Abstract
Objective
Left ventricular (LV) dysfunction after successful cardiopulmonary resuscitation contributes to early death following resuscitation. Proinflammatory cytokines are known to depress myocardial function and TNF-alpha has been shown to increase after successful resuscitation. We hypothesized that blocking the effects of TNF-alpha with infliximab would prevent or minimize postresuscitation cardiac dysfunction.
Design
Randomized, placebo-controlled comparative study.
Setting
Large animal research laboratory.
Subjects
Twenty-eight anesthetized and instrumented domestic male swine (Yorkshire and Yorkshire/Hampshire mix, weight 35-45 kg).
Interventions
Infusion of infliximab (5 mg/kg) or normal saline after resuscitation from VF cardiac arrest.
Measurements and Main Results
Hemodynamic variables, indices of LV function, and TNF-alpha were measured before and after 8 min of cardiac arrest during the early post-resuscitation period (3 hrs). Within 5 min of restoration of spontaneous circulation, 14 animals received infliximab, 5 mg/kg, infused over 30 min. Fourteen animals received an infusion of normal saline. Inotropes and vasopressors were not administered to either group following resuscitation. TNF-alpha increased following restoration of circulation and remained elevated throughout the observation period. Differences between groups were not significant. IL-1β concentration did not change significantly during the observation period in either study group. Mean arterial pressure and stroke work were significantly greater in the infliximab group within 30 mins of resuscitation and these differences were sustained throughout the 3 hr postresuscitation period. The effect of TNF-α blockade was evident only in animals with a significant increase (doubling) in plasma TNF-α at 30 minutes post-arrest.
Conclusion
TNF-α plays a role in post-arrest cardiac dysfunction and Infliximab may attenuate or prevent postresuscitation myocardial dysfunction when given immediately after resuscitation.
Keywords: heart arrest, ventricular fibrillation, cardiopulmonary resuscitation, tumor-necrosis factor, interleukins, hemodynamics
Introduction
Approximately 60% of all cardiac deaths are due to out-of-hospital sudden cardiac death. Although resuscitation efforts are effective in restoring a pulse in 30-40% of victims of cardiac arrest, the eventual hospital discharge rate is <5%.(1,2) In-hospital death is usually the result of ischemic brain injury, multi-organ failure, or cardiac dysfunction.(3) Post-resuscitation myocardial dysfunction has been reported in experimental cardiac arrest models and the clinical population and potential mechanisms defined.(4) Despite the prevalence of post-resuscitation myocardial dysfunction in successfully resuscitated patients and its potential contribution to early mortality, few studies have addressed its etiology or management.(5-8)
Ischemia/reperfusion injury has been characterized as a multifactorial antigen-independent inflammatory condition wherein accelerated proinflammatory cytokine synthesis and release are commonly observed.(9,10) TNF-α and IL-1β are proinflammatory cytokines known to depress myocardial contractility.(11) Observational clinical studies in small series of patients have demonstrated elevated TNF-α and IL-1β levels in patients resuscitated from cardiac arrest.(12,13) TNF-α is elevated at the time of initial sampling after resuscitation and hospital admission and remains elevated in those patients who eventually die in-hospital or who require catecholamines for hemodynamic support. A recent laboratory study in a porcine cardiac arrest and resuscitation model demonstrated an inverse relationship between plasma TNF-α concentrations and myocardial contractility, measured as LV dp/dt.(14)
The purpose of this study was to determine if blocking TNF-α following resuscitation from prolonged cardiac arrest would prevent post-resuscitation myocardial dysfunction.
Methods
This investigation was approved by the Animal Care and Utilization Review Committee of our institution and conformed to the National Institutes of Health policy on humane care and use of laboratory animals.
Twenty-eight domestic male swine (Yorkshire and Yorkshire/Hampshire mix, weight 35-45 kg) were premedicated with ketamine (20 mg/kg) and xylazine (2 mg/kg). General anesthesia was induced with isoflurane via nose cone and, following endotracheal intubation, maintained with inhaled isoflurane and nitrous oxide in a 1:1 mixture with oxygen. Minute ventilation was adjusted to maintain an end-tidal CO2 of 35-45 mm Hg. Standard lead II of the surface ECG was monitored during instrumentation and throughout the study protocol.
Both external jugular veins and a femoral artery were surgically exposed and micromanometer-tipped catheters (Millar Instruments, Houston, TX) were inserted and positioned in the RA, LV, and Ao arch. The tip of a bipolar pacing catheter was positioned in contact with the right ventricular endocardium for induction of VF. A multilumen catheter thermistor (Edwards Lifesciences, Irvine, CA) was positioned in a branch of the pulmonary artery for thermodilution CO determinations. Standard adhesive defibrillation electrode patches were applied to the left and right lateral aspects of the shaved thorax (Quick-Combo, Medtronic Emergency Response Systems, Redmond, WA). Transthoracic impedance was measured using a tetrapolar constant current impedance measuring system (THRIM®, Morro Bay, CA). A small value non-inductive resistor (30 Ω) was then placed in series with the truncated exponential biphasic defibrillation waveform defibrillator (LifePak 12, Medtronic Emergency Response Systems, Redmond, WA).
Following instrumentation, heart rate, core temperature (measured with thermistor catheter), systolic and diastolic Ao pressure, LVEDP, and CO (measured in triplicate) were recorded and arterial blood was analyzed (I-Stat CG8+, I-Stat Corp, Princeton, NJ). Mean arterial pressure (MAP) and stroke volume (SV) were derived using standard formulae. The time constant of isovolumic LV relaxation (tau) was determined using commercially available software (Blood Pressure Module, v 1.0.3, ADInstruments, Colorado Springs, CO). Hemodynamic data were recorded and stored on a lap-top computer using PowerLab Chart v. 5.2 (ADInstruments, Colorado Springs, CO). VF was then induced with a brief 60 Hz AC current pulse delivered to the right ventricular endocardium via the pacing catheter. After 7 min of untreated VF, mechanical closed-chest compressions (Thumper®, Michigan Instruments, Grand Rapids, MI) were begun with the animal in the supine position and were administered at a rate of approximately 100/min with force sufficient to depress the sternum 1.5 to 2.0 inches. Ventilation was not performed during the one minute interval preceding the first countershock. After one minute, electrical defibrillation was attempted with a biphasic waveform defibrillator (Medtronic Physio-Control Corporation, LifePak 12). Three shocks were administered in an escalating energy sequence (200-300-360 J), if necessary. Chest compressions were performed between countershocks. If one of the first three countershocks terminated VF but resulted in a nonperfusing spontaneous cardiac rhythm, i.e., asystole or PEA or if VF persisted after the first three shocks, chest compressions were restarted and continued for two minutes or ROSC. Animals not resuscitated within two minutes were given intravenous epinephrine (0.01 mg/kg) every 5-8 min and CPR continued until spontaneous perfusing rhythm was established.
Following resuscitation, animals were randomized into two groups using permuted block design. Control animals (n = 14) were given 250 cc of normal saline over 30 min, beginning 5 min after ROSC. Animals in the treatment group (n = 14) were given infliximab, a monoclonal anti-TNF-α antibody, at a dose of 5 mg/kg in 250 ml of normal saline infused over 30 min beginning 5 min after ROSC. No other drugs were given or interventions performed during the ensuing 3 hr post-ROSC observation period. Hemodynamic measurements and core temperature were made at 30, 60, 120, and 180 min following ROSC.
Prior to VF induction and at 30, 60, 120, and 180 min following restoration of circulation, arterial blood was sampled, placed in sterile, chilled (0° C), heparinized tubes, and centrifuged at 5000 rpm for 10 min. Plasma was immediately separated and stored at -80° C until analysis. TNF-α and IL1-β concentrations were determined by a quantitative sandwich ELISA using commercially available kits specific for these porcine cytokines (R&D Systems, Inc., Minneapolis, MN).
Data Analyses
Statistical analyses were conducted using SAS Version 9.1.3 (SAS Institute, Cary, NC). Summary measures are reported as means and standard deviations for normal distributions or medians and inter-quartile ranges for non-normal distributions. Student's t-tests or Mann-Whitney Rank Sum tests were used for comparing fixed time-point variables between saline and infliximab treated animals.
Generalized linear mixed models were employed to evaluate associations between variables while accounting for the autocorrelation inherent in repeated measure designs. The SAS procedure, Proc Mixed, was employed for this purpose, with time included as a random effect and other variables included as fixed effects. Variable selection was based on a priori assumptions and iterative fitting of potential models. Model fit and correlation structure specification was assessed by Akaike Information Criteria. When making multiple post-resuscitation comparisons with prearrest values, p values were adjusted according to the method of Dunnett-Hsu.(15) We considered an alpha level of 0.05 to be significant.
Results
Differences in prearrest hemodynamic variables were not observed between treatment groups (table 1). Similarly, variables associated with resuscitation outcome and post-resuscitation ventricular function were likewise not significantly different (table 2).
Table 1. Prearrest variables for the treatment groups.
| Control (n = 14) | Infliximab (n = 14) | P value | |||
|---|---|---|---|---|---|
| HR (beats per minute) | 86 | (17) | 96 | (10) | 0.06 |
| MAP (mmHg) | 87 | (8) | 92 | (10) | 0.12 |
| LVEDP (mmHg) | 6.29 | (1.90) | 5.43 | (2.10) | 0.27 |
| Stroke work (gm-m) | 47.1 | (6.92) | 50.3 | (7.38) | 0.25 |
| Temperature (°C) | 37.3 | (1.1) | 37.0 | (0.7) | 0.38 |
| TNF-α level (pg/ml) | 135 | (82-210) | 113 | (65-159) | 0.45 |
| IL-1β level (pg/ml) | 0 | (0-15) | 0 | (0-16) | 0.65 |
Values are the mean ± SD or median and interquartile range.
Table 2. Resuscitation variables for the treatment groups.
| Control (n = 14) | Infliximab (n = 14) | P value | |||
|---|---|---|---|---|---|
| Transthoracic impedance (Ohms) | 67.5 | (2.87) | 69 | (4.14) | 0.20 |
| Coronary Perfusion Pressure (mmHg) | 12.9 | (3.55) | 13.2 | (5.00) | 0.86 |
| Countershocks to defibrillate | 1.5 | (1-2) | 1 | (1-2) | 0.28 |
| Total countershocks delivered | 2 | (1-3) | 1 | (1-2) | 0.28 |
| Joules delivered | 500 | (200-800) | 200 | (200-500) | 0.22 |
| Epinephrine administered (mg) | 0.5 | (0.5-0.5) | 0.5 | (0.5-0.5) | 0.21 |
| Time to ROSC* (seconds) | 114 | (84-163) | 99 | (84-134) | 0.44 |
Return of Spontaneous Circulation
Values are the mean ± standard deviation or median and interquartile range.
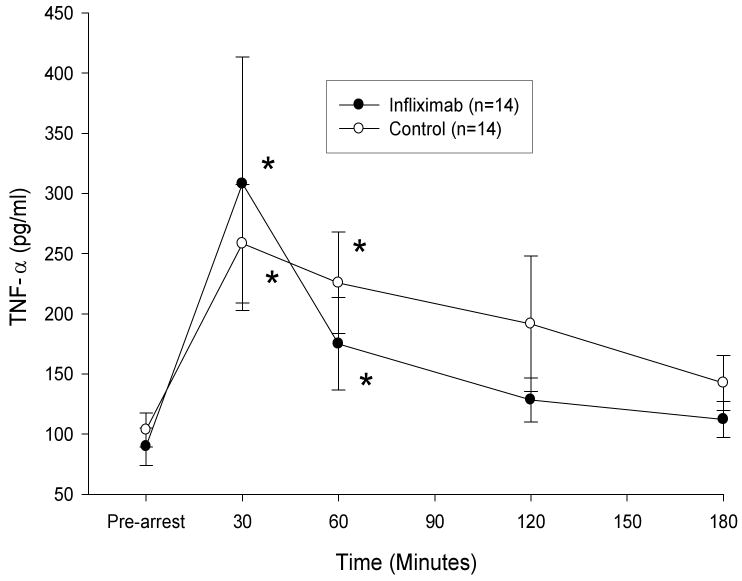
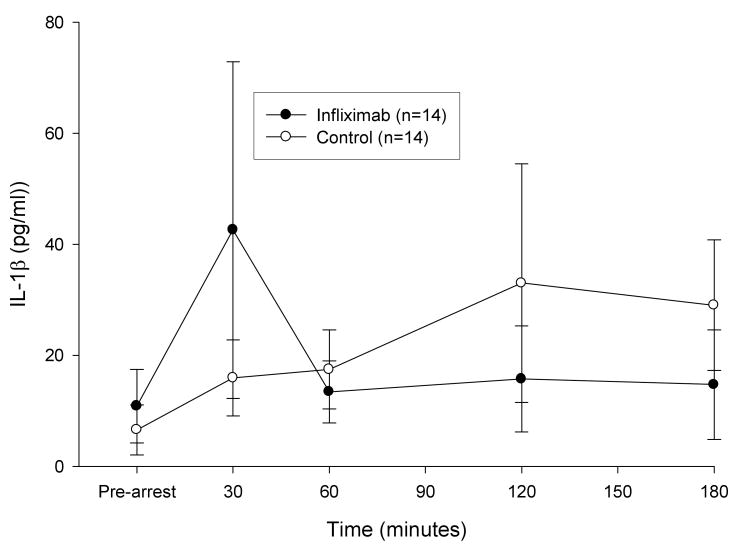
Following resuscitation, TNF-α concentration 30 and 60 min after ROSC were significantly greater than pre-arrest values for both study groups. However, IL-1β levels did not vary significantly from pre-arrest values in either group. There was no statistically significant difference in the response of either TNF-α or IL-1β between treatment groups over time (figures 1 and 2). At 3 hrs post-resuscitation, core temperature was not significantly different between groups (control group, 35.5 ± 1.3 °C, infliximab group, 35.6 ± 3 °C).
Figure 1. Plasma TNF-α Concentrations Following Resuscitation.
Plasma TNF-α concentrations (mean ± SE) were not significantly different between control and infliximab treatment groups during the 3 hour post-arrest observation period. TNF-α levels were greater than pre-arrest values (*p < 0.02) at 30 and 60 min following resuscitation in both groups.
Figure 2. Plasma IL-1β Concentrations Following Resuscitation.
Plasma IL-1β concentrations (mean ± SE) did not increase significantly from pre-arrest values following resuscitation and differences were not observed between groups.
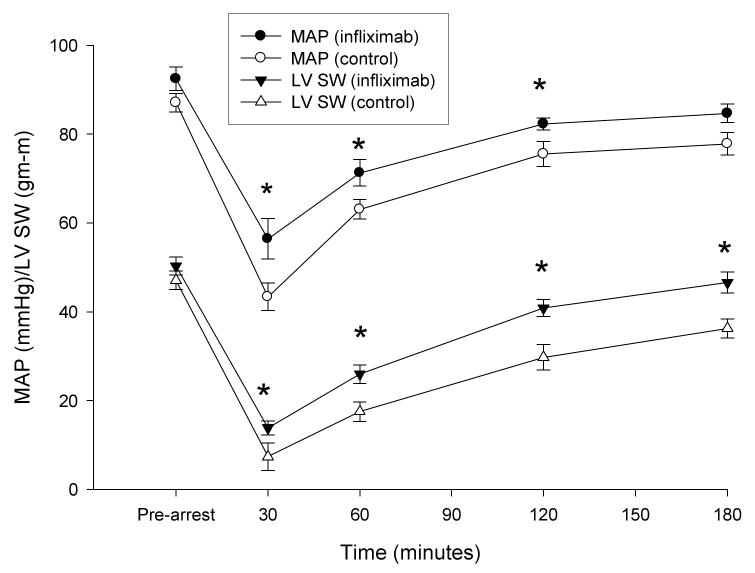
In a generalized linear mixed model, infliximab treated animals had a MAP that was, on average, 8.7 mmHg (95% confidence interval [CI] 4.5-13.0 mmHg, p=0.0001) higher than control animals. Treated animals also had a mean SW that was, on average, 9.1 gm-m (95% CI 5.4-12.8 gm-m, p<0.0001) higher than control animals. Postresuscitation hemodynamic variables are shown in table 3. Differences in mean values for MAP and SW are illustrated in figure 3. Systolic dp/dt was significantly greater in the infliximab group at all postresuscitation time points and stroke volume was greater from 30 to 120 min. TNF-α levels were inversely correlated with MAP (r= −0.41, p<0.0001) and with SW (r= −0.37, p<0.0001). IL-1β, however, was not associated with either response (p>0.11 for both associations).
Table 3. Hemodynamic Variables Following Resuscitation.
| Prearrest | 30 min | 60 min | 120 min | 180 min | |
|---|---|---|---|---|---|
| Control | |||||
| HR (beat/min) | 86 ± 17 | 94 ± 16 | 85 ± 15 | 80 ± 13 | 77 ± 13 |
| LVEDP (mm Hg) | 6 ± 2 | 11 ± 5 | 12 ± 2 | 14 ± 3 | 13 ± 3 |
| SV (ml) | 41 ± 5 | 16 (5-18) | 25 (21-28) | 35 ± 9 | 43 ± 9 |
| S dp/dt (mm Hg/s) | 1099 ± 156 | 420 ± 155 | 728 ± 104 | 954 ± 179 | 1000 (952-1180) |
| tau (msec) | 30 ± 4 | 66 ± 18 | 45 ± 6 | 45 (41-49) | 44 ± 13 |
| Inflximab | |||||
| HR (beats/min) | 96 ± 10 | 99 ± 9 | 90 ± 10 | 83 ± 9 | 79 ± 11 |
| LVEDP (mmHg) | 5 ± 2 | 11 ± 4 | 10 ± 3 | 12 ± 4 | 11 ± 5 |
| SV (ml) | 42 ± 5 | 21 (18-24)† | 31 (528-33)* | 41 ± 6* | 46 ± 7 |
| S dp/dt (mm Hg/s) | 1230 ± 216 | 603 ± 204** | 883 ± 194** | 1130 ± 255* | 1174 (1042-1250)* |
| tau (msec) | 33 ± 4 | 58 ± 14 | 45 ± 5 | 44 (42-50) | 46 ± 7 |
Values are the mean ± standard deviation or median and interquartile range.
HR = heart rate; LVEDP = Left ventricular end-diastolic pressure; S dp/dt = systolic dp/dt; SV = stroke volume
p < 0.05,
p < 0.025,
p < 0.005 versus control group
Figure 3. Mean Arterial Pressure (MAP) and Left Ventricular Stroke Work (LV SW) Following Resuscitation.
MAP and SW (mean ± SE) were significantly decreased from pre-arrest values in both groups within 30 min of reperfusion. Values were significantly higher at all time points in the infliximab group. (*p < 0.002, Dunnet-Hsu adjusted for differences between groups).
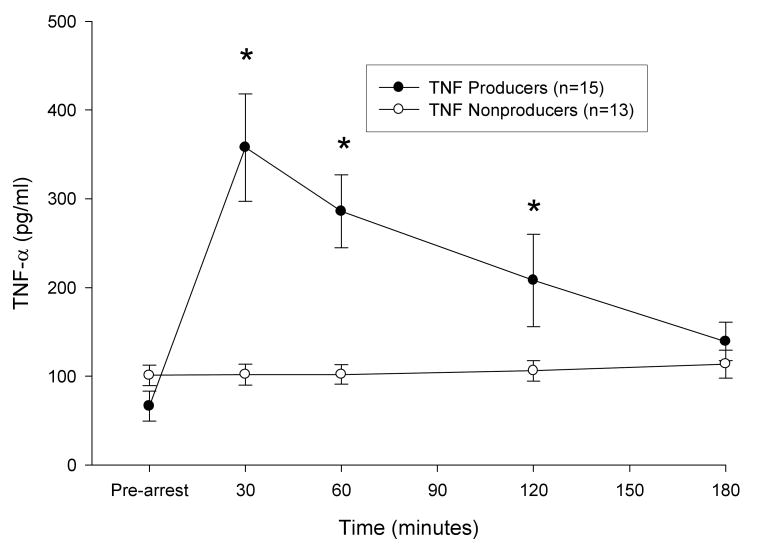
When animals were classified into TNF-α producers (defined as at least a doubling of TNF-α at 30 minutes when compared to prearrest values, n = 15) and non-producers (n = 13) (figure 4), an association between infliximab treatment and higher MAP and SW was seen only within the TNF-α producer group. In this group, infliximab treated animals had, on average, 10.8 mmHg higher MAP (95% CI 5.5-16.1 mmHg, p=0.0002) than controls. There was also an interaction between time and treatment assignment. This interaction resulted in a lessening of the treatment effect on MAP over time until there was no statistical difference in MAP between the two groups at the 180 minute measurement. Infliximab treatment was also associated with higher SW in producers, but not non-producers. SW among infliximab treated animals was, on average, 11.2 gm-m (95% CI 5.7-16.7 gm-m, p=0.0002) higher than controls. This effect was not time-varying in the model with infliximab-treated animals having a consistently higher SW across all time points.
Figure 4. Plasma TNF-α Concentrations in TNF Producers and Nonproducers.
A dichotomy in the TNF-α response to reperfusion was observed in animals of both treatment groups. Fifty four percent of animals (n=15) demonstrated at least a doubling of TNF-α at 30 min post-arrest. *p < 0.002 compared to pre-arrest value. Data are mean ± SE.
Discussion
This study in a conventional porcine cardiac arrest model demonstrates that infliximab, a monoclonal anti-TNF-α antibody, administered after resuscitation, is effective in preserving hemodynamic indices of left ventricular function. Left ventricular stroke work, a global measure of cardiac function, was significantly greater in the treatment group within 30 min of successful resuscitation and hemodynamic differences persisted throughout the observation period. When animals were subsequently classified based upon the post-arrest TNF-α response, the beneficial effect of infliximab was most prominent in those animals with a significant increase in TNF following restoration of spontaneous circulation. These findings suggest that TNF-α plays a contributory role in post-arrest myocardial dysfunction, one of the hallmarks of the post-arrest syndrome.
TNF-α is a known myocardial depressant, presumably by disrupting calcium homeostasis or calcium sensitivity and the normal myocardial contraction-relaxation cycle.(16,17) TNF-α may also induce a state of relative catecholamines insensitivity or refractoriness.(18-20) The hemodynamic effects of TNF-α include depressed myocardial contractility, decreased ejection fraction, and ventricular dilatation. The use of monoclonal antibodies to TNF-α in low doses (1 mg/kg) has been shown to minimize cardiac dysfunction in in vivo and isolated heart models of acute global and regional myocardial ischemia.(21).
Infliximab is produced by a recombinant cell line and is a chimeric IgG1κ monoclonal antibody composed of human constant and murine variable regions of TNF-α. Porcine TNF shares a similar structure with that of human and murine TNF and exhibits cytotoxicity to target indicator cells (PK(15) and L929) at similar concentrations.(22) Porcine TNF-α cytotoxic activity can be totally neutralized with anti-human TNF monoclonal antibody.(23) Porcine TNF-α receptors likewise share a structure similar to that of humans and mice and human soluble TNF-α receptors bind porcine TNF-α.(24) Considering these characteristics, binding to and neutralization of porcine TNF-α by infliximab would be expected.
We observed considerable variability in the TNF-α response to ischemia and reperfusion within the control and treatment groups. We postulate that this variable response is likely due to genetic polymorphism in the promoter region of the TNF-α gene. TNF-α single nucleotide polymorphism has been extensively studied in humans and is postulated to play a role in susceptibility and response to infection as well as outcome.(25-27) Polymorphism would be expected in the typical outbred, mixed breed swine used in research laboratories.
This study has several limitations. Although the anatomy of the porcine coronary circulation is similar to that of humans, the absence of significant underlying atherosclerotic coronary artery disease and prior myocardial injury, both of which are usually present in resuscitated patients, is likely to have affected the extent of cardiac dysfunction following resuscitation. We used only male swine in this study due to prior observations suggesting that there are gender differences in the proinflammatory response following resuscitation and that the response is more dramatic and more predictable in males than females.(28) We evaluated one cardiac arrest duration (7 min) prior initiation of resuscitation efforts. A long duration of ischemia may have resulted in a more pronounced inflammatory response. The anesthetic agent necessary to conduct the study may also have impacted coronary vascular tone as well as baseline and postresuscitation LV function. Although statistically significant differences in energy required for defibrillation, time to restoration of spontaneous circulation, and epinephrine dose were not demonstrated, small differences might be physiologically significant and may have affected postresuscitation ventricular function. We evaluated only one dose of infliximab in this study. We selected a dose of 5 mg/kg because prior work in clinical trials indicated that 5 mg/kg did not produce immediate significant side effects when administered intravenously and approximates the dose used in large clinical trials of TNF-α blockade in sepsis.(29,30) We did not evaluate the potential role of other cytokine inhibitors, e.g., an anti-IL-1β inhibitor, on post-arrest cardiac dysfunction. However, IL-1β concentrations did not significantly vary during the 3 hour post-arrest study period, were not associated with hemodynamic variables, and thus is unlikely to have contributed substantially to the observed hemodynamic depression. We did not attempt to control body temperature during the observation period and observed statistically insignificant differences in temperature between groups. Prior work in whole animal models subjected to hypothermia following cardiac arrest and hemorrhagic shock has not demonstrated a significant effect of hypothermia on the inflammatory response.(31,32)
Anti-cytokine therapy has not been shown to be beneficial in some disease states characterized by increased cytokine production, specifically TNF-α. Notably, infliximab and etanercept have not been shown to be consistently effective in the setting of chronic congestive heart failure or the sepsis syndrome and reasons for the lack of a positive effect have been enumerated.(33,34) However, it is likely that the organism resuscitated from an acute, short-lived, global (total body) ischemic insult, i.e., cardiac arrest, represents a unique physiologic state characterized by an early and sustained maximal stress response and a unique proinflammatory cytokine response when compared to other more indolent insults.(35) The findings of this investigation in a commonly used porcine cardiac arrest model suggest that TNF-α plays a role in postresuscitation myocardial contractile dysfunction and that therapeutic interventions that inhibit its effects may attenuate to some degree, but not prevent its myocardial depressant properties. Other factors contribute to the postresuscitation myocardial dysfunction.(4) Hemodynamic benefit appears to be dependent upon the TNF-α response after resuscitation and reperfusion and are likely genotypically determined. TNF-α blockers, either alone or in combination with drugs that block other cytokines or their receptors, may be useful adjuncts in the management of the post-arrest syndrome, particularly if the TNF-α or other cytokine blood levels are known early after resuscitation.
Acknowledgments
Supported, in part, by a grant from the National Institutes of Health, NHLBI R01 HL076671.
References
- 1.Nichol G, Steen P, Herlitz J, et al. International resuscitation network registry: design, rationale and preliminary results. Resuscitation. 2005;65:265–277. doi: 10.1016/j.resuscitation.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 2.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–31. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome. Epidemiology, pathophysiology, treatment, and prognostication. Circulation. 2008;118:2452–83. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 4.Gazmuri RJ, Weil MH, Kern KB, et al. Prevention and therapy of postresuscitation myocardial dysfunction. In: Paradis NA, Halperin HR, Kern KB, Wenzel V, Chamberlain DA, editors. Cardiac Arrest. The Science and Practice of Resuscitation Medicine. Second. New York: Cambridge University Press; 2007. pp. 829–847. [Google Scholar]
- 5.Gazmuri RJ, Ayoub IM, Kolarova J. Myocardial protection during resuscitation from cardiac arrest. Curr Opin Crit Care. 2003;9:199–204. doi: 10.1097/00075198-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Kern KB, Hilwig RW, Berg RA, et al. Postresuscitation left ventricular systolic and diastolic dysfunction. Treatment with dobutamine. Circulation. 1997;95:2610–13. doi: 10.1161/01.cir.95.12.2610. [DOI] [PubMed] [Google Scholar]
- 7.Niemann JT, Garner D, Khaleeli E, Lewis RJ. Milrinone facilitates resuscitation from cardiac arrest and attenuates postresuscitation myocardial dysfunction. Circulation. 2003;108:1032–36. doi: 10.1161/01.CIR.0000101925.37174.85. [DOI] [PubMed] [Google Scholar]
- 8.Vasquez A, Kern KB, Hilwig RW, Heidenreich J, Berg RA, Ewy GA. Optimal dosing of dobutamine for treating post-resuscitation left ventricular dysfunction. Resuscitation. 2004;61:199–207. doi: 10.1016/j.resuscitation.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Boros P, Bromber JS. New cellular and molecular immune pathways in ischemia/reperfusion injury. Am J Tranplant. 2006;6:652–58. doi: 10.1111/j.1600-6143.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- 10.Stangl V, Baumann G, Stangl K, Felix SB. Negative inotropic mediators from heart after myocardial ischaemia-reperfusion. Cardiovasc Res. 2002;53:12–30. doi: 10.1016/s0008-6363(01)00420-5. [DOI] [PubMed] [Google Scholar]
- 11.Kan H, Finkel MS. Inflammatory mediators and reversible myocardial dysfunction. J Cell Physiol. 2003;195:1–11. doi: 10.1002/jcp.10213. [DOI] [PubMed] [Google Scholar]
- 12.Ito T, Saitoh D, Fukuzuka K, et al. Significance of elevated serum interleukin-8 in patients resuscitated after cardiac arrest. Resuscitation. 2001;51:47–53. doi: 10.1016/s0300-9572(01)00382-3. [DOI] [PubMed] [Google Scholar]
- 13.Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–68. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 14.Niemann JT, Garner D, Lewis RJ. Tumor necrosis factor-α is associated with early postresuscitation myocardial dysfunction. Crit Care Med. 2004;32:1753–58. doi: 10.1097/01.ccm.0000132899.15242.d3. [DOI] [PubMed] [Google Scholar]
- 15.Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Amer Statist Assoc. 1955;50:1096–1121. [Google Scholar]
- 16.Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;174:R577–95. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama T, Vaca L, Rossen RD, Durante W, Hazarika P, Mann DL. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J Clin Invest. 1993;92:2303–12. doi: 10.1172/JCI116834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulick T, Chung M, Pieper SJ, Lange LG, Schreiner GF. Interleukin 1 and tumor necrosis factor inhibit cardiac myocytes beta-adrenergic responsiveness. Proc Natl Acad Sci. 1989;86:6753–57. doi: 10.1073/pnas.86.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A, Kosuri R, Kandula P, Dimou C, Allen J, Parrillo JE. Effects of epinephrine and amrinone on contractility and cyclic adenosine monophosphate generation of tumor necrosis factor alpha-exposed cardiac myocytes. Crit Care Med. 1999;27:286–92. doi: 10.1097/00003246-199902000-00032. [DOI] [PubMed] [Google Scholar]
- 20.Bucher M, Kees F, Taeger K, Kurtz A. Cytokines down-regulate α-1 adrenergic receptor expression during endotoxemia. Crit Care Med. 2003;31:566–71. doi: 10.1097/01.CCM.0000048621.36569.69. [DOI] [PubMed] [Google Scholar]
- 21.Li D, Zhao L, Liu M, et al. Kinetics of tumor necrosis factor α and the cardioprotective effect of a monoclonal antibody to tumor necrosis factor α in acute myocardial infarction. Am Heart J. 1999;137:2245–52. doi: 10.1016/s0002-8703(99)70375-3. [DOI] [PubMed] [Google Scholar]
- 22.Pauli U. Porcine TNF: a review. Vet Immunol Immunopathol. 1995;47:187–201. doi: 10.1016/0165-2427(94)05405-h. [DOI] [PubMed] [Google Scholar]
- 23.Pauli U, Beutler B, Peterhans E. Porcine tumor necrosis factor alpha: cloning with the polymerase chain reaction and determination of the nucleotide sequence. Gene. 1989;81:185–91. doi: 10.1016/0378-1119(89)90350-8. [DOI] [PubMed] [Google Scholar]
- 24.Pauli U, Bertoni G, Duerr M, Peterhans E. A bioassay for the detection of tumor necrosis factor from eight different species: evaluation of neutralization rates of monoclonal antibody against human TNF-α. J Immunol Meth. 1994;171:263–65. doi: 10.1016/0022-1759(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 25.Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor α promoter on transcriptional activation. Proc Natl Acad Sci. 1997;94:3195–99. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmes CL, Russell JA, Walley KR. Genetic polymorphisms in sepsis and septic shock. Role in prognosis and potential for therapy. Chest. 2003;124:1103–15. doi: 10.1378/chest.124.3.1103. [DOI] [PubMed] [Google Scholar]
- 27.Imahara SD, O'Keefe GE. Genetic determinants of the inflammatory response. Curr Opin Crit Care. 2004;10:318–24. doi: 10.1097/01.ccx.0000140942.42247.7e. [DOI] [PubMed] [Google Scholar]
- 28.Niemann JT, Rosborough JP, Youngquist S. Is the tumor necrosis factor-alpha response following resuscitation gender dependent in the swine model? Resuscitation. 2008;77:258–63. doi: 10.1016/j.resuscitation.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT, ATTACH Investigators Randomized double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate –to-severe heart failure. Results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) Trial. Circulation. 2003;107:3133–40. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 30.Abraham E, Anzueto A, Guitierrez G, et al. Double-blind randomised controlled trial of monclonal antibody to human tumor necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351:929–33. [PubMed] [Google Scholar]
- 31.Wu X, Stezoski J, Safar P, et al. Mild hypothermia during hemorrhagic shock in rats improves survival without significant effects on inflammatory responses. Crit Care Med. 2003;31:195–202. doi: 10.1097/00003246-200301000-00030. [DOI] [PubMed] [Google Scholar]
- 32.Callaway CW, Rittenberger JC, Logue ES, McMichael MJ. Hypothermia after cardiac arrest does not alter serum inflammatory markers. Crit Care Med. 2008;36:2607–2612. doi: 10.1097/CCM.0b013e318184443b. [DOI] [PubMed] [Google Scholar]
- 33.Anker SD, Coates AJS. How to RECOVER from RENAISSANCE? The significance of the results of RECOVER, RENAISSANCE, RENEWAL, and ATTACH. Int J Cardiol. 2002;86:123–30. doi: 10.1016/s0167-5273(02)00470-9. [DOI] [PubMed] [Google Scholar]
- 34.Abraham E. Why immunomodulatory therapies have not worked in sepsis. Intens Care Med. 1999;25:556–66. doi: 10.1007/s001340050903. [DOI] [PubMed] [Google Scholar]
- 35.Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis. Results of the genetic and inflammatory markers of sepsis (GemIMS) study. Arch Intern Med. 2007;167:1655–63. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]