Abstract
The existence of sex-based differences in tendon and ligament injury rates has led investigators to test the hypothesis that sex plays a significant role in modulating tendon and ligament composition and material properties. To date, no studies have attempted to characterize how such differences develop during the course of normal tissue maturation and growth. Thus, the primary aim of the present study was to use a murine model to test the hypothesis that sex based differences in the normal age-related development of tendon composition and material properties exist by assessing these parameters in the Achilles and tail tendons from 4, 6, 9, 12, and 15 week-old male and female C57Bl/6J mice. Despite significantly lower levels of total collagen content in females subsequent to sexual maturity (p < 0.0001), as well as a significant effect of sex on glycosaminoglycan content (p < 0.0001), Achilles tendon elastic modulus was not compromised in females. Female Achilles tendons did exhibit a significantly higher failure strain (p = 0.0201) and strain energy density (p = 0.0004) than did males, as well as a trend toward higher ultimate strength (p = 0.0556). In contrast to the high load-bearing environment of the Achilles tendon site, sex did not have a statistically significant effect on any compositional or material property in the low load bearing tendon fascicles of the tail. These data support recent studies by others, which suggest that male and female tendons have a differential adaptational response to their local mechanical loading environment.
Keywords: tendon, sex, mouse, material properties, composition
INTRODUCTION
The existence of sex-based differences in tendon and ligament injury rates is well established, although the precise nature of this sex-bias appears to be dependent on anatomical location1-5. In the Anterior Cruciate Ligament (ACL) of the knee, for example, the incidence of non-contact injury is roughly 4-6 times greater for women than men1-3. The prevailing theories used to explain the higher ACL injury rate among women include anatomical differences6-10, neuromuscular differences11-14 and hormonal effects on the metabolism of the tissue15-18 that could potentially result in inferior material properties, thereby rendering the ligament more susceptible to failure. In contrast to the ACL, injury rates for the Achilles tendon are roughly four times greater in men than women4,5,19-21, with the primary theory put forward being the differential participation of men and women in certain sports5.
Despite the notably higher incidence of Achilles tendon rupture in males, no studies to date have examined whether there are sex-based differences in the material properties of the Achilles tendon. Further, among the studies that have investigated possible sex-based differences in the material properties of other tendons and ligaments9,10, 22, none has attempted to examine how such differences develop during the course of normal tissue maturation and growth. The primary aim of the present study, then, was to use a murine model to test the hypothesis that sex based differences in the normal age-related development of Achilles tendon composition and material properties exist. A secondary aim was to test whether such differences are influenced by local in vivo mechanical loading environment by examining two distinct anatomical locations: the highly loaded Achilles tendon, and the less loaded tendon fascicles of the tail.
METHODS
Study Design
Ten male and ten female C57BL/6J mice (Jackson Labs strain #000664) at each age of 4, 6, 9, 12, and 15 weeks were euthanized via CO2 inhalation in accordance with IACUC guidelines. Animals were quarantined in single sex cohorts of the same age for a minimum of one week after being received from the vendor and were kept on a 12 hour light/dark cycle, at 72°F and 40% humidity with free cage activity and food and water provided ad libitum. After being euthanized, animals were immediately weighed, and tissue allocated as follows: right Achilles tendons were processed to assess biochemical composition; left Achilles tendons were used for mechanical testing; one tail tendon fascicle bundle was used for compositional analysis; and the remaining three tail tendon fascicle bundles were used for mechanical testing.
Compositional Analysis
Immediately after sacrifice, the right Achilles tendon and one tail tendon fascicle bundle were dissected free and each placed in 500μl of sterile papain solution (125 μg/ml in 1X PBE, pH 6.5, with papain first diluted in PBE containing 5mM L-cysteine HCl) for 18 hours at 60°C. DNA content was determined fluorometrically on a BioTek FLX 800 Multi-Detection Microplate Reader (BioTek Instruments Inc., Winooski, VT) using the Hoechst 33258 Dye Method with calf thymus DNA as a standard23. Sulfated glycosaminoglycan (GAG) content was determined using a dimethylmethylene blue (DMMB) assay, with dermatan sulphate as a standard24. Lastly, hydroxyproline (Hyp) content, indicative of total collagen content, was determined subsequent to acid hydrolysis in 6N hydrochloric acid at 110°C for 24 hours using a chloramine-T/dimethylaminobenzaldehyde (DMBA) method, with purified hydroxy-L-proline as a standard25. Each sample and standard was processed in duplicate for all assays, and average values used for analysis. GAG and Hyp values were normalized to DNA to account for sample-to-sample size differences. DNA (rather than wet weight) normalization was chosen for the present study due to lower scatter in DNA values in pilot study data (standard deviation was approximately 15% of mean DNA value, whereas wet weight SD/mean was approximately twice that value). An additional cohort of ten male and ten female C57BL/6J mice at twelve weeks of age were utilized to verify that cell density was comparable between males and females (thus making DNA normalization a reasonable substitute for wet weight normalization). For each Achilles tendon, wet weight was determined using a microbalance, DNA content determined as described above, and DNA/wet weight calculated as an indicator of cell density.
Mechanical Testing of Achilles Tendons
Detailed methods regarding the tensile testing of murine Achilles tendons have been published previously26,27. In summary, each left hind limb was dissected free at the hip immediately after sacrifice, wrapped in saline-soaked gauze and frozen in a sealed bag at −20°C until the time of testing. After thawing at room temperature for one hour, tendons were prepared and measured as described elsewhere26,27, then loaded to failure (without preconditioning) at a strain rate of 100%/sec using the same materials testing system as in previous studies26,27, keeping tendons moist throughout. A strain rate of 100%/sec was chosen for physiological relevance28. Gauge length was determined by zeroing the testing system displacement with the fixtures in contact (0.1N compression) and recording the vertical displacement after the sample was inserted and pre-loaded to 0.05N. Displacement and load were then re-zeroed, test performed, and failure mode recorded. Grip displacement normalized to initial gage length was used to calculate strain, while stress was calculated as recorded load divided by tendon cross-sectional area, assuming a circular cross-section with diameter as determined via LED micrometer as previously described27. The following material properties were then calculated: (1) Ultimate Strength; (2) Modulus of Elasticity; (3) Failure Strain; and (4) Strain Energy Density. Modulus was determined using linear regression to the near-linear region of the stress-strain curve with a point inclusion criterion of r2 ≥ 0.999. Yield data were not calculated, as murine Achilles tendons do not exhibit a yield point at high strain rates.
Mechanical Testing of Tail Tendon Fascicles
Subsequent to removal of the tail tendon bundle that was used for compositional analysis, the remainder of the tail was immediately wrapped in saline-soaked gauze and frozen at −20°C in a sealed plastic bag until the day of testing. After thawing for one hour at room temperature, each of the remaining three bundles was dissected free, and fascicles teased apart under a dissecting microscope, maintaining hydration throughout. Detailed methods for tensile testing of tail tendon fascicles have been previously published29. In brief, five individual tendon fascicles were randomly chosen by alternating between the three remaining bundles and tested to failure in tension at a strain rate of 50%/sec, without preconditioning, in phosphate buffered saline using the same materials testing system as in previous studies29. Fascicles were sandwiched between two pieces of sandpaper that were securely taped to the top and bottom of a laser-cut card-frame used to mount the specimens29. Using the pre-set 20 mm gage length card frame and cross-sectional area (determined using a reticulated eye-piece under a dissecting microscope at 40X and assuming a circular cross section, with fascicle diameter measured in triplicate), force-displacement data were converted to stress-strain data, and the following material properties were calculated: (1) Ultimate Strength; (2) Modulus of Elasticity; (3) Failure Strain; (4) Strain Energy Density; and (5) Yield Strain. As with the Achilles tendons, strain was determined using the grip displacement normalized to gage length, and modulus was determined using linear regression to the near-linear region of the stress-strain curve with a point inclusion criterion of r2 ≥ 0.999. For each dependent variable, values from all five fascicles per tail were averaged together to serve as the representative value for any given animal.
Statistical Analysis
All dependent parameters were analyzed statistically using the statistical software package Statview 5.0 (SAS Institute, Cary, NC). A two-factor ANOVA was employed, with sex and age as the two independent variables, and a cutoff value of p ≤ 0.05 for statistical significance. If sex was statistically significant for a given dependent variable, post-hoc analyses were performed with corrections for multiple comparisons via Bonferroni/Dunn. Given the observed variance (error MS) for each measured compositional and material parameter, power analyses indicated a minimum detectable difference between means of 10-15% with the sample sizes that were employed (n = 10 per group), assuming α = 0.05 and 1-β = 0.80. All data are reported as mean ± standard deviation.
RESULTS
Morphometry
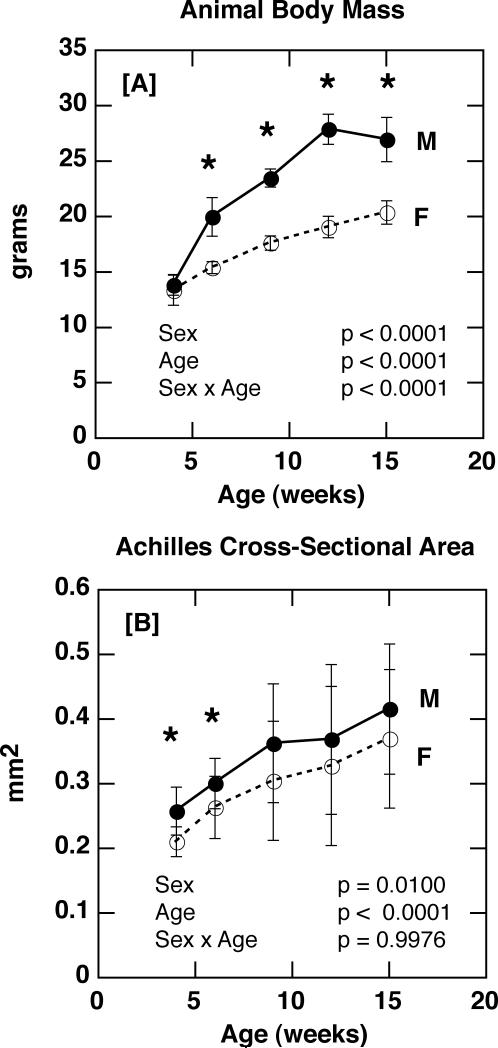
After four weeks of age, male body mass was significantly greater than that of females (p < 0.0001 for sex), with body mass increasing more rapidly with age among males than females (p < 0.0001 for age × sex; Figure 1 A). As expected based on body mass differences, sex had a significant effect on the cross-sectional area of the Achilles tendon, with males having larger tendons than females (p = 0.01 for sex; Figure 1 B).
Figure 1.
[A] Animal body mass in male and female C57Bl/6J mice with age (p < 0.0001 for sex; p < 0.0001 for age; p < 0.0001 for sex × age). [B] Achilles tendon cross-sectional area in male and female C57Bl/6J mice with age (p = 0.01 for sex; p < 0.0001 for age; p = 0.9976 for sex × age). [* p ≤ 0.05]
Achilles Tendon Composition and Material Properties
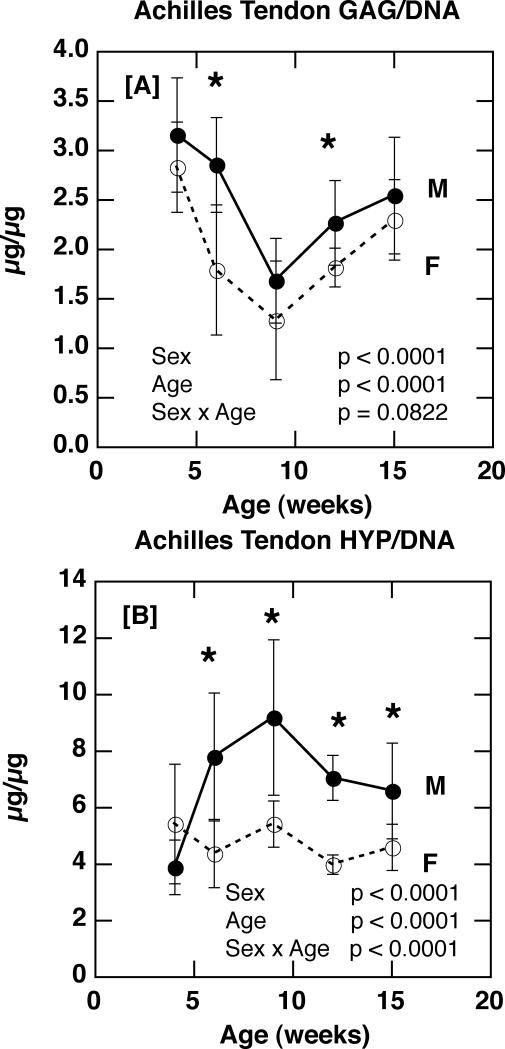
Animal sex had a highly significant effect on Achilles tendon composition, with female tendons exhibiting lower proteoglycan (GAG/DNA) and total collagen (Hyp/DNA) than males (Figure 2 A & B; p < 0.0001 for sex). While the age-related changes in proteoglycan content were not significantly modulated by sex (p = 0.0822 for age × sex; Figure 2 A), the age-related changes in collagen were (p < 0.0001 for age × sex): male and female collagen content were comparable at four weeks of age, but subsequent to sexual maturity (5-6 weeks of age for C57BL/6J mice), female values were significantly lower than male values (Figure 2 B).
Figure 2.
[A] Achilles tendon glycosaminoglycan content (GAG/DNA) in male and female C57Bl/6J mice with age (p < 0.0001 for sex; p < 0.0001 for age; p = 0.0822 for sex × age). [B] Achilles tendon collagen content (Hydroxyproline/DNA) in male and female C57Bl/6J mice with age (p < 0.0001 for sex; p < 0.0001 for age; p < 0.0001 for sex × age). [* p ≤ 0.05]
For the 12-week time point at which DNA/wet weight was assessed, sex did not have a significant effect (1.73 ± 0.46 μg/mg (F) vs. 1.50 ± 0.28 μg/mg (M); p = 0.1455), thus suggesting comparable cell density between males and females at this age. Sample size calculations indicated that 24 mice per group would be required for this difference between means to reach statistical significance with α = 0.05 and 1-β = 0.80.
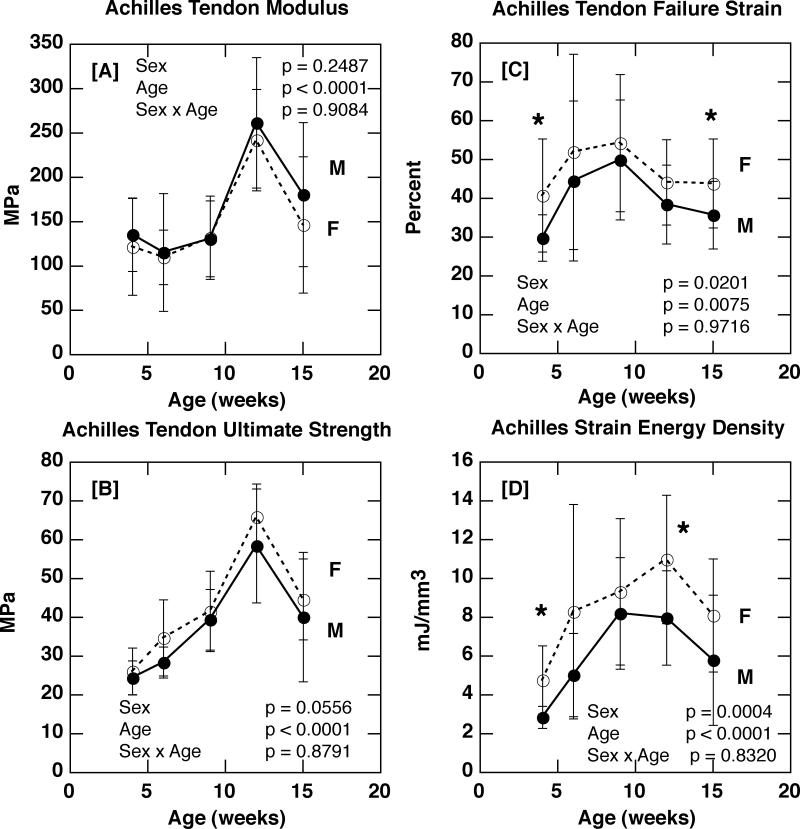
Despite the significantly lower levels of both collagen and proteoglycan content, female Achilles tendon modulus of elasticity was comparable to that of males (Figures 3 A). Female tendons did exhibit a significantly greater failure strain (p = 0.0201 for sex; Figure 3 C), strain energy density (p = 0.0004 for sex; Figure 3 D), and a strong trend towards greater ultimate failure strength (p = 0.0556 for sex; Figure 3 B) compared to males, however. Logistic regression (i.e. the logistic likelihood ratio test) demonstrated that sex did not have a significant effect on tendon failure mode (avulsion vs. tendon mid-substance) at any age (S-Table 1).
Figure 3.
[A] Achilles tendon modulus of elasticity in male and female C57Bl/6J mice with age (p = 0.2487 for sex; p < 0.0001 for age; p = 0.9084 for sex × age). [B] Achilles tendon ultimate tensile strength in male and female C57Bl/6J mice with age (p = 0.0556 for sex; p < 0.0001 for age; p = 0.8791 for sex × age). [C] Achilles tendon failure strain in male and female C57Bl/6J mice with age (p = 0.0201 for sex; p = 0.0075 for age; p = 0.9716 for sex × age). [D] Achilles tendon strain energy density in male and female C57Bl/6J mice with age (p = 0.0004 for sex; p < 0.0001 for age; p = 0.8320 for sex × age). [* p ≤ 0.05]
Tail Tendon Composition and Material Properties
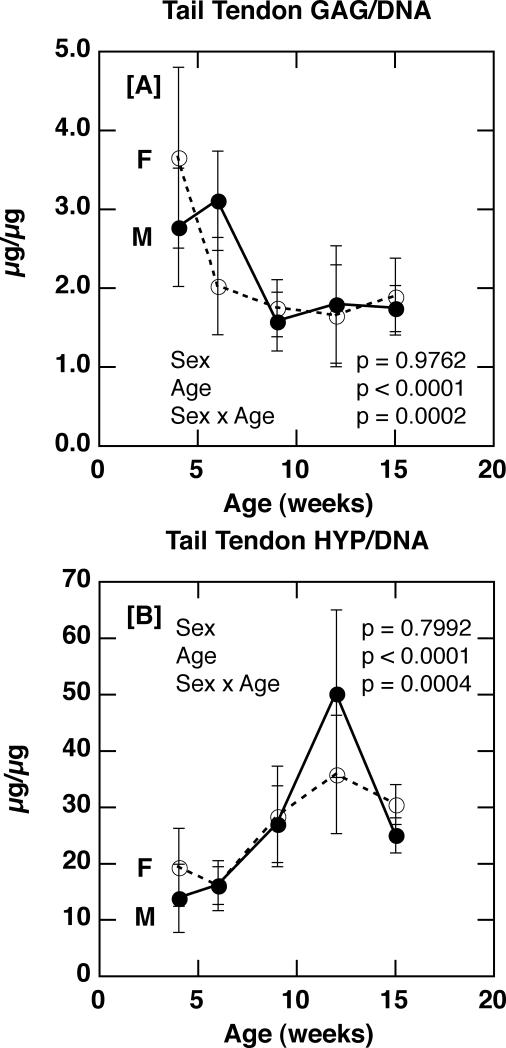
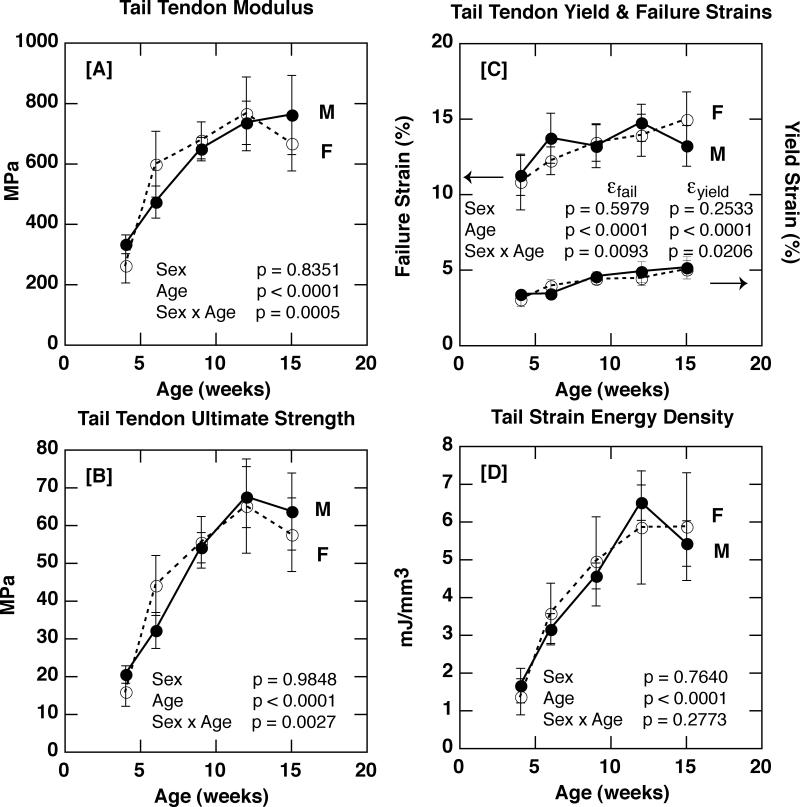
In contrast to the Achilles tendon site, sex did not have a significant effect on either compositional parameter in tail tendon fascicles (p = 0.9762 for GAG/DNA; p = 0.7992 for Hyp/DNA; Figure 4 A & B). Consistent with the lack of sex-based differences in composition, sex was not found to have a significant effect on tail tendon modulus of elasticity, ultimate tensile strength, yield strain, failure strain, or strain energy density (Figures 5). Thus, while sex was a significant factor in influencing tendon composition and some material properties (i.e. strain energy density and failure strain), this effect was only detected in the Achilles tendon, and not the tendon fascicles of the tail.
Figure 4.
[A] Tail tendon glycosaminoglycan content (GAG/DNA) in male and female C57Bl/6J mice with age (p = 0.9762 for sex; p < 0.0001 for age; p = 0.0002 for sex × age). [B] Tail tendon collagen content (Hydroxyproline/DNA) in male and female C57Bl/6J mice with age (p = 0.7992 for sex; p < 0.0001 for age; p = 0.0004 for sex × age).
Figure 5.
[A] Tail tendon elastic modulus in male and female C57Bl/6J mice with age (p = 0.8351 for sex; p < 0.0001 for age; p = 0.0005 for sex × age). [B] Tail tendon ultimate tensile strength in male and female C57Bl/6J mice with age (p = 0.9848 for sex; p < 0.0001 for age; p = 0.0027 for sex × age). [C] Tail tendon yield (right axis) and failure (left axis) strains in male and female C57Bl/6J mice with age (Yield Strain: p = 0.2533 for sex, p < 0.0001 for age, p = 0.0206 for sex × age; Failure Strain: p = 0.5979 for sex, p < 0.0001 for age, p = 0.0093 for sex × age). [D] Tail tendon strain energy density in male and female C57Bl/6J mice with age (p = 0.7640 for sex; p < 0.0001 for age; p = 0.2773 for sex × age).
DISCUSSION
The primary aim of the present study was to test the hypothesis that there are significant sex-based differences in the composition and material properties of tendons in growing C57BL/6J mice. Despite significantly lower levels of total (DNA-normalized) collagen and GAG content in female Achilles tendons, sex did not have a statistically significant effect on Achilles tendon elastic modulus. Failure strain and strain energy density were significantly greater in females, however, and there was a strong trend for ultimate failure strength to be greater for the females compared to the males (55% power).
It is intriguing to note that sex was found to modulate the normal age-related increases in Achilles tendon collagen content. At four weeks of age, male and female collagen levels are comparable. Subsequent to sexual maturity, however (5-6 weeks of age in C57BL/6J mice30,31), significant sex-based differences arise, with male levels increasing two-and-a-half fold to their peak at nine weeks, while female values remained roughly constant. It is well-established that estrogen receptors are present in tendons and ligaments15,32-36, and that estrogen can decrease the amount of collagen present in ACL tissue in culture37. Miller and colleagues recently demonstrated that in human patellar tendons, resting tendon collagen synthesis rates are significantly lower in females than males38. Although the relationship between estrogen and collagen production is likely to be dependent on species, these results from humans are consistent with the lower levels of collagen observed in female C57BL/6J mice in the present study. While hormonal levels were not measured in the current study, it is noteworthy that these sex-based differences in collagen only arise subsequent to the reported onset of sexual maturity in this strain of mice (5-6 weeks of age) 30,31. In mice, baseline serum estrogen levels rise sharply with the onset of puberty (sexual maturity) and remain high throughout adulthood, with cyclical increases leading into the preoestrus phase of the regular estrus cycle, followed by a return to baseline levels with ovulation39. Baseline estrogen levels then decline precipitously during estropause (the rodent equivalent of menopause), at approximately 12 months of age39. In the present study, it is likely that the 4 week old animals are pre-pubertal. Given that the oldest cohort (15 weeks) is still far from the age at which anestrus occurs, it is also likely that the remaining age groups fall into the age range corresponding to higher baseline levels of serum estrogen, prior to the decline that accompanies estropause. To verify this, however, a carefully controlled serum estrogen profiling would need to be performed over this age span in the C57Bl6/J strain.
The precise nature of the relationship between tendon composition and material behavior is undoubtedly complex, however, studies in the literature support an obvious correlation between collagen content and material strength and stiffness40,41. Thus, it is surprising that, despite significantly lower collagen levels in female mice, Achilles tendon modulus was not adversely affected in the present study. Comparable modulus in the face of lower collagen cannot be explained by differential Achilles tendon failure modes between males and females, as no significant sex-based difference in failure mode was detected at any age. An alternative explanation is that sex-based differences in Achilles tendon cell-density could exist, thereby artificially resulting in lower DNA-normalized levels of collagen and GAG in females when in fact mass-normalized compositional measures might be comparable in both sexes. While a comprehensive assessment of cell density changes with age was not included as part of the present study, our limited measure of DNA/wet weight in 12 week old Achilles tendons found no significant difference between males and females at that age with the sample size that was used. A larger sample size, as outlined in the Results section, may have revealed differences. Our preliminary QRT-PCR studies (not shown) demonstrate that Col1α1 gene expression levels in 15 week old female Achilles tendons are only 20% of male levels, whereas expression levels are comparable between sexes in tail tendons at this age. These preliminary gene expression data support the interpretation that the observed compositional differences in the present study are not an artifact of DNA-normalization. Further investigation into sex-based differences in the presence (and age-related changes in) collagen cross-linking, as well as a detailed ultrastructural characterization of collagen fibril diameter distribution, area fraction, and numerical density will be required to fully elucidate the structural mechanisms by which female Achilles tendons are able to achieve comparable modulus in the face of lower total collagen and GAG content than males. A more comprehensive characterization of cell density as a function of age in both sites would also provide valuable additional information.
The second aim of the present study was to determine whether the observed sex-based differences in tendon composition and/or material properties might be influenced by local in vivo mechanical loading environment. This was accomplished by examining two distinct anatomical locations: the highly loaded Achilles tendon as well as the less loaded tendon fascicles of the tail. Although sex-based differences in composition and some material properties were present in the Achilles, no such differences were found in the less-loaded tail site.
Like all tissues of the musculoskeletal system, tendons exhibit a substantial degree of mechanosensitivity and are capable of adapting to changes in mechanical loading environment42,43. In general, ECM turnover (both collagen synthesis as well as degradation via MMPs) increases in response to physical activity44-46. Over time, such changes modify the material properties of the tendon itself43. Of interest to the present study is the finding that tendon adaptation to changes in mechanical loading environment differs in men and women38,47. Not only is the resting collagen synthesis rate lower in female patellar tendons, but so is the rate of collagen synthesis 72 hours subsequent to exercise: synthesis rates rise in males after exercise, whereas this response is mitigated in females38. Further, this mitigated exercise-induced increase in the rate of tendon collagen synthesis in females appears to be additionally dampened when estradiol levels are elevated48. More recent studies by the same group suggest that the ability of Achilles and patellar tendons to adapt to habitual exercise (i.e. running) is also attenuated in women49. The results of the present study are thus consistent with the growing body of evidence suggesting sex-based modulation of tendon adaptation to local mechanical loading environment. Such a differential response of males and females to changes in loading will likely prove to be critical in understanding sex-based differences in the etiology and treatment of tendon and ligament injuries42, 47.
One weakness of the present study is the fact that Achilles tendons and tail tendons were not tested at the same strain rate. This raises the question of whether sex-based differences might become evident if the tail fascicles had been loaded at 100%/sec rather than 50%/sec: the rate at which no sex-based differences in any material properties were seen. While additional experiments would be required to rule out such a possibility (and such high speed tests would exceed the capabilities of our testing system with a 20 mm tendon gage length and a testing system displacement limit of 1000 mm/min), it is unlikely that we would see differences in material properties in light of the identical compositional parameters in males and females. Thus, our tail fascicle material and compositional findings are consistent with one another. As an alternative, the Achilles tendon tests could have been performed at 50%/sec. However, in our experience, such a reduction in strain rate would result in a higher incidence of bone avulsion failures, rather than mid-substance failures, which would further confound the interpretation of the ultimate strength data. Lastly, 100%/sec was chosen for its physiologic relevance in the Achilles28, while a lower rate is likely to be more physiologically appropriate in the non-load bearing site of the tail.
A second weakness of the present study is the somewhat low statistical power associated with certain dependent variables. In particular, the results associated with the effect of sex on cell density and Achilles tendon ultimate strength, as well as the interaction between sex and age and its effect on Achilles tendon GAG/DNA could be more conclusive with greater sample sizes.
In conclusion, this study in growing C57BL/6J mice demonstrates that sex has a significant effect on Achilles tendon composition, with female mice exhibiting significantly lower levels of proteoglycan and collagen than males. Despite the lower levels of important ECM constituents, however, modulus values obtained at a high strain rate were not compromised in female Achilles tendons. The results of this study suggest that the effect of sex on murine tendon properties may be influenced by the local mechanical loading environment, for no sex-based differences were detected in the low-load environment of the tail tendon, in contrast to the higher-magnitude loading environment of the Achilles tendon. Whether sex-based differences are present during tendon and ligament healing remains to be determined.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by Grant Number AR049745 from the National Institutes of Health (NIH-NIAMS).
REFERENCES
- 1.Arendt EA, Agel J, Dick R. Anterior cruciate ligament injury patterns among collegiate men and women. Journal of Athletic Training. 1999;34:86–92. [PMC free article] [PubMed] [Google Scholar]
- 2.Hewett TE, Lindenfeld TN, Riccobene JB, Noyes FR. The effect of neuromuscular control about the knee with maturation in female athletes. The American Journal of Sports Medicine. 1999;27:699–706. doi: 10.1177/03635465990270060301. [DOI] [PubMed] [Google Scholar]
- 3.Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. The American Journal of Sports Medicine. 1996;24:427–436. doi: 10.1177/036354659602400405. [DOI] [PubMed] [Google Scholar]
- 4.Leppilahti J, Puranen J, Orava S. Incidence of Achilles tendon rupture. Acta Orthop Scand. 1996;67:277–279. doi: 10.3109/17453679608994688. [DOI] [PubMed] [Google Scholar]
- 5.Nyyssön T, Lüthje P, Kröger H. The increasing incidence and difference in sex distribution of Achilles tendon rupture in Finland in 1987-1999. Scand J Surg. 2008;97:272–275. doi: 10.1177/145749690809700312. [DOI] [PubMed] [Google Scholar]
- 6.Ford KR, Myer GD, Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Medicine and Science in Sports & Exercise. 2003;35:1745–1750. doi: 10.1249/01.MSS.0000089346.85744.D9. [DOI] [PubMed] [Google Scholar]
- 7.Ireland ML. The female ACL: why is it more prone to injury? Orthopaedic Clinics of North America. 2002;33:637–651. doi: 10.1016/s0030-5898(02)00028-7. [DOI] [PubMed] [Google Scholar]
- 8.Moul JL. Differences in selected predictors of anterior cruciate ligament tears between male and female NCAA Division I collegiate basketball players. Journal of Athletic Training. 1998;33:118–121. [PMC free article] [PubMed] [Google Scholar]
- 9.Hashemi J, Chandrasheker N, Slauterbeck J. The mechanical properties of the human patellar tendon are correlated to its mass density and are independent of sex. Clinical Biomechanics. 2005;20:645–652. doi: 10.1016/j.clinbiomech.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Chandrashekar N, Mansouri H, Slauterbeck J, Hashemi J. Sex-based differences in the tensile properties of the human anterior cruciate ligament. Journal of Biomechanics. 2006;30:2943–2950. doi: 10.1016/j.jbiomech.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 11.Anderson AF, Dome DC, Gautam S, et al. Correlation of anthropometric measurements, strength, anterior cruciate ligament size, and intercondylar notch characteristics to sex differences in anterior cruciate ligament tear rates. American J Sports Med. 2001;29:58–65. doi: 10.1177/03635465010290011501. [DOI] [PubMed] [Google Scholar]
- 12.Ford KR, Myer GD, Toms HE, Hewett TE. Gender differences in the kinematics of unanticipated cutting young athletes. Medicine and Science in Sports & Exercise. 2005;37:124–129. [PubMed] [Google Scholar]
- 13.Hewett TE, Myer GD, Ford KR. Decrease in neuromuscular control about the knee with maturation in female athletes. The Journal of Bone and Joint Surgery, Incorporated. 2004;86:1601–1608. doi: 10.2106/00004623-200408000-00001. [DOI] [PubMed] [Google Scholar]
- 14.McLean SG, Walker KB, van den Bogert AJ. Effect of gender on lower extremity kinematics during rapid direction changes: an integrated analysis of three sports movements. J Sci Med Sport. 2005;8:411–422. doi: 10.1016/s1440-2440(05)80056-8. [DOI] [PubMed] [Google Scholar]
- 15.Liu SH, Al-Shaikh RA, Panossian V, et al. Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. Journal of Orthopaedic Research. 1996;14:526–533. doi: 10.1002/jor.1100140405. [DOI] [PubMed] [Google Scholar]
- 16.Schultz SJ, Kirk SE, Johnson ML, et al. Relationship between sex hormones and anterior knee laxity across the menstrual cycle. Med Sci Sports Exerc. 2004;36:1165–1174. doi: 10.1249/01.MSS.0000132270.43579.1A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultz SJ, Sander TC, Kirk SE, Perrin DH. Sex differences in knee joint laxity change across the female menstrual cycle. J Sports Med Phys Fitness. 2005;45:594–603. [PMC free article] [PubMed] [Google Scholar]
- 18.Slauterbeck JR, Clevenger C, Lundberg W, Burchfield DM. Estrogen level alters failure load of the rabbit anterior cruciate ligament. J Orthop Res. 1999;17:405–408. doi: 10.1002/jor.1100170316. [DOI] [PubMed] [Google Scholar]
- 19.Carden DG, Jonathan N, Chalmers J, et al. Rupture of the calcaneal tendon. J Bone Joint Surg (Br) 1987;69:416–420. doi: 10.1302/0301-620X.69B3.3294839. [DOI] [PubMed] [Google Scholar]
- 20.Jozsa L, Kvist M, Balint BJ, et al. The role of recreational sports activity in Achilles tendon rupture: a clinical, pathoanatomical, and sociological study of 292 cases. Am J Sports Med. 1989;17:338–343. doi: 10.1177/036354658901700305. [DOI] [PubMed] [Google Scholar]
- 21.Puddu G, Ippolito E, Postacchini F. A classification of Achilles tendon disease. Am J Sports. 1976;4:145–150. doi: 10.1177/036354657600400404. [DOI] [PubMed] [Google Scholar]
- 22.Kubo K, Kanehisa H, Fukunago T. Gender differences in the viscoelastic properties of tendon structures. Eur J Appl Physiol. 2002;88:520–526. doi: 10.1007/s00421-002-0744-8. [DOI] [PubMed] [Google Scholar]
- 23.Kim YJ, Sah RL, Doong JY, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Ana Biochem. 1988;174:168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 24.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochemica et Biophysics Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 25.Creemers LB, Jansen DC, van Veen-Reurings A, et al. Microassay for the assessment of low levels of Hydroxyproline. BioTechniques. 1997;22:656–658. doi: 10.2144/97224bm19. [DOI] [PubMed] [Google Scholar]
- 26.Mikic B, Bierwert L, Tsou D. Achilles tendon characterization in GDF-7 deficient mice. J Orthop Res. 2006;24:831–41. doi: 10.1002/jor.20092. [DOI] [PubMed] [Google Scholar]
- 27.Mikic B, Schalet BJ, Clark RT, Gaschen V, Hunziker EB. Altered Achilles tendon composition, mechanical properties, and ultrastructure in GDF-5 deficient mice. J Orthop Res. 2001;19:365–371. doi: 10.1016/S0736-0266(00)90018-4. [DOI] [PubMed] [Google Scholar]
- 28.Belkoff SM, Haut RC. Experimental methods in biological tissue testing. In: Sharpe WN Jr, editor. Handbook of Experimental Solid Mechanics. Springer Science & Business Media; Boston: 2008. pp. 871–890. [Google Scholar]
- 29.Mikic B, Entwistle R, Rossmeier K, Bierwert L. Effect of GDF-7 deficiency on tail tendon phenotype in mice. J Orthop Res. 2008;26:834–9. doi: 10.1002/jor.20581. [DOI] [PubMed] [Google Scholar]
- 30.Beattie JH, Wood AM, Newman AM, et al. Obesity and hyperleptinemia in metallothionein (-I and –II) null mice. Proc Ntl Acad Sci USA. 1998;95:358–363. doi: 10.1073/pnas.95.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burich RL, Ewing KL. Age effects on lactic- and malic-acid dehydrogenase in C57Bl/6 mouse testes. The Ohio Journal of Science. 1970;70:306–310. [Google Scholar]
- 32.Faryniarz DA, Bhargava M, Lajam C, et al. Quantitation of estrogen receptors and relaxin binding in human anterior cruciate ligament fibroblasts. In Vitro Cell Dev Biol Anim. 2006;42:176–181. doi: 10.1290/0512089.1. [DOI] [PubMed] [Google Scholar]
- 33.Jönsson D. The biological role of the female sex hormone estrogen in the periodontium—studies on human periodontal ligament cells. Swed Dent J Suppl. 2007;187:11–54. [PubMed] [Google Scholar]
- 34.Liang L, Yu JF, Ding Y. Estrogen regulates expression of osteoprotegrin and RANKL in human periodontal ligament cells through estrogen receptor beta. J Periodontol. 2008;79:1745–1751. doi: 10.1902/jop.2008.070437. [DOI] [PubMed] [Google Scholar]
- 35.Sciore P, Frank CB, Hart DA. Identification of sex hormone receptors in human and rabbit ligaments of the knee by reverse transcription-polymerase chain reaction: evidence that receptors are present in tissue from both male and female subjects. J Orthop Res. 1998;16:604–610. doi: 10.1002/jor.1100160513. [DOI] [PubMed] [Google Scholar]
- 36.Rau MD, Renouf D, Benfield D, et al. Examination of the failure properties of the anterior cruciate ligament during the estrous cycle. The Knee. 2005;12:37–40. doi: 10.1016/j.knee.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Liu SH, Al-Shaikh RA, Panossian V, et al. Estrogen affects the cellular metabolism of the anterior cruciate ligament. A potential explanation for female athletic injury. The American Journal of Sports Medicine. 1997;25:704–709. doi: 10.1177/036354659702500521. [DOI] [PubMed] [Google Scholar]
- 38.Miller BF, Hansen M, Olesen JL, et al. Tendon collagen synthesis at rest and after exercise in women. J Appl Physiol. 2007;102:541–546. doi: 10.1152/japplphysiol.00797.2006. [DOI] [PubMed] [Google Scholar]
- 39.Maffucci JA, Gore AC. Age-related changes in hormones and their receptors in animal models of female reproductive senescence. In: Conn PM, editor. Handbook of models for human aging. Elsevier; San Diego: 2006. pp. 533–552. [Google Scholar]
- 40.Haut RC, Lancaster RL, Decamp CE. Mechanical properties of the canine patellar tendon: some correlations with age and the content of collagen. J Biomech. 1992;25:163–173. doi: 10.1016/0021-9290(92)90273-4. [DOI] [PubMed] [Google Scholar]
- 41.Woo SL, Gomez MA, Amiel D, et al. The effects of exercise on the biomechanical and biochemical properties of swine digital flexor tendons. J Biomech Eng. 1981;103:51–56. doi: 10.1115/1.3138246. [DOI] [PubMed] [Google Scholar]
- 42.Kjaer M, Magnussen P, Krogsgaard M, et al. Extracellular matrix adaptation of tendon and muscle to exercise. J Anat. 2006;208:445–450. doi: 10.1111/j.1469-7580.2006.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reeves ND. Adaptation of the tendon to mechanical usage. J Musculoskelet Neuronal Interact. 2006;6:174–180. [PubMed] [Google Scholar]
- 44.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 45.Koskinen SO, Heinemeier KM, Olesen JL, et al. Physical exercise can influence local levels of matrix metalloproteinases and their inhibitors in tendon related connective tissue. J Appl Physiol. 2004;96:861–864. doi: 10.1152/japplphysiol.00489.2003. [DOI] [PubMed] [Google Scholar]
- 46.Miller BF, Olesen JL, Barbraj J, et al. Muscle and tendon collagen and non-collagen protein synthesis rates are synchronized after strenuous exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magnussen SP, Hansen M, Langberg H, et al. The adaptability of tendon to loading differs in men and women. Int J Exp Path. 2007;88:237–240. doi: 10.1111/j.1365-2613.2007.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langberg H, Skovgaard D, Peterson LJ, et al. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol. 1999;521:299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westh E, Kongsgaard M, Bojsen-Moller J, et al. Effect of habitual exercise on the structural and mechanical properties of human tendon, in vivo, in men and women. Scand J Med Sports. 2008;18:23–30. doi: 10.1111/j.1600-0838.2007.00638.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.