Abstract
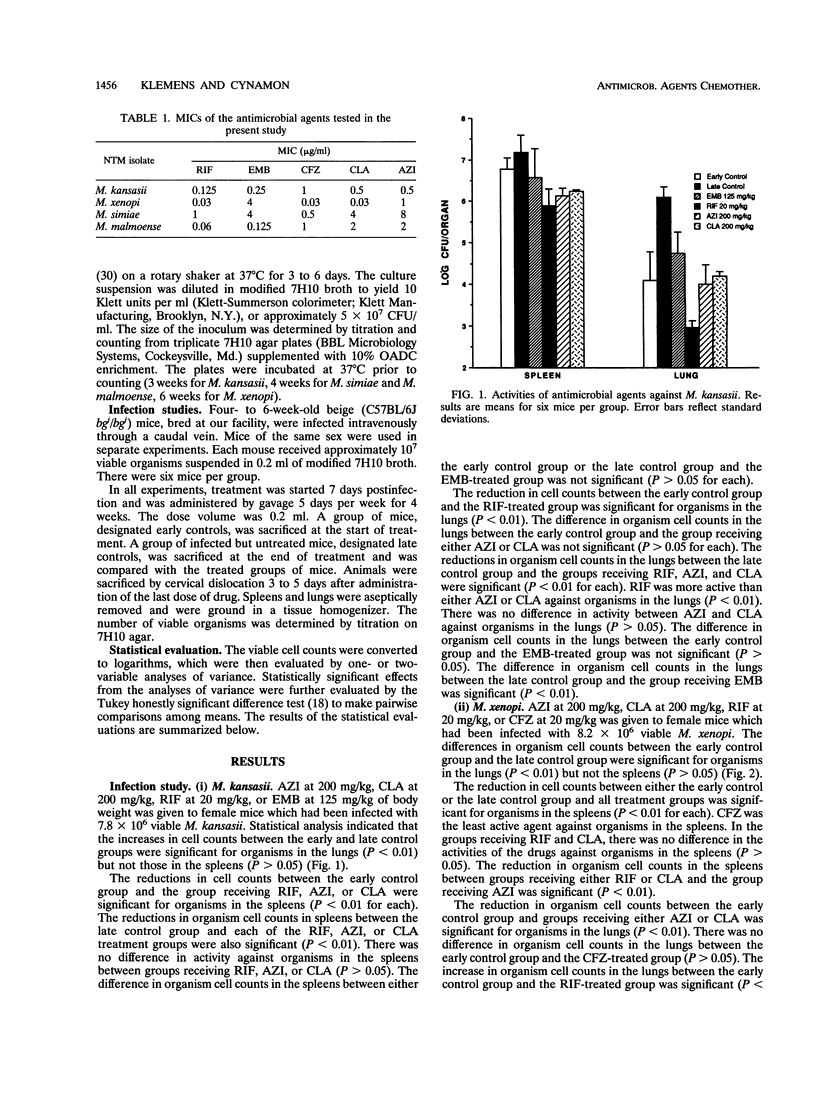
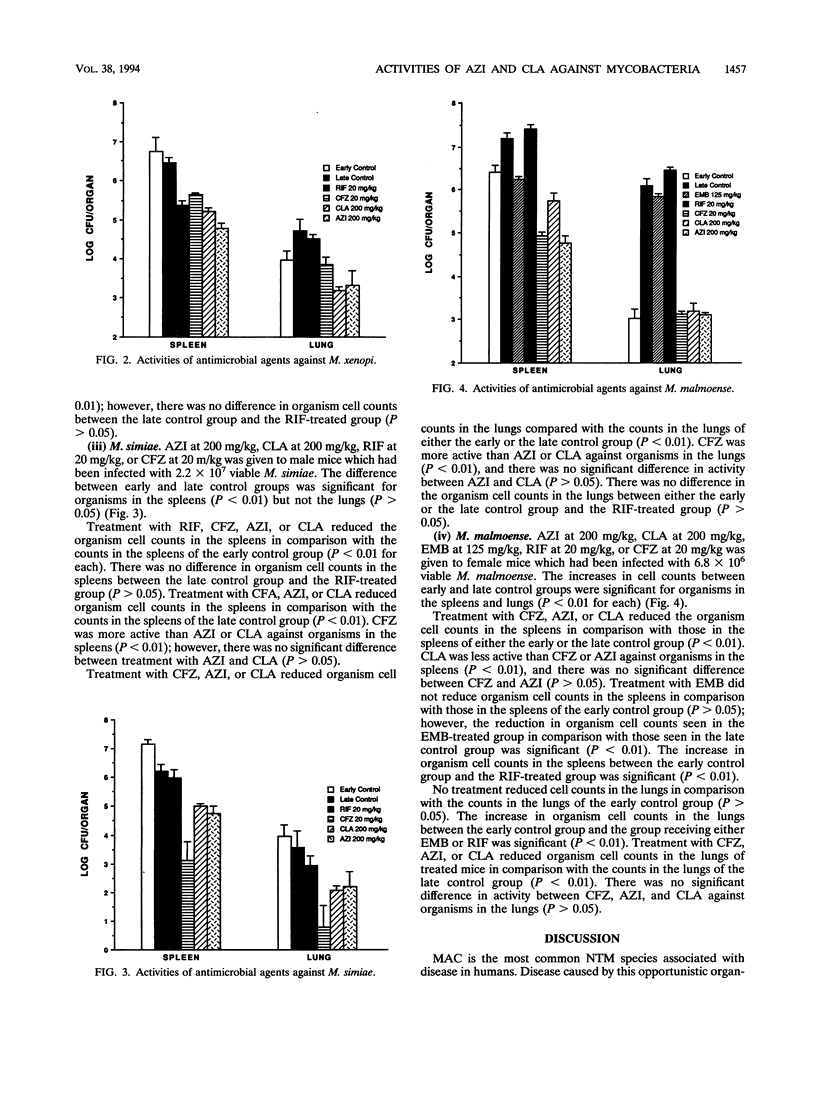
The comparative activities of azithromycin (AZI) and clarithromycin (CLA) were evaluated against nontuberculous mycobacteria in a murine model of disseminated infection. Four-week-old beige mice (C57BL/6J bgj/bgj) were infected intravenously with approximately 10(7) viable Mycobacterium kansasii, M. xenopi, M. simiae, or M. malmoense. Treatment with AZI at 200 mg/kg, CLA at 200 mg/kg, ethambutol at 125 mg/kg, rifampin at 20 mg/kg, or clofazimine at 20 mg/kg of body weight was started 7 days postinfection, and the treatments were administered 5 days per week for 4 weeks. Control groups were sacrificed at the start and end of the treatments. Spleens and lungs were homogenized, and viable cell counts were determined by serial dilution and plating onto 7H10 agar. AZI and CLA had activities comparable to or better than that of rifampin, ethamutol, or clofazimine against these nontuberculous mycobacteria in the beige mouse test system. AZI at 200 mg/kg was more active than CLA at 200 mg/kg against organisms in the spleens for M. xenopi and M. malmoense. The activities of AZI and CLA were comparable against organisms in the spleens for M. kansasii and M. simiae. The activities of these two agents were comparable against organisms in the lungs for all four nontuberculous mycobacterial species. AZI or CLA in combination with other agents may be useful for the therapy of nontuberculous mycobacterial infections in humans.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn C. H., Lowell J. R., Ahn S. S., Ahn S., Hurst G. A. Chemotherapy for pulmonary disease due to Mycobacterium kansasii: efficacies of some individual drugs. Rev Infect Dis. 1981 Sep-Oct;3(5):1028–1034. doi: 10.1093/clinids/3.5.1028. [DOI] [PubMed] [Google Scholar]
- Akiyama H., Maruyama T., Uetake T., Kawaguchi K., Sakamaki H., Onozawa Y. Systemic infection due to atypical mycobacteria in patients with chronic myelogenous leukemia. Rev Infect Dis. 1991 Sep-Oct;13(5):815–818. doi: 10.1093/clinids/13.5.815. [DOI] [PubMed] [Google Scholar]
- Banks J., Hunter A. M., Campbell I. A., Jenkins P. A., Smith A. P. Pulmonary infection with mycobacterium xenopi: review of treatment and response. Thorax. 1984 May;39(5):376–382. doi: 10.1136/thx.39.5.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. A., Wallace R. J., Jr, Onyi G. O., De Rosas V., Wallace R. J., 3rd Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and M. chelonae-like organisms. Antimicrob Agents Chemother. 1992 Jan;36(1):180–184. doi: 10.1128/aac.36.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynamon M. H. Comparative in vitro activities of MDL 473, rifampin, and ansamycin against Mycobacterium intracellulare. Antimicrob Agents Chemother. 1985 Sep;28(3):440–441. doi: 10.1128/aac.28.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynamon M. H., Klemens S. P. Activity of azithromycin against Mycobacterium avium infection in beige mice. Antimicrob Agents Chemother. 1992 Aug;36(8):1611–1613. doi: 10.1128/aac.36.8.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautzenberg B., Truffot C., Legris S., Meyohas M. C., Berlie H. C., Mercat A., Chevret S., Grosset J. Activity of clarithromycin against Mycobacterium avium infection in patients with the acquired immune deficiency syndrome. A controlled clinical trial. Am Rev Respir Dis. 1991 Sep;144(3 Pt 1):564–569. doi: 10.1164/ajrccm/144.3_Pt_1.564. [DOI] [PubMed] [Google Scholar]
- Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am Rev Respir Dis. 1990 Oct;142(4):940–953. doi: 10.1164/ajrccm/142.4.940. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Hardy D. J., McDaniel D., Hanson C. W., Swanson R. N. In vitro and in vivo activities of clarithromycin against Mycobacterium avium. Antimicrob Agents Chemother. 1989 Sep;33(9):1531–1534. doi: 10.1128/aac.33.9.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelber R. H., Siu P., Tsang M., Murray L. P. Activities of various macrolide antibiotics against Mycobacterium leprae infection in mice. Antimicrob Agents Chemother. 1991 Apr;35(4):760–763. doi: 10.1128/aac.35.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzynski E. A., Gutman S. I., Allen W. Comparative antimycobacterial activities of difloxacin, temafloxacin, enoxacin, pefloxacin, reference fluoroquinolones, and a new macrolide, clarithromycin. Antimicrob Agents Chemother. 1989 Apr;33(4):591–592. doi: 10.1128/aac.33.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets L. B., Lindholm-Levy P. J., Comstock R. D. Clarithromycin minimal inhibitory and bactericidal concentrations against Mycobacterium avium. Am Rev Respir Dis. 1992 Apr;145(4 Pt 1):856–858. doi: 10.1164/ajrccm/145.4_Pt_1.856. [DOI] [PubMed] [Google Scholar]
- Hoffner S. E., Hjelm U., Källenius G. Susceptibility of Mycobacterium malmoense to antibacterial drugs and drug combinations. Antimicrob Agents Chemother. 1993 Jun;37(6):1285–1288. doi: 10.1128/aac.37.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Selik R. M. The epidemiology of disseminated nontuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). Am Rev Respir Dis. 1989 Jan;139(1):4–7. doi: 10.1164/ajrccm/139.1.4. [DOI] [PubMed] [Google Scholar]
- Ji B., Perani E. G., Grosset J. H. Effectiveness of clarithromycin and minocycline alone and in combination against experimental Mycobacterium leprae infection in mice. Antimicrob Agents Chemother. 1991 Mar;35(3):579–581. doi: 10.1128/aac.35.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemens S. P., DeStefano M. S., Cynamon M. H. Activity of clarithromycin against Mycobacterium avium complex infection in beige mice. Antimicrob Agents Chemother. 1992 Nov;36(11):2413–2417. doi: 10.1128/aac.36.11.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemens S. P., DeStefano M. S., Cynamon M. H. Therapy of multidrug-resistant tuberculosis: lessons from studies with mice. Antimicrob Agents Chemother. 1993 Nov;37(11):2344–2347. doi: 10.1128/aac.37.11.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuze F., Kurasawa T., Bando K., Lee Y., Maekawa N. In vitro and in vivo susceptibility of atypical mycobacteria to various drugs. Rev Infect Dis. 1981 Sep-Oct;3(5):885–897. doi: 10.1093/clinids/3.5.885. [DOI] [PubMed] [Google Scholar]
- Lillo M., Orengo S., Cernoch P., Harris R. L. Pulmonary and disseminated infection due to Mycobacterium kansasii: a decade of experience. Rev Infect Dis. 1990 Sep-Oct;12(5):760–767. doi: 10.1093/clinids/12.5.760. [DOI] [PubMed] [Google Scholar]
- Meissner G. The value of animal models for study of infection due to atypical mycobacteria. Rev Infect Dis. 1981 Sep-Oct;3(5):953–959. doi: 10.1093/clinids/3.5.953. [DOI] [PubMed] [Google Scholar]
- Naik S., Ruck R. In vitro activities of several new macrolide antibiotics against Mycobacterium avium complex. Antimicrob Agents Chemother. 1989 Sep;33(9):1614–1616. doi: 10.1128/aac.33.9.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzia W., Raleigh J. W., Bailey M. C., Toth E. A., Silverblatt J. Treatment of pulmonary disease due to Mycobacterium kansasii: recent experience with rifampin. Rev Infect Dis. 1981 Sep-Oct;3(5):1035–1039. doi: 10.1093/clinids/3.5.1035. [DOI] [PubMed] [Google Scholar]
- Shafer R. W., Sierra M. F. Mycobacterium xenopi, Mycobacterium fortuitum, Mycobacterium kansasii, and other nontuberculous mycobacteria in an area of endemicity for AIDS. Clin Infect Dis. 1992 Jul;15(1):161–162. doi: 10.1093/clinids/15.1.161. [DOI] [PubMed] [Google Scholar]
- Truffot-Pernot C., Ji B., Grosset J. Effect of pH on the in vitro potency of clarithromycin against Mycobacterium avium complex. Antimicrob Agents Chemother. 1991 Aug;35(8):1677–1678. doi: 10.1128/aac.35.8.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valainis G. T., Cardona L. M., Greer D. L. The spectrum of Mycobacterium kansasii disease associated with HIV-1 infected patients. J Acquir Immune Defic Syndr. 1991;4(5):516–520. [PubMed] [Google Scholar]
- Wallace R. J., Jr The clinical presentation, diagnosis, and therapy of cutaneous and pulmonary infections due to the rapidly growing mycobacteria, M. fortuitum and M. chelonae. Clin Chest Med. 1989 Sep;10(3):419–429. [PubMed] [Google Scholar]
- Weber J., Mettang T., Staerz E., Machleidt C., Kuhlmann U. Pulmonary disease due to Mycobacterium xenopi in a renal allograft recipient: report of a case and review. Rev Infect Dis. 1989 Nov-Dec;11(6):964–969. doi: 10.1093/clinids/11.6.964. [DOI] [PubMed] [Google Scholar]
- Weiszfeiler J. G., Karasseva V., Karczag E. Mycobacterium simiae and related mycobacteria. Rev Infect Dis. 1981 Sep-Oct;3(5):1040–1045. doi: 10.1093/clinids/3.5.1040. [DOI] [PubMed] [Google Scholar]
- Wolinsky E. Mycobacterial diseases other than tuberculosis. Clin Infect Dis. 1992 Jul;15(1):1–10. doi: 10.1093/clinids/15.1.1. [DOI] [PubMed] [Google Scholar]
- Woods G. L., Washington J. A., 2nd Mycobacteria other than Mycobacterium tuberculosis: review of microbiologic and clinical aspects. Rev Infect Dis. 1987 Mar-Apr;9(2):275–294. doi: 10.1093/clinids/9.2.275. [DOI] [PubMed] [Google Scholar]
- Young L. S., Wiviott L., Wu M., Kolonoski P., Bolan R., Inderlied C. B. Azithromycin for treatment of Mycobacterium avium-intracellulare complex infection in patients with AIDS. Lancet. 1991 Nov 2;338(8775):1107–1109. doi: 10.1016/0140-6736(91)91965-w. [DOI] [PubMed] [Google Scholar]
- Zaugg M., Salfinger M., Opravil M., Lüthy R. Extrapulmonary and disseminated infections due to Mycobacterium malmoense: case report and review. Clin Infect Dis. 1993 Apr;16(4):540–549. doi: 10.1093/clind/16.4.540. [DOI] [PubMed] [Google Scholar]



