Abstract
Ischemia predisposes orthopaedic trauma patients to delayed fracture healing or non-union. The goal of this study was to test the ability of bone morphogenetic protein 7 (BMP7) to stimulate fracture repair in an ischemic environment. Ischemic fractures were generated in male adult mice by resecting the femoral artery prior to the creation of a non-stabilized tibia fracture. Recombinant human BMP7 (rhBMP7, 50µg) was injected into the fracture site immediately after surgery. Histomorphometric analyses revealed that rhBMP7 induced more cartilage at day 7, more callus and bone at days 14 and 28, and more adipose tissue and fibrous tissue at days 7, 14 and 28 compared to controls (n=5/group/time). At day 28, all fractures treated with rhBMP7 (50µg, n=5) healed, whereas only 3 of 5 control fractures exhibited slight bony bridging. In addition, we found that rhBMP7 (both 10 and 50µg) significantly increased the amount of cartilage compared to controls in stabilized fractures, confirming its chondrogenic effect. No significant effects of rhBMP7 on tissue vascularization were observed at 3 days after fracture. Lastly, using bone marrow transplantation we determined that no donor-derived osteocytes or chondrocytes were present in rhBMP7 treated fractures, suggesting rhBMP7 did not recruit mesenchymal stem cells from the bone marrow to the fracture site. In conclusion, our results indicate that rhBMP7 is a promising treatment for fractures with severely disrupted blood supply.
Keywords: fracture, ischemia, non-union, bone morphogenetic protein 7, osteogenic protein-1
Introduction
Inadequate blood supply is a major cause of delayed- and non-union during fracture healing. The rate of impaired bone healing is nearly 50% in patients with concomitant vascular injuries or when fractures occur in areas with decreased perfusion1, and this is significantly higher than the 10% non-union rate observed in general fractures2. Therefore, developing therapies to enhance fracture healing in ischemic environments is of great importance. Previously, we developed an ischemic fracture model by resecting the femoral artery prior to creating a tibial fracture. In this model, the blood supply slowly returns to the lower limb via collateral blood vessel formation 3. However, femoral artery resection creates an anoxic environment during the early stages of repair 4 that leads to cell death within the periosteum, delayed chondrocyte and osteoblast differentiation, and ultimately, delayed bone repair 3. Therefore, this model is ideal for testing novel interventions for treating fractures that are accompanied by acute severe ischemia, such as those with extensive vascular damage that is beyond repair or those resulting from crush injuries.
A number of candidate molecules could be used for treating ischemic fractures. We chose to focus on bone morphogenetic protein 7 (BMP7, also named osteogenic protein 1 (OP-1)), because this molecule has been used to treat other non-union fracture models in rodents 5 and is approved as a humanitarian device to treat recalcitrant fractures in humans. BMP7 induces cartilage and bone formation, and the effect of BMP7 on fracture healing and spinal fusion has been illustrated experimentally 6–9 and clinically (See a review by White et al. 10). However, these studies were performed in tissues with adequate blood supply, and the capacity of BMP7 to improve bone repair in an ischemic environment has not been assessed. In this work, we hypothesized that rhBMP7 can accelerate fracture healing in severe ischemic fractures by inducing bone and cartilage formation, improving tissue vascularization, and recruiting circulating stem cells. We first tested the ability of BMP7 to enhance bone repair in our model of ischemic fracture. We then examined potential mechanisms by analyzing the effects of BMP7 on cartilage formation and tissue vascularization in stabilized fractures and stem cell recruitment in chimeric mice created by bone marrow transplantation.
Methods
Generation of ischemic tibia fractures and treatment with rhBMP7
Three-month-old male 129J/B6 mice (25–30g) were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California at San Francisco. Animals were anesthetized by intraperitoneal injection of Ketamine/Medetomidine. Femoral artery resection was performed as described previously 3. Briefly, the right femoral artery and its branches between the inguinal ligament and saphenous/popliteal bifurcation were separated and removed. A closed transverse mid-diaphyseal tibial fracture was then created by three-point bending. Animals were allowed to move freely after recovery from anesthesia. This model of non-stabilized tibia fracture allows us to study intramembranous ossification and endochondral ossification in the same animal 3. Pre-operative antibiotic (Ancef, 25mg/kg) and post-operative pain killer (Buprenex, 0.01mg/kg) were provided. Recombinant human BMP7 (rhBMP7 (OP-1, Stryker Biotech, Hopkinton, MA)) was suspended in 10% lactose solution at a concentrations of 0.33 or 1.66µg /µl. Thirty microliters of rhBMP7 solutions, equivalent to 10 or 50µg, was injected directly into the fracture site immediately after the creation of fractures. Control animals received 30µl of 10% lactose solution only. Animals were sacrificed at 7, 14, and 28 days (n=4–5/time/group) for histological analysis. Two mice treated with rhBMP7 died at 4 and 23 days after surgery and one animal from the control group developed foot necrosis. These animals were excluded from further analyses.
Generation of stabilized tibia fractures and treatment with rhBMP7
Animals were anesthetized as described above. Two sets of insect pins were place in the proximal and distal ends of tibia, 11mm apart. An external fixator was then applied to these pins as previously described 11. Closed transverse mid-diaphyseal fractures of the tibia were created with a three-point bending apparatus. Radiographs were taken immediately after injury to confirm the extent of fracture. The external fixator provides rigid fixation of the tibial fracture. After recovery, animals were allowed to ambulate ad libitum and analgesics were provided for the first 48 hours (Buprenorphine, ZT Sigma, St. Louis, MO). No femoral artery resection was performed in this set of animals. Immediately after fracture, the fracture site was injected with 10 or 50µg of rhBMP7 or 10% lactose as control. Animals were sacrificed at 3 days after fracture for blood vessel quantification and 10 days post-fracture for histological analysis of bone repair (n=5/group/time).
Tissue processing and histological analyses
Fractured tibiae were fixed in 4% paraformaldehyde (PFA) at 4°C overnight, decalcified in 19% EDTA, dehydrated, and embedded in paraffin. Sagittal sections (10µm) through the entire block were prepared. To analyze the effects of rhBMP7 on fracture healing, every tenth slide, or every twentieth slide for samples with large calluses, was stained by the Hall’s and Brunt’s Quadruple (HBQ) staining (bone is red and cartilage is blue) 12. Histomorphometry was performed using stereology. The volume of callus (Vcallus) and the volumes of bone (Vbone), cartilage (Vcartilage), fibrous tissue (Vfibrous), and adipose tissue (Vadipose) within the callus were estimated using the Cavalier method 13. To assess chondrocyte differentiation and maturation, in situ hybridization using probes to Collagen type II and Collagen type X, markers of chondrocytes and hypertrophic chondrocytes respectively, was performed on sections adjacent to those used by histological staining 14.
Immunohistochemistry of members of BMP signaling pathway
Goat polyclonal antibodies against BMP7 and BMP receptors (BMPR-IA, BMPR-IB, BMPR-II) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibody against p-Smad-1/5/8 was purchased from Chemicon (Millipore, Billerica, MA). Paraffin sections (10μm thick) were dewaxed, rehydrated, and incubated with primary antibodies in PBS (1:100 dilution) at 4°C overnight. Sections were then incubated with a biotinylated secondary antibody (Vector Labs) for 30 min at room temperature followed by staining with Vectastain ABC Reagent (Vector Labs). Antibody complex was visualized with DAB reaction. Sections incubated with secondary antibody only without primary antibodies were used as controls.
Stereology analysis of tissue vascularization
Stereology is a widely used technique to analyze blood vessels. It gives unbiased estimation of the length density (the length per unit volume of the reference space, Lv) and surface density (the surface area of the endothelium per unit volume of the reference space, Sv) of tissue vasculature 13, 15. Mice with femoral artery resection and tibial fracture were treated with 50µg of rhBMP-7 or lactose solution as control. Animals were sacrificed at 7 days after fracture (n=6/group). The entire tissue including fractured bone and surrounding muscles between the knee joint and ankle was collected, fixed in 4% PFA, and decalcified in 19% EDTA. Tissues were embedded in OCT and vertical, uniform, and random sections (10µm) were prepared through the whole block. For each sample, 6–9 sections were selected using systematic random sampling for blood vessel analysis. Immunohistochemistry was performed to visualize blood vessels using an anti-PECAM (Platelet Endothelial Cell Adhesion Molecule, a marker of endothelial cells) antibody. The length density and surface density of blood vessels were quantified as described 16. The total volume of tissues between the knee and ankle (Vref) was estimated using the Cavalieri method 13. The total length and surface area of vasculature were calculated by multiplying Lv and Vref or Sv and Vref respectively.
Generating bone marrow chimeras for lineage analysis
We created chimeric mice in which host bone marrow was replaced by bone marrow from Rosa26 mice as previously described 17. Briefly, bone marrow was collected from tibiae and femora of 6-week old male Rosa26 mice (Jackson Laboratories, Bar Harbor, ME) that express the β-galactosidase transgene ubiquitously. Red blood cells were lysed (RBC lysis buffer, Sigma, St. Louis, MO), and the remaining cells were washed and suspended in PBS. Six-week-old male congenic C57BL/6J mice were irradiated (12 Gray, γ-ray, 137Cs irradiator), and then donor bone marrow was injected into lateral tail vein (107 cells/ mouse). Fractures were created in the chimeric animals 4 weeks after bone marrow transplantation. Donor cells were distinguished from hosts by standard X-gal staining to detect β-galactosidase activity as described 17.
Assessing osteogenic and chondrogenic capacity of transplanted bone marrow from chimeric mice
To determine whether donor bone marrow mesenchymal stem cells (MSCs) were present in chimeric animals, bone marrow cells were isolated from chimeric mice 6 weeks after transplantation. The ability of the adherent cells to undergo osteogenesis and chondrogenesis in vitro was tested. The standard practice of isolating cells that adhere to plastic was used to isolate and culture the putative MSCs 18–20. Briefly, bone marrow cells were collected from femora and tibiae. Cells were plated and cultured for three days. The adherent cells were allowed to form colonies (CFU-F) and expanded through 3 passages. X-gal staining was performed on these cells to test whether they are donor–derived.
The capacity of these adherent cells to form bone in vitro was tested. Cells were collected, seeded (105 cells/well) in a 24-well plate, and cultured in low-glucose DMEM containing 10% FBS, 0.1 µM Dexamethasone, 0.2 mM Ascorbic Acid 2-Phosphate and 10 mM Glycerol 2-Phosphate in a humidified incubator at 37°C and 5% CO2. The medium was changed every 3 days for 21 days. Then the cells were fixed in 0.2% Glutaraldehyde at room temperature for 15 minutes and stained with Alizarin Red to assess mineralization and/or X-gal staining solution to determine if the osteoblasts were derived from the donor bone marrow.
The capacity of these adherent cells to form cartilage in vitro was also tested. Once adherent cells had reached the 3rd passage, they were collected, centrifuged at 300g for 5 minutes to form a cell pellet in a 15-ml polypropylene tube, then cultured in a low-glucose DMEM medium containing 10% FBS, 0.1 µM Dexamethasone, 0.2 mM Ascorbic Acid 2-Phosphate, 10ng/ml TGF-β1, 10µg/ml insulin and ITS (insulin-transferrin-selenium) premix (Sigma). The media was changed every 3 days for 21 days. Then the cell pellet was fixed in 0.2% Glutaraldehyde at room temperature for 30 minutes and then embedded in OCT (Sakura Finetechnical Co., Tokyo, Japan). Frozen sections were prepared and stained with X-gal to assess the presence of donor cells and Safranin O/Fast Green to visualize cartilage matrix.
Assessing the ability of rhBMP7 to stimulate donor MSCs in ischemic fractures
Six weeks after bone marrow transplantation, ischemic tibial fractures were created in chimeric mice, and then rhBMP7 (50µg) was injected directly into the fracture site immediately after the creation of ischemic fractures. The mice were sacrificed at 14 days after fracture. The tibias and surrounding callus tissues were collected and fixed in 4% paraformaldehyde solution at 4 °C for 24 hours. The samples were decalcified in 19% EDTA at 4 °C for 10–14 days, then cryo-embedded in OCT. Consecutive frozen sections (10µm) were cut through the entire callus and stored at −20°C. Sections were subjected to Safranin O/Fast Green staining for histological analysis and X-gal staining for cell lineage analysis.
Statistics
Single factor ANOVA and t-test were used to determine the effects of rhBMP7 on fracture healing. Data are presented as mean ± one standard deviation.
Results
RhBMP7 improves early fracture healing in an severe ischemic environment
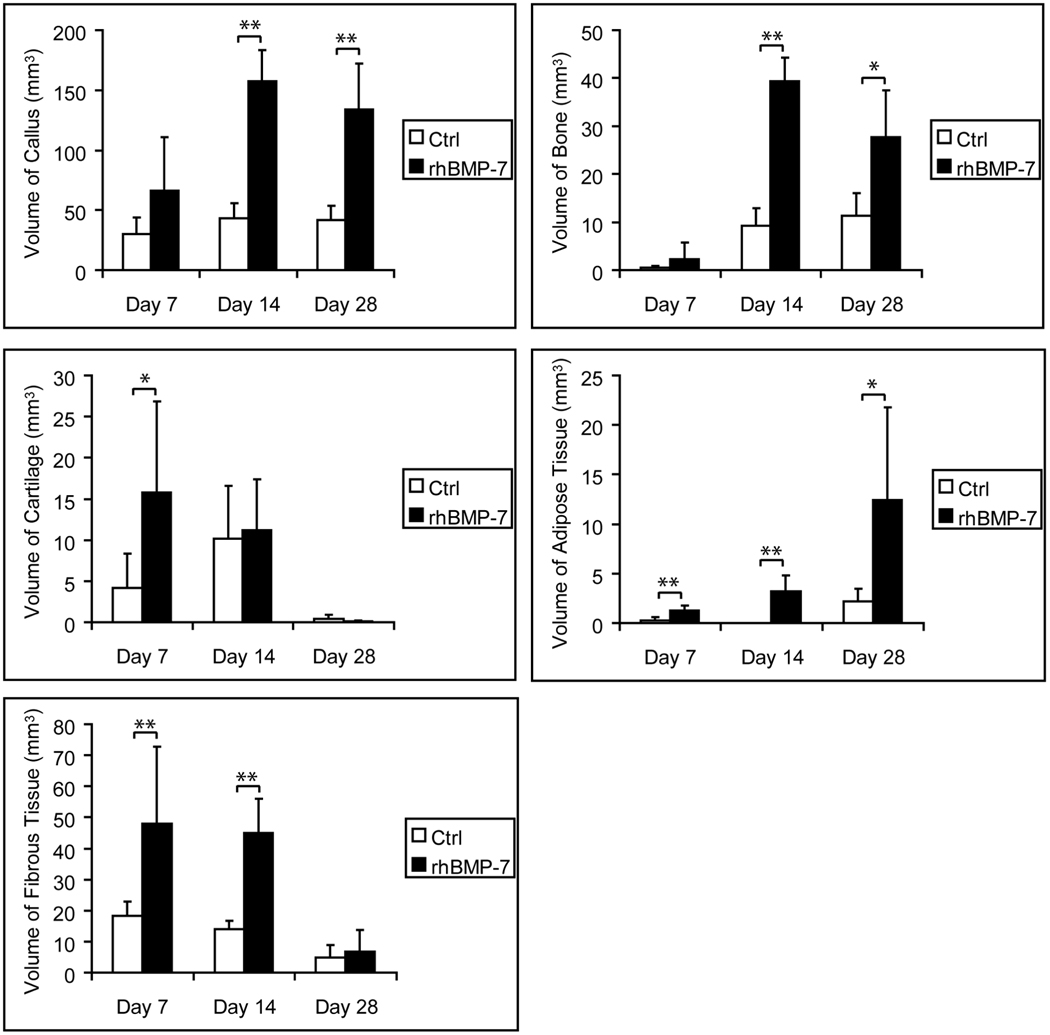
Non-stabilized, ischemic tibial fractures were created and treated with a single injection of rhBMP7 (50µg) or control solution at the time of injury. At 7 days after injury, rhBMP7-treated fractures exhibited significantly more cartilage, fibrous, and adipose tissues compared to controls (Fig. 1). RhBMP7 also increased callus and bone formation at this time point, but this increase was not statistically significant (Fig.1). Histological analysis revealed a large amount of dead muscles at the fracture site in both treated and control fractures (Fig. 2B, F). Cartilage was observed in the area of periosteal reaction and at the periphery of dead muscles (Fig. 2C, G). Further molecular analysis using in situ hybridization showed similar expression patterns of Collagen II and X transcripts between treated and control fractures (data not shown), suggesting the maturation of cartilage was not significantly affected.
Fig. 1. Histomorphometric analyses of fracture healing in rhBMP7 treated ischemic fractures.
Animals with tibia fracture and femoral artery resection were treated with 50µg of rhBMP7. * p<0.05, ** p<0.01.
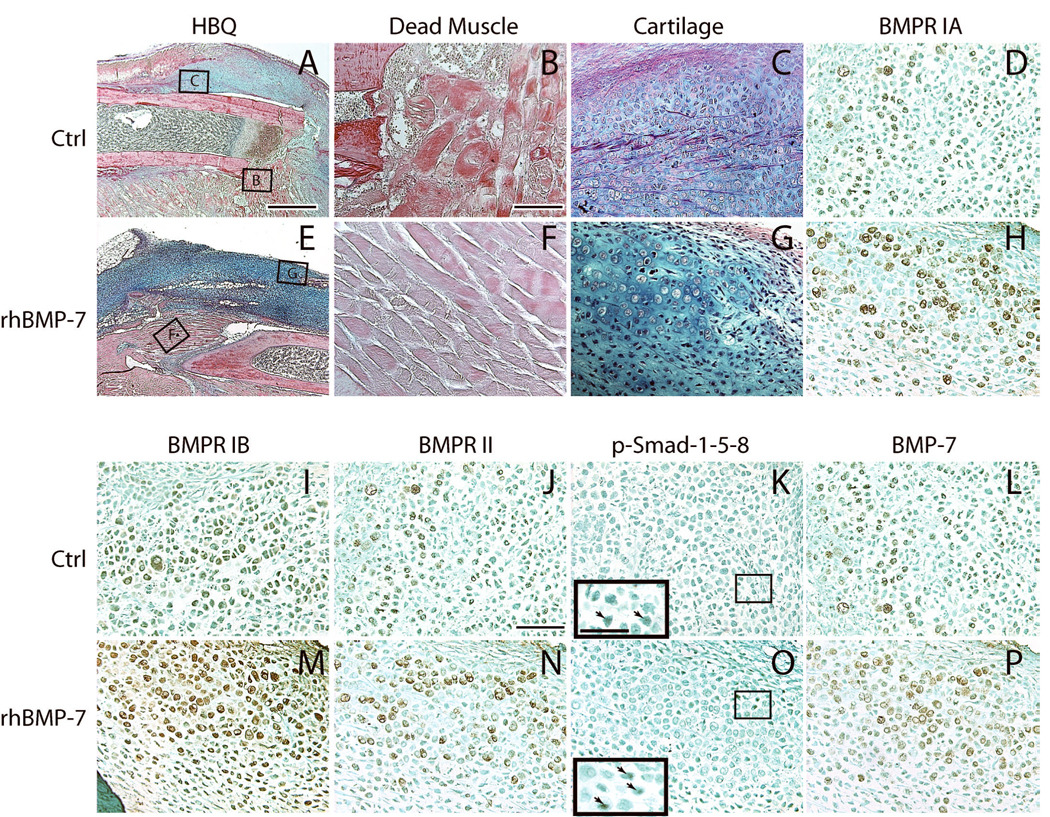
Fig. 2. Increased activation of Bmp signaling pathway after 50µg of rhBMP7 treatment: 7 days after fracture and femoral artery resection.
(A-D, I-L) Representative micrographs of control fractures. (A) A fracture callus stained by Hall’s and Brunt’s Quadruple stain method (HBQ). Cartilage appears blue. (B) Necrotic myofibers at the fracture site. (C) A cartilage island formed in the callus. (D, I-L) Bmp receptors IA (BMPR IA), IB, II, phosphorylated Smad-1-5-8, and Bmp7 are detected in chondrocytes. Insert in (K) is a high magnification of the box area, showing p-Smad-1-5-8 positive cells (arrows). (E-H, M-P) Representative micrographs of ischemic fractures treated with rhBMP7. (E) A fracture callus of rhBMP7 treated fracture. (F) Necrotic myofibers at the fracture site. (G) A cartilage island. (H, M-P) rhBMP7 treated fractures have stronger immunostaining for Bmp receptors IA, IB, II, phosphorylated Smad-1-5-8, and Bmp7 in chondrocytes compared to the control. Insert in (O) is a high magnification of the box area, showing p-Smad-1-5-8 positive cells (arrows). Scale bars: (A, E) = 1mm, (B-D, F-H, I-L, M-P) = 100µm, inserts in (K and O) = 50 µm.
Activation of the BMP signaling pathway appeared to be enhanced in response to the exogenous rhBMP7 at 7 days after treatment. Bmp receptors (IA, IB, and II) and phosphorylated Smad-1-5-8 were detected in chondrocytes within fracture calluses, and the immunostaining appeared more intense in fractures treated with rhBMP7 (Fig. 2). In addition, stronger intracellular BMP7 staining was observed in chondrocytes in treated animals (Fig. 2L, P), indicating there was an increase in the expression of endogenous Bmp7. These results demonstrate that exogenous rhBMP7 enhances the early stages of ischemic fracture healing and activates the Bmp signaling pathway directly and/or indirectly. The increased pSmad and Bmp7 expression could be also due to the increased amount of cartilage (Fig. 1).
RhBMP7 improves late fracture healing in an severe ischemic environment
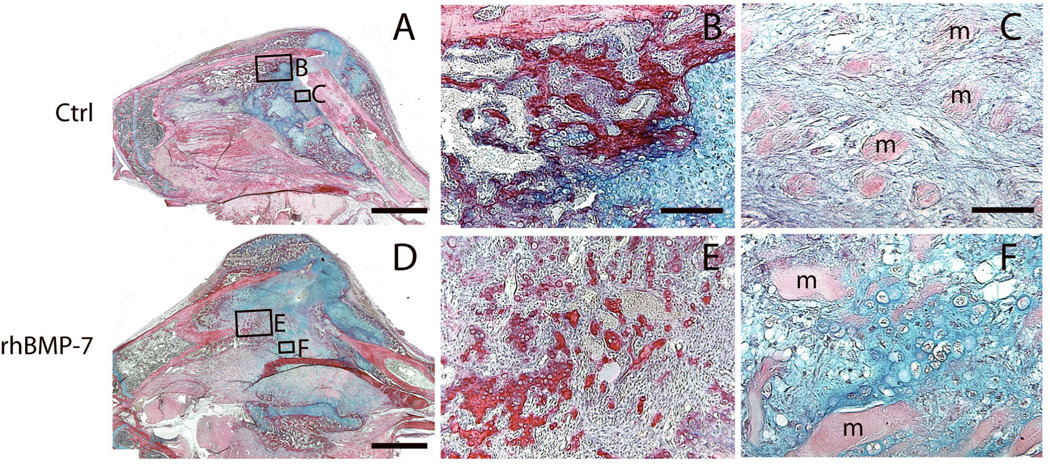
The next objective was to determine if treating ischemic fractures with rhBMP7 had long-term effects on the process of bone healing. At 14 days after fracture, dead muscle fibers were observed in both control and treated fractures. In treated fractures, rhBMP7 led to the formation of significantly larger calluses, more bone, fibrous tissue, and adipose tissues within the fracture calluses compared to controls (Fig. 1). Interestingly, equal amounts of cartilage were observed between the treated and control animals at this time (Fig. 1). Considering rhBMP7 treated fractures had significantly more cartilage at day 7, the equal amounts of cartilage at day 14 may suggest that rhBMP7 accelerated endochondral ossification during this period. There were also signs of ectopic bone and cartilage formation in rhBMP7 treated fractures. In control ischemic fractures, newly formed bone was usually observed adjacent to periosteum (Fig. 3B) and fibrous tissue was present between dead muscle fibers within fracture callus (Fig. 3C). In comparison, rhBMP7 treated fractures exhibited new trabecular bone within fibrous tissue (Fig. 3E) and cartilage islands between dead muscle fibers (Fig. 3F).
Fig. 3. Histology of rhBMP7 treated ischemic fractures at 14 days after injury: HBQ staining.
(A) A representative fracture callus of control animals. (B) New bone (red) in control fractures is well organized. (C) Fibrous tissue is present between dead muscle fibers (m). (D) A representative fracture callus of rhBMP7 treated animals. (E) rhBMP7-induced new bone has a different morphology compared to trabeculae in control fractures (B). (F) Cartilage islands (blue) are observed between dead muscle fibers (m). Scale bars: (A and D) = 2mm, (B, E) = 200µm, (C, F) = 100µm.
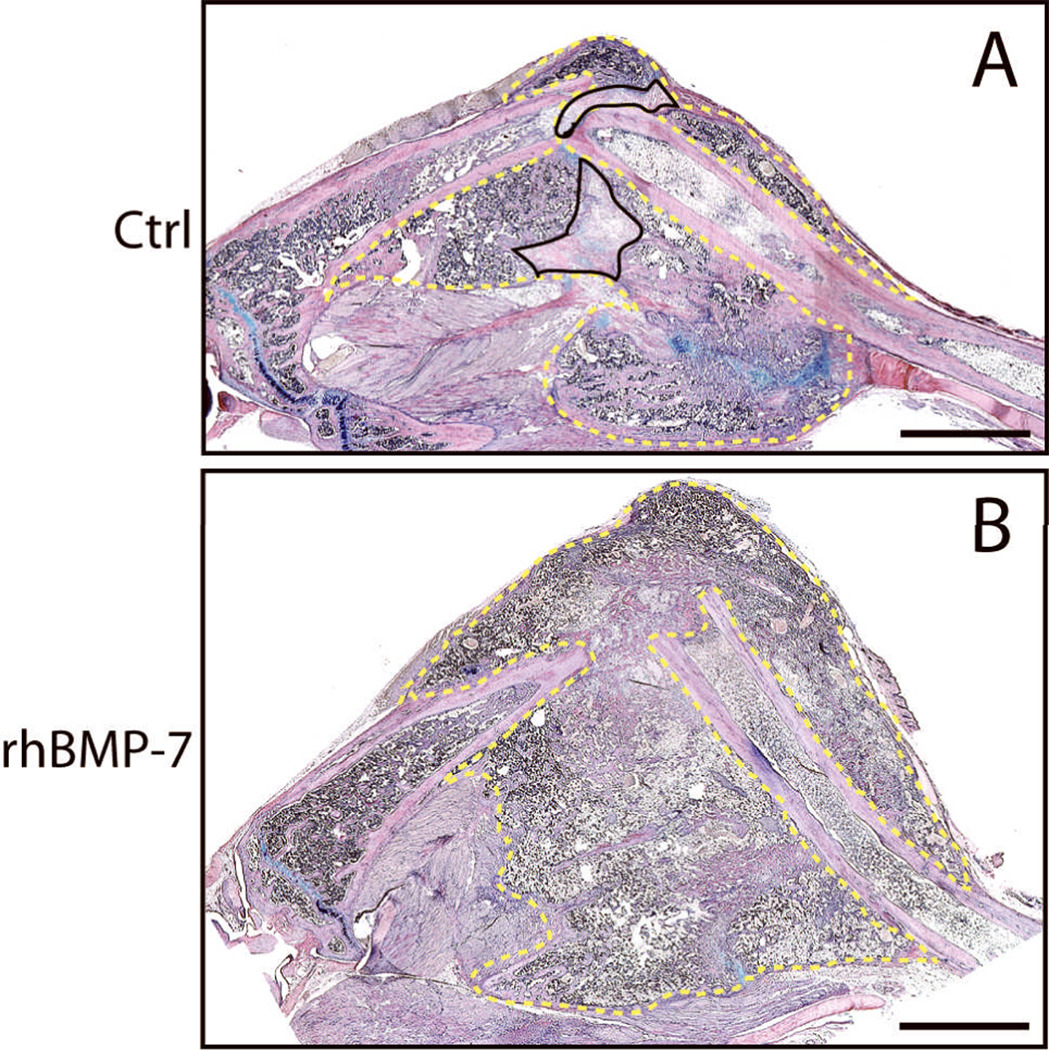
Normally, tibial fractures in mice heal by day 28, but resection of the femoral artery prior to injury delays healing beyond this time point 3. Therefore, we wanted to assess the ability of rhBMP7 to restore the normal time course of healing in ischemic fractures. Indeed, at 28 days after injury, ischemic fractures treated with 50µg of rhBMP7 had significantly larger calluses and more bone within the callus compared to controls (Fig. 1). All rhBMP7 treated fractures (n=5) exhibited a large bony callus that bridged the fractured bone ends, whereas only 3 of 5 control fractures exhibited some degree of bony bridging with a large amount of fibrous tissue in the fracture gap (Fig. 4).
Fig. 4. Histology of rhBMP7 treated ischemic fractures at 28 days after injury: HBQ staining.
(A) In the controls, fibrous tissue (outlined areas with solid line) and cartilage (blue) are present in callus. (B) rhBMP7 treated fractures have large bony calluses with minimal amount of fibrous tissue and cartilage. Area outlined with dashed line is fracture callus. Scale bar = 2mm.
Finally, we assessed the ability of lower doses of rhBMP7 to facilitate repair of ischemic fractures. We treated ischemic animals with 10µg of rhBMP7 and analyzed fracture healing at day 28. All animals (n=5) receiving this low dose of rhBMP7 exhibited bony bridging between fracture ends. Further histomorphometric analysis showed that 10µg of rhBMP7 had efficacy that was similar to 50µg, inducing a large amount of callus and bone formation (See supplemental Fig. 1).
RhBMP7 induces bone and cartilage formation in stabilized fractures
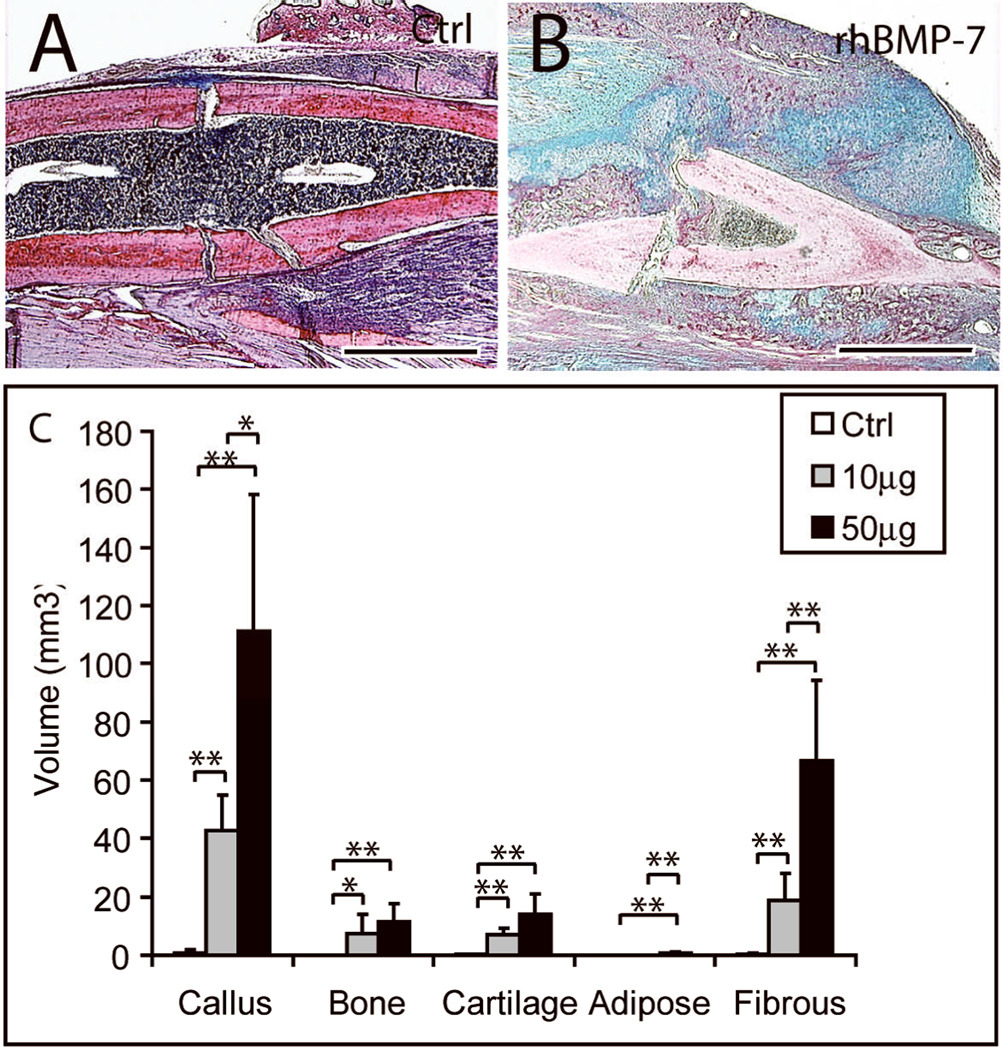
While our findings demonstrated that rhBMP7 increased cartilage formation and enhances endochondral ossification in ischemic fractures, these data do not provide direct evidence that rh-BMP7 directs stem cells to differentiate along chondrogenic pathways in vivo. Those ischemic fractures were not stabilized and a non-stable environment allows stem cells to differentiate into chondrocytes. Therefore, to test the ability of rhBMP7 to directly induce chondrogenesis and endochondral ossification during fracture healing, we assessed fracture healing in a rigidly stabilized environment. In this model, healing occurs primarily through intramembranous ossification (11 and see Fig. 5). Tibial fractures were rigidly stabilized with an external fixator and treated with 10 or 50µg of rh-BMP7 or control solution. At 10 days after injury, control fractures exhibited a small callus and little newly formed bone at the fracture sites (Fig. 5). Cartilage was not detected in 2 out of 5 control fractures and the other 3 animals had a minimal amount of cartilage. In contrast, both doses of rh-BMP7 induced formation of large calluses comprised of a large amount of bone and cartilage at 10 days after fracture (Fig. 5 and data not shown). Histomorphometric analyses illustrate that the volumes of callus, bone, cartilage, and fibrous tissue in rhBMP7 treated fractures are all significantly higher than those in the controls (Fig. 5C).
Fig. 5. Stabilized fractures treated with rhBMP7.
Fracture healing was analyzed at 10 days after injury. (A) A representative callus of the controls showing minimal bone formation at fracture site. (B) Fifty micrograms of rhBMP7 induces a large amount of bone and cartilage formation. (C) Histomorphometric analyses. Scale bar = 1mm. * p<0.05, ** p<0.01.
RhBMP7 increases tissue vascularization
To assess the effects of rhBMP7 on vascular repair, ischemic fractures were treated with 50 µg of rhBMP7 and vascularization of the fractured limbs were analyzed at 7 days after injury using stereology techniques. No significant difference in length density or surface density of vasculature was detected between rhBMP7 treated and control fractures (Table 1). However, rhBMP7 treated mice exhibited larger volume of tissue in the fractured lower hind limbs. The total length and surface area of vasculature in rhBMP7 treated fractures were significant larger than those in the controls (Table 1).
Table 1.
Effect of rhBMP7 on tissue vascularization in ischemic fractures at 7 days after injury.
| Length Density (mm/mm3) |
Surface Density (mm2/mm3) |
Total Volume of Tissues (mm3) |
Total Length (103mm) |
Total Surface Area (mm2) |
|
|---|---|---|---|---|---|
| Control | 285.5±68.2 | 16.1±2.8 | 247.3±44.6 | 69.7±16.9 | 3891±191.5 |
| rhBMP7 (50µg) | 326.7±44.8 | 16.3±3.9 | 470.2±81.3** | 153.2±29.8** | 7830.9±2883.2** |
Data are shown as X±SD.
p<0.01.
Transplanted bone marrow cells do not contribute to bone and cartilage formation in rhBMP7 treated ischemic fractures
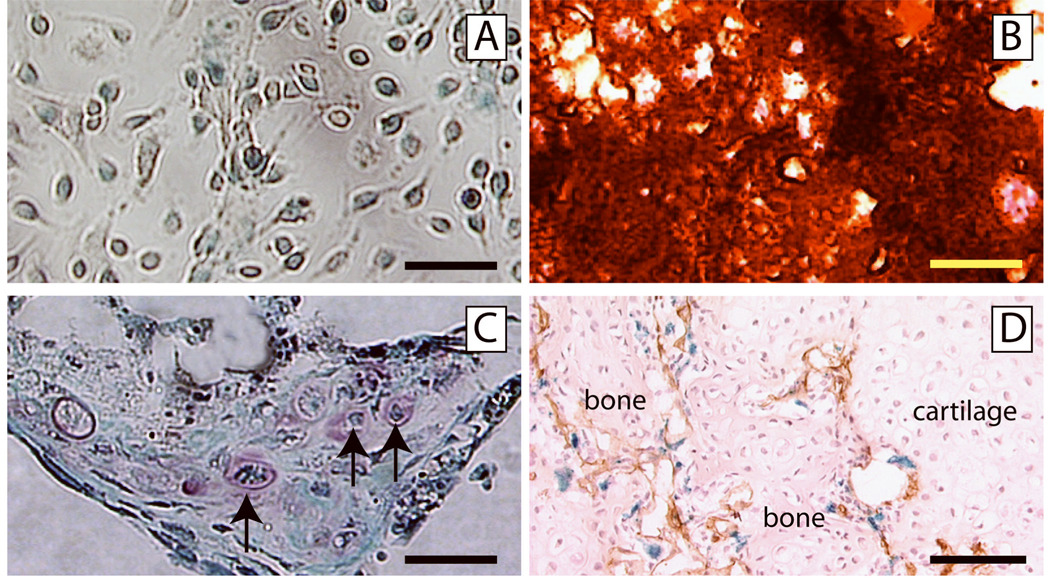
In our final experiment we wanted to assess the extent to which rhBMP7 was able to recruit mesenchymal stem cells (MSCs) from the bone marrow cavity. To achieve this we created chimeric animals by transplanting bone marrow that was genetically labeled with the β-galactosidase transgene into wild type animals. As a first step, we assessed whether donor MSCs were present in these chimeric animals by examining the extent to which donor cells could be isolated from the chimeric marrow and form colonies in vitro (CFU-F). Cells were harvested from the bone marrow of chimeric animals 6 weeks after transplantation and cultured. The adherent cells formed colonies (CFU-F), and β-galactosidase activity was detected in these cells indicating that they were derived from the transplanted donor bone marrow cells (Fig. 6A). Further, when cultured in osteogenic or chondrogenic media, these cells exhibited ability to differentiate into osteoblasts (Fig. 6B) and chondrocytes (Fig. 6C) respectively. Hence, donor-derived MSCs were present in the chimeric animals at the time that we created fractures in these animals.
Fig. 6. Osteogenic/chondrogenic progenitor cells are not recruited from bone marrow in rhBMP7 treated fractures.
(A) Bone marrow cells harvested from chimeric mice formed colonies. A majority of cells in the colony are positive for X-gal staining (blue staining). (B) These colony forming cells differentiated into osteogenic cells, producing mineralized matrix which is stained red by Alizarin Red solution. (C) These colony forming cells were also capable of differentiating into chondrocytes (arrows, Safranin O/Fast Green staining). (D) Ischemic tibia fracture was created in chimeric mice and treated with 50µg of rhBMP7. At 14 days after injury, blood vessels (brown, PECAM immunohistochemistry) invaded a cartilage island (cartilage), with new bone forming (bone). Chondrocytes in the cartilage, osteocytes in the new bone, and endothelial cells (brown staining) are negative for X-gal staining. Some X-gal positive cells (blue) are present at the endochondral front. Scale bars: A, C = 50µm, B = 200µm, D = 100µm.
Using this model of bone marrow transplantation, we assessed whether rhBMP7 could recruit donor mesenchymal stem cells from the bone marrow and induce them to form bone and cartilage during fracture repair. Ischemic fractures were created in chimeric mice, and at the time of fracture rhBMP7 or lactose was injected into the fracture site. At 14 days after injury, we did not detect osteocytes or chondrocytes that expressed β-galatosidase in the fracture callus of animals injected with the control lactose solution (data not shown). As expected, when we injected rhBMP7, we observed a larger fracture callus. However, the osteocytes and chondrocytes in the callus remained negative for X-gal staining (Fig. 6D). These results suggest that transplanted bone marrow mesenchymal stem cells did not contribute to bone or cartilage formation during the repair of ischemic fractures, and that these cells were not induced to form bone or cartilage in the presence of rhBMP7.
Discussion
Results from the current study demonstrate that rhBMP7 induces both bone and cartilage formation in un-ischemic stabilized fractures and non-stabilized ischemic fractures. Femoral artery resection leads to massive cell death in the lower part of the leg 3. Therefore, the ability of rhBMP7 to stimulate repair in this environment is quite striking. This is underscored by our observation that even at the lower dose that we used rhBMP7 stimulated skeletal repair. Several mechanisms may underlie the osteogenic and chondrogenic potentials of rhBMP7 in a severe ischemic environment. Since we observed a large amount of dead tissues in both control and rhBMP7 treated fractures at 7 and 14 days after injury, it is unlikely that rhBMP7 could rescue cells at the fracture site from cell death. Thus, our further analysis of the underlying mechanisms was focused on the effects of rhBMP7 on stem cell recruitment and cell fate decision.
Potential effects of BMP7 on skeletal cell recruitment in a severe ischemic fracture environment
Our data provide significant evidence for the ability of rhBMP7 to stimulate healing of fractured bone in an ischemic environment. However, the source of the progenitor cells in this experimental system needs to be determined. We previously demonstrated that in ischemic environments the periosteum immediately adjacent to the fractured bone end and cells in the bone marrow undergo apoptosis. Further, the muscles in the ischemic limbs appeared necrotic 3. Recent reports indicate that there are skeletal progenitor cells in the circulating blood21. They can form bone lining cells which co-localize with alkaline phosphatase or osteocalcin staining, suggesting they may be able to differentiate into osteoblasts 22–24. These cells may originate from bone marrow 22. Therefore, rhBMP7 may be able to recruit bone marrow-derived stem cells to the fracture site to contribute to bone and cartilage formation in an ischemic environment. We chose to address this in our experiments by examining the contribution of bone marrow-derived MSCs to fracture healing using a bone marrow transplantation approach. Our in vitro data first demonstrated that donor cells with characteristics of MSCs were present in the bone marrow in irradiated and reconstituted chimerae and these cells were able to differentiate into osteogenic and chondrogenic cells in vitro. Further in vivo experiments illustrated that transplanted bone marrow cells were recruited to the fracture site. However, these cells did not form osteocytes or chondrocytes, even in the presence of ischemia and exogenous rhBMP7. These results suggest that bone marrow derived mesenchymal stem cells might not be a major player in rhBMP7 induced bone/cartilage formation in our ischemic fracture model.
These results must be interpreted cautiously, because we do not know definitively whether the donor-derived mesenchymal stem cells were incorporated into a niche from which they can be recruited. Further, other studies have reported a contribution of circulating and/or bone marrow-derived cells to the skeletal tissues forming at the fracture site. Other studies used co-localization of alkaline phosphatase or osteocalcin staining with staining to detect donor cells 22–24. Since strong alkaline phosphatase and osteocalcin staining is observed along the surface of newly formed bone. We found that it was difficult to unequivocally distinguish osteoblasts from other cell types such as osteoclasts that also line bone surfaces. Hence, we used very strict criterion to determine whether the transplanted bone marrow cells contributed to bone and cartilage by examining the presence of X-gal positive osteocytes and chondrocytes. In another study25, a pluripotent cell line was injected systemically into mice after femoral fracture and the distribution of these cells was determined at various times after injury. The investigators found that these cells homed to the fracture callus and over time cells that remained at the fracture site were encapsulated into the newly formed bone. However, whether these cells differentiated into osteocytes and produced bone matrix is not known. Hence, further experiments are required to determine the role of bone marrow-derived cells as a major source of progenitor cells during fracture healing.
Can BMP7 re-direct cell differentiation in an ischemic fracture environment?
Other scenarios may explain the cellular mechanism by which rhBMP7 stimulated bone and cartilage formation in these experiments. We observed a large amount of fibrous tissue in ischemic fractures treated with rhBMP7, and the majority of the fibrous tissue appeared to be replaced by bone at later time points (day 28, Fig. 1 and Fig. 4). Application of rhBMP7 may have accelerated the maturation and resorption of these fibrous tissues, which then allowed bone and cartilage formation to proceed more rapidly than in controls. Alternatively, rhBMP7 is capable of converting fibroblasts into bone forming cells 26. Therefore, rhBMP7 may have enhanced tissue granulation and fibrogenesis at early time points after injury and converted them to bone and cartilage at later time points. Distinguishing among these possibilities requires a genetic approach to allow definitive fate mapping of the cells in this model, but there are no adequate models that could be used to indelibly and exclusively label the fibroblasts that form in ischemic fractures.
We also observed that rhBMP7 treated fractures exhibited more adipose tissue compared to controls. It’s reported that BMP signaling stimulates the formation of brown adipose tissue in mice 27. An in vitro study by Chen et al. demonstrated that at low concentrations BMP7 stimulates adipocyte differentiation from mouse bone marrow stromal cells, while at high concentrations BMP7 favors osteoblast differentiation 28. Thus, the increased amount of adipose tissue in the rhBMP7 treated fractures could result from enhanced adipogenesis. However, this conclusion requires careful evaluation. As shown before 3, and as we observed in this work, ischemia leads to the formation of a large amount of adipose tissue in injured limbs. Since rhBMP7 induces robust callus formation, more muscle and adipose tissue could have been encapsulated in the callus resulting in increased volume of adipose tissue in the fracture calluses of treated animals.
BMP7 may be involved in regulating the differentiation of chondrocytes under distinct mechanical environments
Mechanical stimulus directs stem cell differentiation during early fracture healing. Unstable fractures heal via endochondral ossification while stabilized fractures heal through exclusively intramembranous ossification11. Results from this study revealed that rhBMP7 stimulated bone and cartilage formation in our ischemic fractures, which were mechanically unstable. We further challenged stabilized fractures with rhBMP7. Our results definitively show that at both doses tested, rhBMP7 directs chondrocyte differentiation during fracture healing. These results suggest that differential signaling by BMPs in stable and non-stable fractures may participate in directing the distinct modes of healing in these two mechanical environments. The different mechanical environment may activate or suppress expression of Bmp genes to varying degrees and thereby direct healing via these different modes. This idea is supported by a recent report that demonstrates expression of Bmp-2, and other osteogenic genes, can be regulated by mechanical forces 29.
Effect of rhBMP-7 on Tissue Vascularization
BMPs are involved in angiogenesis, the process of new blood vessel formation and vascular repair. BMP-7 is capable of inducing new blood vessel formation in chick chorioallantoic membranes 30, and BMP2, which acts similar to BMP7, can increase vascularization of tumors 31, 32. In our study, we found that rhBMP7 treatment significantly increased the total length and surface area of blood vessels in the fractured legs. However, the length density and surface density of blood vessels were not affected by rhBMP7 treatment. Therefore, the increased total length and surface area of vasculature in the treated hind limbs resulted from an increased total volume of tissues after rhBMP7 treatment. These results suggest that rhBMP7 induced more robust callus formation and vascular repair in the ischemic fractures. Further work will be required to determine the causal relationship between increased callus formation and increased vascular repair.
In summary, results of the current study demonstrate that rhBMP7 enhances fracture healing in an ischemic environment. We demonstrated that rhBMP7 induces bone and cartilage formation, increases tissue vascularization, may convert fibroblasts to osteoblasts, and regulates adipogenesis. We did not observe a contribution of bone marrow derived mesenchymal stem cells in rhBMP7-accelerated fracture healing, and we found cartilage in the muscle beds. Whether this ectopic ossification impacts functional outcomes was not directly assessed and remains unknown. Finally, further exploration is required to determine the extent to which BMP signaling affects recruitment and differentiation of skeletal progenitor cells.
Supplementary Material
Animals with tibia fracture and femoral artery resection were treated with 10µg or 50 µg of rhBMP7. rhBMP7 treatment at either doses significantly increased the volume of callus and bone compared to the controls. However, no significant difference in the volumes of callus and bone is detected between the two doses. * p<0.05, **p<0.01.
Acknowledgements
We would like to thank Xiaodong Wang and Diane Hu for technical help and R. Yee for statistical analysis. This work was aided by the Orthopaedic Trauma Association (Basic Research Grant to C.L.), NIH-NIAMS (KO8-AR002164 and RO1-AR053645-01 to T.M.), and Stryker Biotech.
References
- 1.Dickson KF, Katzman S, Paiement G. The importance of the blood supply in the healing of tibial fractures. Contemp Orthop. 1995;30:489–493. [PubMed] [Google Scholar]
- 2.Einhorn TA. Enhancement of fracture-healing. J Bone Joint Surg Am. 1995;77:940–956. doi: 10.2106/00004623-199506000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Lu C, Miclau T, Hu D, Marcucio RS. Ischemia leads to delayed union during fracture healing: a mouse model. J Orthop Res. 2007;25:51–61. doi: 10.1002/jor.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu C, Rollins M, Hou H, et al. Tibial fracture decreases oxygen levels at the site of injury. Iowa Orthop J. 2008;28:14–21. [PMC free article] [PubMed] [Google Scholar]
- 5.Hak DJ, Makino T, Niikura T, et al. Recombinant human BMP-7 effectively prevents nonunion in both young and old rats. J Orthop Res. 2006;24:11–20. doi: 10.1002/jor.20022. [DOI] [PubMed] [Google Scholar]
- 6.den Boer FC, Bramer JA, Blokhuis TJ, et al. Effect of recombinant human osteogenic protein-1 on the healing of a freshly closed diaphyseal fracture. Bone. 2002;31:158–164. doi: 10.1016/s8756-3282(02)00816-5. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Kidder LS, Lew WD. Osteogenic protein-1 induced bone formation in an infected segmental defect in the rat femur. J Orthop Res. 2002;20:142–150. doi: 10.1016/S0736-0266(01)00060-2. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Bhargav D, Wei AQ, Diwan A. Posterolateral intertransverse spinal fusion possible in osteoporotic rats with BMP-7 in a higher dose delivered on a composite carrier. Spine. 2008;33:242–249. doi: 10.1097/BRS.0b013e318162451b. [DOI] [PubMed] [Google Scholar]
- 9.Blattert TR, Delling G, Dalal PS, et al. Successful transpedicular lumbar interbody fusion by means of a composite of osteogenic protein-1 (rhBMP-7) and hydroxyapatite carrier: a comparison with autograft and hydroxyapatite in the sheep spine. Spine. 2002;27:2697–2705. doi: 10.1097/00007632-200212010-00009. [DOI] [PubMed] [Google Scholar]
- 10.White AP, Vaccaro AR, Hall JA, et al. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int Orthop. 2007;31:735–741. doi: 10.1007/s00264-007-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson Z, Miclau T, Hu D, Helms JA. A model for intramembranous ossification during fracture healing. J Orthop Res. 2002;20:1091–1098. doi: 10.1016/S0736-0266(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 12.Hall BK. The role of movement and tissue interactions in the development and growth of bone and secondary cartilage in the clavicle of the embryonic chick. J Embryol Exp Morphol. 1986;93:133–152. [PubMed] [Google Scholar]
- 13.Howard CV, Reed MG. Unbiased Stereology: Three-Dimensional Measurement in Microscopy. New York: Springer-Verlag New York Inc.; 1998. pp. 107–132. [Google Scholar]
- 14.Lu C, Miclau T, Hu D, et al. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23:1300–1307. doi: 10.1016/j.orthres.2005.04.003.1100230610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dockery P, Fraher J. The quantification of vascular beds: a stereological approach. Exp Mol Pathol. 2007;82:110–120. doi: 10.1016/j.yexmp.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Lu C, Hansen E, Sapozhnikova A, et al. Effect of age on vascularization during fracture repair. J Orthop Res. 2008;26:1384–1389. doi: 10.1002/jor.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colnot C, Huang S, Helms J. Analyzing the cellular contribution of bone marrow to fracture healing using bone marrow transplantation in mice. Biochem Biophys Res Commun. 2006;350:557–561. doi: 10.1016/j.bbrc.2006.09.079. [DOI] [PubMed] [Google Scholar]
- 18.Neuhuber B, Swanger SA, Howard L, et al. Effects of plating density and culture time on bone marrow stromal cell characteristics. Exp Hematol. 2008;36:1176–1185. doi: 10.1016/j.exphem.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sung JH, Yang HM, Park JB, et al. Isolation and characterization of mouse mesenchymal stem cells. Transplant Proc. 2008;40:2649–2654. doi: 10.1016/j.transproceed.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Tropel P, Noel D, Platet N, et al. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004;295:395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 21.Eghbali-Fatourechi GZ, Lamsam J, Fraser D, et al. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352:1959–1966. doi: 10.1056/NEJMoa044264. [DOI] [PubMed] [Google Scholar]
- 22.Otsuru S, Tamai K, Yamazaki T, et al. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells. 2008;26:223–234. doi: 10.1634/stemcells.2007-0515. [DOI] [PubMed] [Google Scholar]
- 23.Taguchi K, Ogawa R, Migita M, et al. The role of bone marrow-derived cells in bone fracture repair in a green fluorescent protein chimeric mouse model. Biochem Biophys Res Commun. 2005;331:31–36. doi: 10.1016/j.bbrc.2005.03.119. [DOI] [PubMed] [Google Scholar]
- 24.Kumagai K, Vasanji A, Drazba JA, et al. Circulating cells with osteogenic potential are physiologically mobilized into the fracture healing site in the parabiotic mice model. J Orthop Res. 2008;26:165–175. doi: 10.1002/jor.20477. [DOI] [PubMed] [Google Scholar]
- 25.Devine MJ, Mierisch CM, Jang E, et al. Transplanted bone marrow cells localize to fracture callus in a mouse model. J Orthop Res. 2002;20:1232–1239. doi: 10.1016/S0736-0266(02)00051-7. [DOI] [PubMed] [Google Scholar]
- 26.Krebsbach PH, Gu K, Franceschi RT, Rutherford RB. Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo. Hum Gene Ther. 2000;11:1201–1210. doi: 10.1089/10430340050015248. [DOI] [PubMed] [Google Scholar]
- 27.Tseng YH, Kokkotou E, Schulz TJ, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen TL, Shen WJ, Kraemer FB. Human BMP-7/OP-1 induces the growth and differentiation of adipocytes and osteoblasts in bone marrow stromal cell cultures. J Cell Biochem. 2001;82:187–199. doi: 10.1002/jcb.1145. [DOI] [PubMed] [Google Scholar]
- 29.Rath B, Nam J, Knobloch TJ, et al. Compressive forces induce osteogenic gene expression in calvarial osteoblasts. J Biomech. 2008;41:1095–1103. doi: 10.1016/j.jbiomech.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramoshebi LN, Ripamonti U. Osteogenic protein-1, a bone morphogenetic protein, induces angiogenesis in the chick chorioallantoic membrane and synergizes with basic fibroblast growth factor and transforming growth factor-beta1. Anat Rec. 2000;259:97–107. doi: 10.1002/(SICI)1097-0185(20000501)259:1<97::AID-AR11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 31.Langenfeld EM, Langenfeld J. Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res. 2004;2:141–149. [PubMed] [Google Scholar]
- 32.Raida M, Clement JH, Leek RD, et al. Bone morphogenetic protein 2 (BMP-2) and induction of tumor angiogenesis. J Cancer Res Clin Oncol. 2005;131:741–750. doi: 10.1007/s00432-005-0024-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Animals with tibia fracture and femoral artery resection were treated with 10µg or 50 µg of rhBMP7. rhBMP7 treatment at either doses significantly increased the volume of callus and bone compared to the controls. However, no significant difference in the volumes of callus and bone is detected between the two doses. * p<0.05, **p<0.01.