to humans.
CD36 levels in the liver are normally low in rodents suggesting that FA uptake by the tissue is largely CD36-independent [23]. CD36 liver expression is significantly higher in females where it is downregulated in response to food deprivation [45]. However, in mice, liver CD36 was shown to be a common target of the pro-lipogenic transcription factors, Liver × receptor (LXR), Pregnane × receptor (PXR) and PPARγ [21]. These factors converge to up-regulate CD36, promoting FA uptake and hepatic steatosis. Consistent with this, CD36 expression is increased in livers of humans with non alcoholic fatty liver disease [46].
A role for CD36 in regulating blood pressure [47] was identified in the spontaneously hypertensive rat (SHR) possibly through FA effects on nitric oxide–related pathways [48] in the kidney. CD36 may also contribute to the pathogenesis of human diabetic nephropathy by mediating proximal tubular apoptosis including that induced by FA [31].
CD36 function might contribute to etiology of insulin resistance and the metabolic syndrome
Muscle CD36 plays an important role in the metabolic adaptation to changes in nutrient availability. Studies in rodents show that muscle contraction [13] and activation of FoxO1 [49], a transcription factor induced with fasting, increase sarcolemmal CD36, FA uptake and oxidation. In turn, during fasting, CD36-mediated FA uptake is crucial for upregulating muscle PPARδ and FoxO1, two transcription factors that reinforce reliance on FA utilization. This fasting adaptation is blunted in CD36 deficient muscle [6]. As a result, abnormal CD36 function in muscle in obese and/or diabetic patients [13] might contribute to impaired adaptation to fasting/feeding observed under conditions of insulin resistance [50].
Abnormal FA metabolism, especially in muscle, is linked to insulin resistance of glucose utilization [4]. There is evidence that in muscle from diabetic rodents and humans, more CD36 is recruited to the plasma membrane leading to persistent enhancement of FA uptake and possibly contributing to the impairment of insulin-sensitive glucose utilization [13]. CD36 deletion in mice and the resulting reduction in muscle FA uptake enhances peripheral insulin sensitivity [23]. At the same time, CD36 deletion results in more FA reaching the liver where FA uptake is normally CD36-independent, impairing insulin sensitivity of this tissue [51]. The CD36 deficient mouse is more susceptible to insulin resistance from high carbohydrate diets and is partially protected from resistance induced by diets rich in fat [23]. In apparent contrast to the CD36 null mouse, in the spontaneously hypertensive rat (SHR, NIH colony), a genetically complex model of type two diabetes, insulin resistance, like defective FA uptake, was linked to mutations in the CD36 gene and alleviated by CD36 gene rescue [52]. The above observations cannot be easily integrated, and a good understanding of the impact of CD36 function on insulin signaling is still lacking.
In humans, CD36 deficiency is associated with insulin resistance and with abnormalities of plasma lipids (high triglycerides and FA, with low HDL) [32]. A region along chromosome 7q which encompasses CD36 has been linked to components of the metabolic syndrome in several genome-wide linkage studies [53, 54]. The metabolic syndrome is a cluster of factors (abdominal obesity, high blood glucose, high blood triglycerides, low blow HDL, high blood pressure) that greatly increase the risk of diabetes and cardiovascular disease. An examination of the CD36 gene in a large population of African-Americans identified associations between five common variants in the CD36 gene and the metabolic syndrome [20] suggesting that CD36 may significantly contribute to the 7q linkage. Interestingly, subjects heterozygous for CD36 deficiency appeared protected, while those homozygous exhibited a more deleterious phenotype. Multiple single nucleotide polymorphisms that significantly impacted blood HDL (high density lipoproteins, “good cholesterol”) levels [20] were identified, suggesting an important role of CD36 in human HDL metabolism. In Caucasians, common polymorphisms in the CD36 gene are associated with high blood FA and an increased risk of diabetes-linked cardiovascular disease [22]. Whether variants in the CD36 gene contribute to determine individual susceptibility to diet-induced pathology remains to be fully evaluated.
Regulation of CD36
Rafts, caveolae and CD36-facilitated FA uptake
CD36 on the cell surface is recovered in lipid rafts [55], which are detergent-resistant membranes (DRMs) high in sphingolipids and cholesterol. Caveolae, small intracellular invaginations of the membrane, are formed from lipid rafts that contain the structural caveolin proteins (Caveolin-1, 2 and 3). Caveolin-1 and -2 are ubiquitously expressed, whereas caveolin-3 is muscle-specific. Caveolae and rafts are organizational centers that regulate entry of molecules into the cell and cluster proteins implicated in uptake of nutrients such as glucose and cholesterol [56]. There is now evidence for existence of a caveolae subclass that is potentially involved in FA uptake [15] and early steps of FA incorporation into lipids [56]. Caveolin-1 is especially abundant in adipocytes where it participates in delivery of FA to their storage sites or lipid droplets [57, 58]; indeed, there is recent evidence that Caveolin-1 interacts functionally with CD36. For example, caveolin-1 expression is necessary for post-translational stabilization and membrane expression of CD36. In caveolin-1 deficient mouse embryo fibroblasts, CD36 is mistargeted away from the membrane, reducing FA uptake. Adenoviral rescue of caveolin-1 in these cells induces caveolae formation, directs CD36 to the membrane and normalizes FA uptake [59]. On the flip side, caveolin function is modulated by phosphorylation by Src kinases [60] that associate with the C-terminal cytoplasmic domain of CD36. Caveolin-1 deficient mice have reduced CD36 levels [61] and share phenotypic features with CD36 null mice, such as elevated plasma triglyceride and FA levels and impaired triglyceride clearance after an oral fat load [62]. The above findings suggest that modification of CD36 that influences its localization in membrane lipid rafts and/or caveolae would then be associated with changes in FA uptake.
CD36 trafficking
In addition to residing on the cell surface, CD36 localizes on intracellular vesicles and on mitochondria where it interacts with carnityl palmitoyl transferase 1, the key mitochondrial enzyme regulating FA transport into mitochondria and oxidation [63]. Mitochondrial CD36 content correlates with mitochondrial FA oxidation in human muscle [64] and is increased by treatment with rosiglitazone, the insulin sensitizing agonist of PPARγ [65]. It is still unknown whether CD36 might be involved in FA delivery to mitochondria or in actual FA transfer across mitochondrial membranes. The function of CD36 in other intracellular compartments (e.g., ER) is also not well-defined. In rat hepatoma cells, a CD36 mutant lacking the last 10 amino acids of the C-terminal tail is excluded from the plasma membrane and retained in ER-like organelles [66]. In fact, cells expressing wild-type but not mutant CD36 exhibited enhanced oleate uptake and incorporation into diacylglycerol versus a decrease in incorporation into triacylglycerol, suggesting that ER CD36 might have a role in FA esterification and storage.
Insulin and muscular contraction acutely cause translocation of CD36 from intracellular stores to the plasma membrane and enhance FA uptake [13]. Activation of FoxO1 [11] also recruits CD36 to the membrane [49]. Importantly, muscle contraction increases FA uptake and oxidation, while insulin stimulation targets FA for lipid synthesis. On the other hand, FoxO1 activation enhances both FA oxidation and triglyceride accumulation. These results indicate the importance of intracellular signaling/trafficking events on CD36 function and the metabolic fates of FAs following uptake. The detailed signaling pathways mediating CD36 trafficking are still unclear, but there is emerging evidence indicating a role for small GTPase Rab proteins [13] and for chemical modification of the protein as discussed in the next section.
CD36 ubiquitination and its relation to trafficking
CD36 trafficking between the plasma membrane and intracellular organelles is initiated by ligand binding. In Chinese hamster ovary cells over-expressing CD36 or in C32 cells with endogenous CD36 expression, CD36 internalizes OxLDL through a non-clathrin, non-caveolar, lipid raft pathway that requires the last six amino-acids of the C-tail and is dynamin-dependent [67].
An important mechanism for acute regulation of many membrane protein levels is ubiquitination. This involves the addition of ubiquitin tags that serve as a sorting signal to lysosomes for degradation, instead of recycling the protein back to the plasma membrane [68]. Using several cell lines including C2C12 muscle cells, we found that CD36 is ubiquitinated on lysines 469 and 472 on its C-terminal domain (Figure 1b). Importantly, this process is inhibited by insulin but enhanced by FA, suggesting physiological mechanisms for CD36 regulation (Figure 2). However, although FA promotes ubiquitination and degradation of CD36, internalization of CD36 does not appear required for FA uptake [69] but may instead be tied to intracellular FA processing [70]. Intracellular trafficking of CD36 and its interaction with signaling molecules may represent novel determinants of FA metabolic fates following uptake. For example, it is possible that partitioning FA between storage and oxidation may partially depend on CD36 subcellular localization.
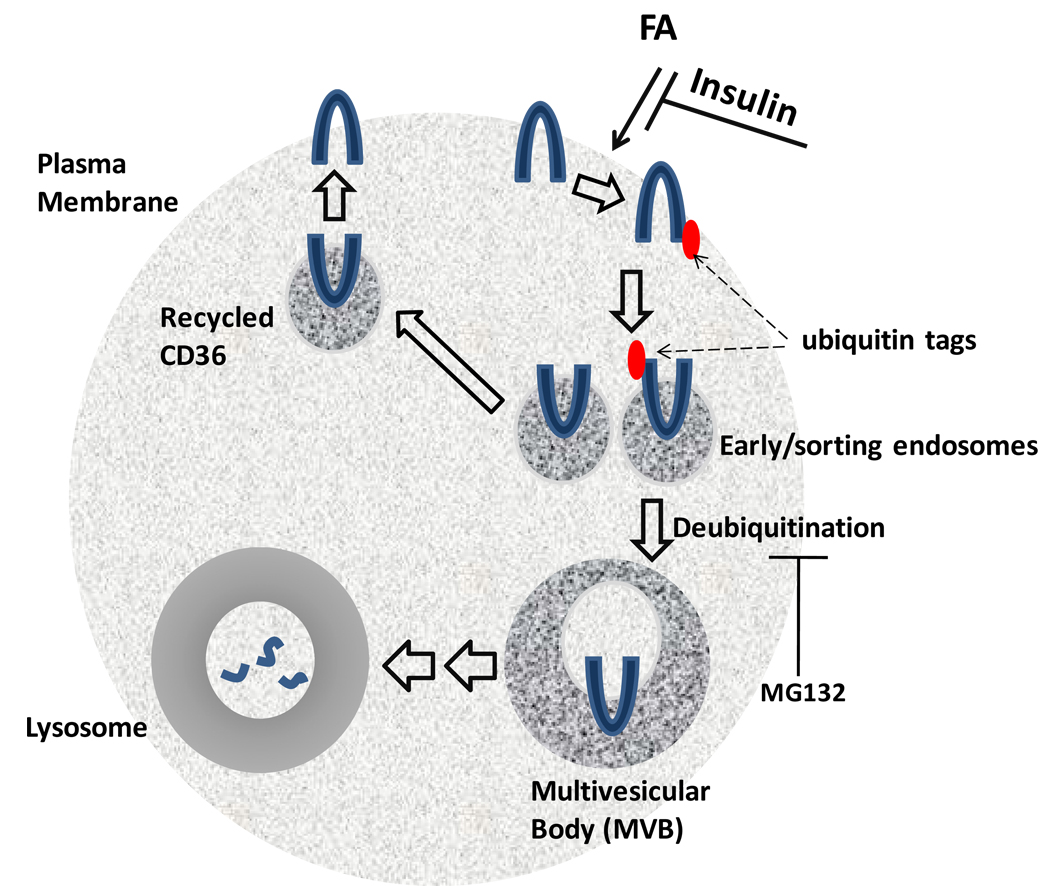
Figure 2. Ubiquitination and trafficking of CD36.
This figure shows a proposed model for uniquitination-mediated CD36 trafficking. Ubiquitination is enhanced by FA and diminished by insulin. (i) Ubiquitinated or non-ubiquitinated CD36 is internalized into early/sorting endosomes, where the ubiquitinated form is selected and (ii) delivered to multivesicular body (MVB) and then to (iii) lysosomes for degradation. (iv) Non-ubiquitinated CD36 in early endosomes is recycled back to the plasma membrane. Deubiquitination of CD36 may be mediated by proteosome activity and is required for trafficking from early endosomes to MVB. This model is supported by the finding that treatment with MG132, a proteosomal inhibitor, leads to the accumulation of ubiquitinated CD36 [70].
Understanding how chemical modification(s) of CD36 impacts CD36 trafficking and FA metabolism will provide information on how FA uptake is regulated and why it is dysfunctional under certain conditions. For example, CD36 turnover is abnormally slow in macrophages from insulin resistant ob/ob mice [71] which may reflect altered CD36 modification by FA or insulin. Conceivably, this could result in more CD36 sorting to the membrane [Figure 2] as is observed in muscle of diabetic rodents and humans [13].
Despite the fact that the two cytoplasmic domains on CD36 are small, each domain contains several residues that are known sites for modification (Figure 1a and b). CD36 is palmitoylated at cysteines 3, 7, 464, and 466 [72]. Palmitoylation plays a key role in protein targeting to membrane lipid rafts and in protein-protein and protein-lipid interactions. Palmitoylation of CD36 at the various cysteines may differentially regulate its localization and function; however, studies using cysteine mutants are not yet available.
Concluding Comments
Recent progress has provided insight into several novel aspects of CD36-facilitated cellular uptake of long chain FA. Uptake might require membrane lipid rafts with initial FA metabolism likely occuring in segregated raft domains or caveolae. Consistent with this, proteins that uptake or metabolize FA [13, 73, 74] and caveolin [58] show changes in localization after metabolic stimuli. FA uptake involves cycling of CD36 between plasma membrane and intracellular organelles, and insulin resistance correlates with persistently increased sarcolemmal CD36 [13]. Recent findings support the interpretation that CD36 sorting to the membrane may be determined by CD36 ubiquitination, a process that is regulated by FA and insulin. Among the important questions that will need to be answered in future studies are the following: Is susceptibility of CD36 to ubiquitination by FA altered in insulin resistant states possibly via other chemical modifications of the protein? How are intracellular trafficking of CD36 and FA and FA metabolism altered by CD36 ubiquitination or palmitoylation states? What are the molecular players involved in CD36 trafficking between organelles and the plasma membrane? How does CD36 localization in mitochondria, the endoplasmic reticulum and lysosomes impact FA utilization? What are the mechanisms involved in tissue specific regulation of CD36 levels or function, and how does this impact metabolic homeostasis? For example, does CD36 function in adipocytes influence ectopic fat distribution and the pathogenesis of insulin resistance in muscle and liver? What is the impact of abnormal CD36 level or trafficking on heart function, and is this linked to cardiomyopathy? Better understanding of the molecular mechanisms mediating and regulating cellular FA uptake and translation of findings from rodents to humans will be crucial to designing approaches that prevent or target abnormal FA utilization and its deleterious consequences. Finally, a more thorough evaluation of the functional impact of polymorphisms in the CD36 gene that contribute to individual variations in lipid metabolism, in susceptibility to diet-induced pathology and in responsiveness to therapeutic interventions will be clinically valuable [75].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. 2006;86:465–514. doi: 10.1152/physrev.00025.2005. [DOI] [PubMed] [Google Scholar]
- 2.Zaid H, Antonescu CN, Randhawa VK, Klip A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem J. 2008;413:201–215. doi: 10.1042/BJ20080723. [DOI] [PubMed] [Google Scholar]
- 3.Silveira LR, Fiamoncini J, Hirabara SM, Procopio J, Cambiaghi TD, Pinheiro CH, Lopes LR, Curi R. Updating the effects of fatty acids on skeletal muscle. J Cell Physiol. 2008;217:1–12. doi: 10.1002/jcp.21514. [DOI] [PubMed] [Google Scholar]
- 4.Prentki M, Murthy Madiraju SR. Glycerolipid Metabolism and Signaling in Health and Disease. Endocr Rev. 2008;29:647–676. doi: 10.1210/er.2008-0007. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder F, Petrescu AD, Huang H, Atshaves BP, McIntosh AL, Martin GG, Hostetler HA, Vespa A, Landrock D, Landrock KK, et al. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids. 2008;43:1–17. doi: 10.1007/s11745-007-3111-z. [DOI] [PubMed] [Google Scholar]
- 6.Nahle Z, Hsieh M, Pietka T, Coburn CT, Grimaldi PA, Zhang MQ, Das D, Abumrad NA. CD36-dependent regulation of muscle FoxO1 and PDK4 in the PPAR delta/beta-mediated adaptation to metabolic stress. J Biol Chem. 2008;283:14317–14326. doi: 10.1074/jbc.M706478200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Sambandam N, Han X, Gross RW, Courtois M, Kovacs A, Febbraio M, Finck BN, Kelly DP. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ Res. 2007;100:1208–1217. doi: 10.1161/01.RES.0000264104.25265.b6. [DOI] [PubMed] [Google Scholar]
- 8.Madrazo JA, Kelly DP. The PPAR trio: regulators of myocardial energy metabolism in health and disease. J Mol Cell Cardiol. 2008;44:968–975. doi: 10.1016/j.yjmcc.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Tontonoz P, Spiegelman BM. Fat and Beyond: The Diverse Biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 10.Buteau J, Accili D. Regulation of pancreatic beta-cell function by the forkhead protein FoxO1. Diabetes Obes Metab. 2007;9 Suppl 2:140–146. doi: 10.1111/j.1463-1326.2007.00782.x. [DOI] [PubMed] [Google Scholar]
- 11.Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 12.Simard JR, Pillai BK, Hamilton JA. Fatty acid flip-flop in a model membrane is faster than desorption into the aqueous phase. Biochemistry. 2008;47:9081–9089. doi: 10.1021/bi800697q. [DOI] [PubMed] [Google Scholar]
- 13.Schwenk RW, Luiken JJ, Bonen A, Glatz JF. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc Res. 2008;79:249–258. doi: 10.1093/cvr/cvn116. [DOI] [PubMed] [Google Scholar]
- 14.Abumrad N, Storch J. Physiology of the Gastrointestinal Tract (Fourth Edition) Burlington: Academic Press; 2006. Role of Membrane and Cytosolic Fatty Acid Binding Proteins in Lipid Processing by the Small Intestine; pp. 1693–1709. [Google Scholar]
- 15.Ehehalt R, Fullekrug J, Pohl J, Ring A, Herrmann T, Stremmel W. Translocation of long chain fatty acids across the plasma membrane--lipid rafts and fatty acid transport proteins. Mol Cell Biochem. 2006;284:135–140. doi: 10.1007/s11010-005-9034-1. [DOI] [PubMed] [Google Scholar]
- 16.Black PN, DiRusso CC. Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation. Biochim Biophys Acta. 2007;1771:286–298. doi: 10.1016/j.bbalip.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Stremmel W, Pohl L, Ring A, Herrmann T. A new concept of cellular uptake and intracellular trafficking of long-chain fatty acids. Lipids. 2001;36:981–989. doi: 10.1007/s11745-001-0809-2. [DOI] [PubMed] [Google Scholar]
- 18.Covington DK, Briscoe CA, Brown AJ, Jayawickreme CK. The Gprotein-coupled receptor 40 family (GPR40–GPR43) and its role in nutrient sensing. Biochem Soc Trans. 2006;34:770–773. doi: 10.1042/BST0340770. [DOI] [PubMed] [Google Scholar]
- 19.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 20.Love-Gregory L, Sherva R, Sun L, Wasson J, Schappe T, Doria A, Rao DC, Hunt SC, Klein S, Neuman RJ, et al. Variants in the CD36 gene associate with the metabolic syndrome and high-density lipoprotein cholesterol. Hum Mol Genet. 2008;17:1695–1704. doi: 10.1093/hmg/ddn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J, Febbraio M, Wada T, Zhai Y, Kuruba R, He J, Lee JH, Khadem S, Ren S, Li S, et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 2008;134:556–567. doi: 10.1053/j.gastro.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 22.Ma X, Bacci S, Mlynarski W, Gottardo L, Soccio T, Menzaghi C, Iori E, Lager RA, Shroff AR, Gervino EV, et al. A common haplotype at the CD36 locus is associated with high free fatty acid levels and increased cardiovascular risk in Caucasians. Hum Mol Genet. 2004;13:2197–2205. doi: 10.1093/hmg/ddh233. [DOI] [PubMed] [Google Scholar]
- 23.Hajri T, Abumrad NA. Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Annu Rev Nutr. 2002;22:383–415. doi: 10.1146/annurev.nutr.22.020402.130846. [DOI] [PubMed] [Google Scholar]
- 24.Febbraio M, Silverstein RL. CD36: implications in cardiovascular disease. Int J Biochem Cell Biol. 2007;39:2012–2030. doi: 10.1016/j.biocel.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazen SL. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J Biol Chem. 2008;283:15527–15531. doi: 10.1074/jbc.R700054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stuart LM, Bell SA, Stewart CR, Silver JM, Richard J, Goss JL, Tseng AA, Zhang A, El Khoury JB, Moore KJ. CD36 signals to the actin cytoskeleton and regulates microglial migration via a p130Cas complex. J Biol Chem. 2007;282:27392–27401. doi: 10.1074/jbc.M702887200. [DOI] [PubMed] [Google Scholar]
- 27.Demers A, Rodrigue-Way A, Tremblay A. Hexarelin Signaling to PPARgamma in Metabolic Diseases. PPAR Res. 2008;2008:364784. doi: 10.1155/2008/364784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 29.Febbraio M. CD36 goes native. Arterioscler Thromb Vasc Biol. 2008;28:1209–1210. doi: 10.1161/ATVBAHA.108.169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Yassimi A, Hichami A, Besnard P, Khan NA. Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J Biol Chem. 2008;283:12949–12959. doi: 10.1074/jbc.M707478200. [DOI] [PubMed] [Google Scholar]
- 31.Susztak K, Ciccone E, McCue P, Sharma K, Bottinger EP. Multiple metabolic hits converge on CD36 as novel mediator of tubular epithelial apoptosis in diabetic nephropathy. PLoS Med. 2005;2:e45. doi: 10.1371/journal.pmed.0020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamashita S, Hirano KI, Kuwasako T, Janabi M, Toyama Y, Ishigami M, Sakai N. Physiological and pathological roles of a multi-ligand receptor CD36 in atherogenesis; insights from CD36-deficient patients. Mol Cell Biochem. 2007;299:19–22. doi: 10.1007/s11010-005-9031-4. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka T, Nakata T, Oka T, Ogawa T, Okamoto F, Kusaka Y, Sohmiya K, Shimamoto K, Itakura K. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J Lipid Res. 2001;42:751–759. [PubMed] [Google Scholar]
- 34.Irie H, Krukenkamp IB, Brinkmann JF, Gaudette GR, Saltman AE, Jou W, Glatz JF, Abumrad NA, Ibrahimi A. Myocardial recovery from ischemia is impaired in CD36-null mice and restored by myocyte CD36 expression or medium-chain fatty acids. Proc Natl Acad Sci U S A. 2003;100:6819–6824. doi: 10.1073/pnas.1132094100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuang M, Febbraio M, Wagg C, Lopaschuk GD, Dyck JR. Fatty acid translocase/CD36 deficiency does not energetically or functionally compromise hearts before or after ischemia. Circulation. 2004;109:1550–1557. doi: 10.1161/01.CIR.0000121730.41801.12. [DOI] [PubMed] [Google Scholar]
- 36.Koonen DP, Febbraio M, Bonnet S, Nagendran J, Young ME, Michelakis ED, Dyck JR. CD36 expression contributes to age-induced cardiomyopathy in mice. Circulation. 2007;116:2139–2147. doi: 10.1161/CIRCULATIONAHA.107.712901. [DOI] [PubMed] [Google Scholar]
- 37.Drover VA, Ajmal M, Nassir F, Davidson NO, Nauli AM, Sahoo D, Tso P, Abumrad NA. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J Clin Invest. 2005;115:1290–1297. doi: 10.1172/JCI21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masuda D, Hirano KI, Oku H, Sandoval JC, Kawase R, Yuasa-Kawase M, Yamashita Y, Takada M, Tsubakio-Yamamoto K, Tochino Y, et al. Chylomicron remnants are increased in the postprandial state in CD36 deficiency. J Lipid Res. 2008 doi: 10.1194/jlr.P700032-JLR200. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem. 2007;282:19493–19501. doi: 10.1074/jbc.M703330200. [DOI] [PubMed] [Google Scholar]
- 40.Drover VA, Nguyen DV, Bastie CC, Darlington YF, Abumrad NA, Pessin JE, London E, Sahoo D, Phillips MC. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J Biol Chem. 2008;283:13108–13115. doi: 10.1074/jbc.M708086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, Vonlehmden SB, Lee D, Jandacek RJ, Abumrad NA, Tso P. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 2006;131:1197–1207. doi: 10.1053/j.gastro.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1823–R1832. doi: 10.1152/ajpregu.00211.2007. [DOI] [PubMed] [Google Scholar]
- 43.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goudriaan JR, den Boer MA, Rensen PC, Febbraio M, Kuipers F, Romijn JA, Havekes LM, Voshol PJ. CD36 deficiency in mice impairs lipoprotein lipase-mediated triglyceride clearance. J Lipid Res. 2005;46:2175–2181. doi: 10.1194/jlr.M500112-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Cheung L, Andersen M, Gustavsson C, Odeberg J, Fernandez-Perez L, Norstedt G, Tollet-Egnell P. Hormonal and nutritional regulation of alternative CD36 transcripts in rat liver--a role for growth hormone in alternative exon usage. BMC Mol Biol. 2007;8:60. doi: 10.1186/1471-2199-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greco D, Kotronen A, Westerbacka J, Puig O, Arkkila P, Kiviluoto T, Laitinen S, Kolak M, Fisher RM, Hamsten A, et al. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1281–G1287. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 47.Pravenec M, Churchill PC, Churchill MC, Viklicky O, Kazdova L, Aitman TJ, Petretto E, Hubner N, Wallace CA, Zimdahl H, et al. Identification of renal Cd36 as a determinant of blood pressure and risk for hypertension. Nat Genet. 2008;40:952–954. doi: 10.1038/ng.164. [DOI] [PubMed] [Google Scholar]
- 48.Zhu W, Smart EJ. Myristic acid stimulates endothelial nitric-oxide synthase in a CD36- and an AMP kinase-dependent manner. J Biol Chem. 2005;280:29543–29550. doi: 10.1074/jbc.M501238200. [DOI] [PubMed] [Google Scholar]
- 49.Bastie CC, Nahle Z, McLoughlin T, Esser K, Zhang W, Unterman T, Abumrad NA. FoxO1 stimulates fatty acid uptake and oxidation in muscle cells through CD36-dependent and -independent mechanisms. J Biol Chem. 2005;280:14222–14229. doi: 10.1074/jbc.M413625200. [DOI] [PubMed] [Google Scholar]
- 50.Larsen TS, Aasum E. Metabolic (In)Flexibility of the Diabetic Heart. Cardiovasc Drugs Ther. 2008;22:91–95. doi: 10.1007/s10557-008-6083-1. [DOI] [PubMed] [Google Scholar]
- 51.Goudriaan JR, Dahlmans VE, Teusink B, Ouwens DM, Febbraio M, Maassen JA, Romijn JA, Havekes LM, Voshol PJ. CD36 deficiency increases insulin sensitivity in muscle, but induces insulin resistance in the liver in mice. J Lipid Res. 2003;44:2270–2277. doi: 10.1194/jlr.M300143-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Pravenec M, Landa V, Zidek V, Musilova A, Kren V, Kazdova L, Aitman TJ, Glazier AM, Ibrahimi A, Abumrad NA, et al. Transgenic rescue of defective Cd36 ameliorates insulin resistance in spontaneously hypertensive rats. Nat Genet. 2001;27:156–158. doi: 10.1038/84777. [DOI] [PubMed] [Google Scholar]
- 53.An P, Freedman BI, Hanis CL, Chen YD, Weder AB, Schork NJ, Boerwinkle E, Province MA, Hsiung CA, Wu X, et al. Genome-wide linkage scans for fasting glucose, insulin, and insulin resistance in the National Heart, Lung, and Blood Institute Family Blood Pressure Program: evidence of linkages to chromosome 7q36 and 19q13 from meta-analysis. Diabetes. 2005;54:909–914. doi: 10.2337/diabetes.54.3.909. [DOI] [PubMed] [Google Scholar]
- 54.Malhotra A, Elbein SC, Ng MC, Duggirala R, Arya R, Imperatore G, Adeyemo A, Pollin TI, Hsueh WC, Chan JC, et al. Meta-analysis of genome-wide linkage studies of quantitative lipid traits in families ascertained for type 2 diabetes. Diabetes. 2007;56:890–896. doi: 10.2337/db06-1057. [DOI] [PubMed] [Google Scholar]
- 55.Ehehalt R, Sparla R, Herrmann T, Kulaksiz H, Fullekrug J, Stremmel W. Uptake of long chain fatty acids is regulated by dynamic interaction of FAT/CD36 with cholesterol/ sphingolipid enriched microdomains (lipid rafts) BMC Cell Biol. 2008;9:45. doi: 10.1186/1471-2121-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ortegren U, Aboulaich N, Ost A, Stralfors P. A new role for caveolae as metabolic platforms. Trends Endocrinol Metab. 2007;18:344–349. doi: 10.1016/j.tem.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 57.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 58.Pilch PF, Souto RP, Liu L, Jedrychowski MP, Berg EA, Costello CE, Gygi SP. Cellular spelunking: exploring adipocyte caveolae. J Lipid Res. 2007;48:2103–2111. doi: 10.1194/jlr.R700009-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Ring A, Le Lay S, Pohl J, Verkade P, Stremmel W. Caveolin-1 is required for fatty acid translocase (FAT/CD36) localization and function at the plasma membrane of mouse embryonic fibroblasts. Biochim Biophys Acta. 2006;1761:416–423. doi: 10.1016/j.bbalip.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 60.Labrecque L, Nyalendo C, Langlois S, Durocher Y, Roghi C, Murphy G, Gingras D, Beliveau R. Src-mediated tyrosine phosphorylation of caveolin-1 induces its association with membrane type 1 matrix metalloproteinase. J Biol Chem. 2004;279:52132–52140. doi: 10.1074/jbc.M409617200. [DOI] [PubMed] [Google Scholar]
- 61.Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- 62.Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, Li M, Tang B, Jelicks LA, Scherer PE, et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem. 2002;277:8635–8647. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- 63.Campbell SE, Tandon NN, Woldegiorgis G, Luiken JJ, Glatz JF, Bonen A. A novel function for fatty acid translocase (FAT)/CD36: involvement in long chain fatty acid transfer into the mitochondria. J Biol Chem. 2004;279:36235–36241. doi: 10.1074/jbc.M400566200. [DOI] [PubMed] [Google Scholar]
- 64.Holloway GP, Thrush AB, Heigenhauser GJ, Tandon NN, Dyck DJ, Bonen A, Spriet LL. Skeletal muscle mitochondrial FAT/CD36 content and palmitate oxidation are not decreased in obese women. Am J Physiol Endocrinol Metab. 2007;292:E1782–E1789. doi: 10.1152/ajpendo.00639.2006. [DOI] [PubMed] [Google Scholar]
- 65.Benton CR, Holloway GP, Campbell SE, Yoshida Y, Tandon NN, Glatz JF, Luiken JJ, Spriet LL, Bonen A. Rosiglitazone increases fatty acid oxidation and fatty acid translocase (FAT/CD36) but not carnitine palmitoyltransferase I in rat muscle mitochondria. J Physiol. 2008;586:1755–1766. doi: 10.1113/jphysiol.2007.146563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eyre NS, Cleland LG, Tandon NN, Mayrhofer G. Importance of the carboxyl terminus of FAT/CD36 for plasma membrane localization and function in long-chain fatty acid uptake. J Lipid Res. 2007;48:528–542. doi: 10.1194/jlr.M600255-JLR200. [DOI] [PubMed] [Google Scholar]
- 67.Sun B, Boyanovsky BB, Connelly MA, Shridas P, van der Westhuyzen DR, Webb NR. Distinct mechanisms for OxLDL uptake and cellular trafficking by class B scavenger receptors CD36 and SR-BI. J Lipid Res. 2007;48:2560–2570. doi: 10.1194/jlr.M700163-JLR200. [DOI] [PubMed] [Google Scholar]
- 68.Miranda M, Sorkin A. Regulation of receptors and transporters by ubiquitination: new insights into surprisingly similar mechanisms. Mol Interv. 2007;7:157–167. doi: 10.1124/mi.7.3.7. [DOI] [PubMed] [Google Scholar]
- 69.Pohl J, Ring A, Korkmaz U, Ehehalt R, Stremmel W. FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts. Mol Biol Cell. 2005;16:24–31. doi: 10.1091/mbc.E04-07-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith J, Su X, El-Maghrabi R, Stahl PD, Abumrad NA. Opposite regulation of CD36 ubiquitination by fatty acids and insulin: effects on fatty acid uptake. J Biol Chem. 2008;283:13578–13585. doi: 10.1074/jbc.M800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang CP, Han S, Okamoto H, Carnemolla R, Tabas I, Accili D, Tall AR. Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest. 2004;113:764–773. doi: 10.1172/JCI19528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tao N, Wagner SJ, Lublin DM. CD36 is palmitoylated on both N- and C-terminal cytoplasmic tails. J Biol Chem. 1996;271:22315–22320. doi: 10.1074/jbc.271.37.22315. [DOI] [PubMed] [Google Scholar]
- 73.Stahl A, Evans JG, Pattel S, Hirsch D, Lodish HF. Insulin causes fatty acid transport protein translocation and enhanced fatty acid uptake in adipocytes. Dev Cell. 2002;2:477–488. doi: 10.1016/s1534-5807(02)00143-0. [DOI] [PubMed] [Google Scholar]
- 74.Lobo S, Wiczer BM, Smith AJ, Hall AM, Bernlohr DA. Fatty acid metabolism in adipocytes: functional analysis of fatty acid transport proteins 1 and 4. J Lipid Res. 2007;48:609–620. doi: 10.1194/jlr.M600441-JLR200. [DOI] [PubMed] [Google Scholar]
- 75.Madden J, Carrero JJ, Brunner A, Dastur N, Shearman CP, Calder PC, Grimble RF. Polymorphisms in the CD36 gene modulate the ability of fish oil supplements to lower fasting plasma triacyl glycerol and raise HDL cholesterol concentrations in healthy middle-aged men. Prostaglandins Leukot Essent Fatty Acids. 2008;78:327–335. doi: 10.1016/j.plefa.2008.04.003. [DOI] [PubMed] [Google Scholar]