Abstract
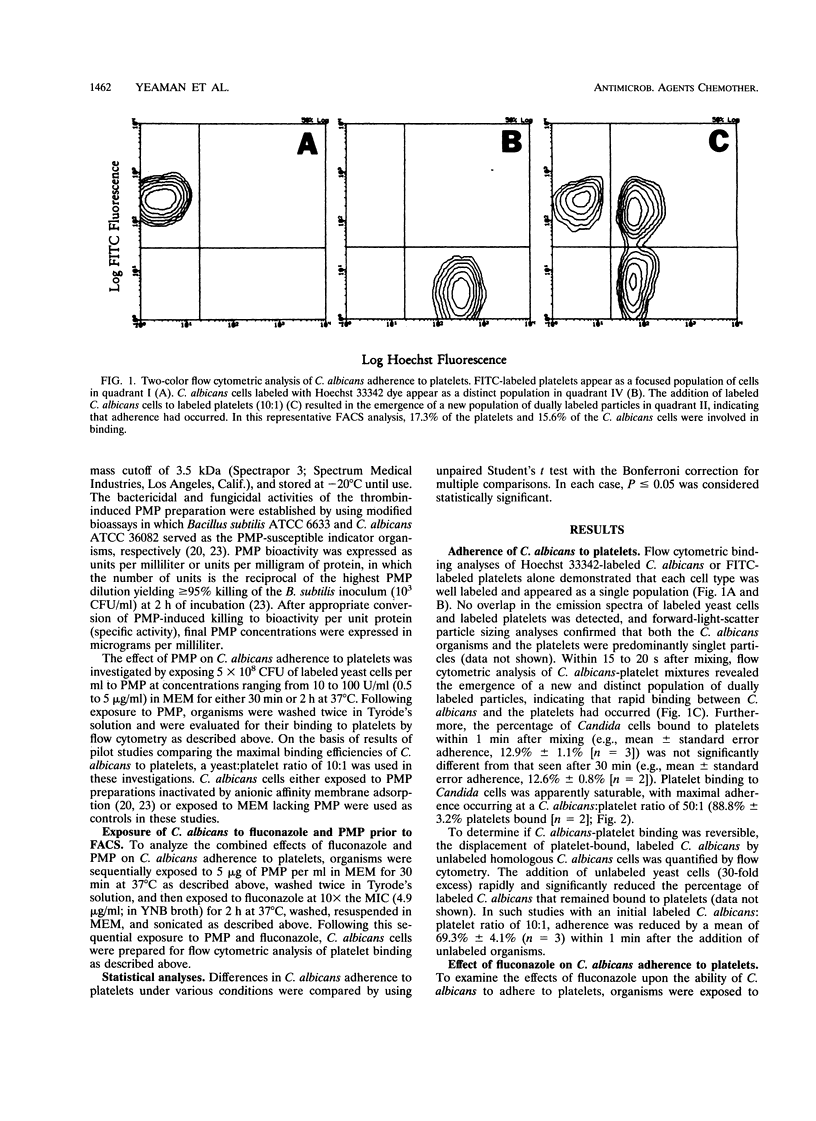
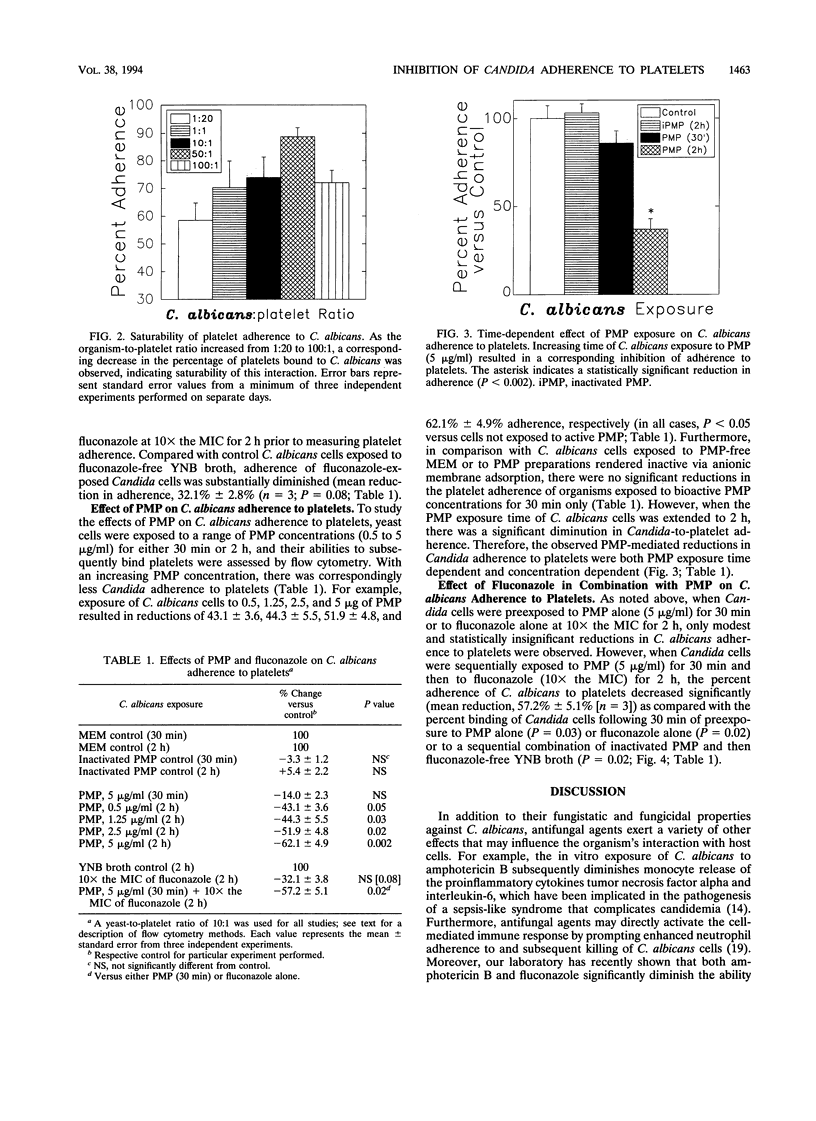
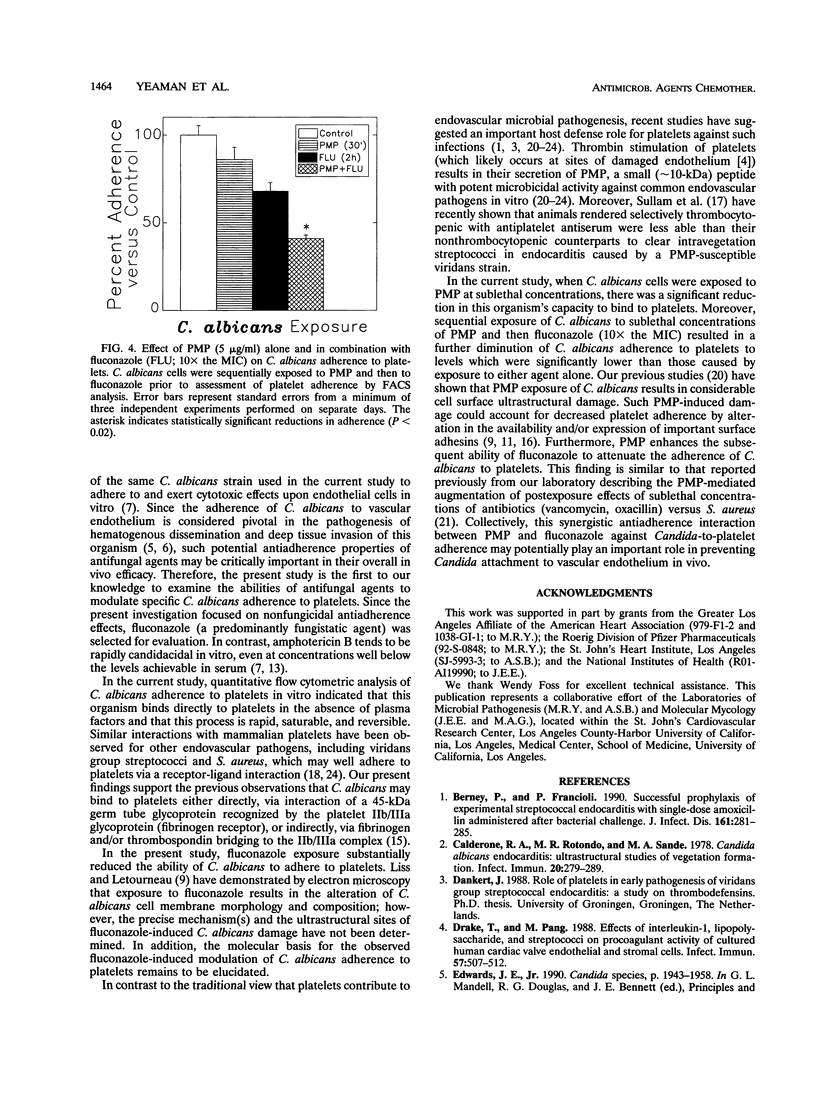
Adherence to vascular endothelium is considered an essential step in the pathogenesis of hematogenously disseminated candidiasis. Platelets have been shown to promote Candida adherence to vascular endothelium in vitro. In contrast, recent studies indicate that platelets may also play a role in the primary host defense against endovascular infection by secretion of alpha granule-derived platelet microbicidal protein (PMP), which possesses both bactericidal and fungicidal activities as well as antiadherence properties. We examined the influences of PMP and the antifungal agent fluconazole on the adherence of Candida albicans to rabbit platelets, as measured by quantitative flow cytometry. In the absence of PMP and fluconazole, adherence of C. albicans to platelets was rapid (complete within 1 min), saturable, and reversible. Following 2 h of exposure to fluconazole at 10x the MIC, platelet binding of C. albicans was substantially reduced (mean reduction, 32.1%; P = 0.08). Similarly, exposure of C. albicans to PMP (range, 0.5 to 5 micrograms/ml) for 2 h (but not 30 min) significantly reduced candidal adherence to platelets 43.1 to 62.1%; (reduction range, P < 0.05). Moreover, exposure of C. albicans to PMP (5 micrograms/ml for 30 min) and then fluconazole (10x the MIC for 2 h) further decreased candidal adherence to platelets in comparison with the adherence after exposure to either agent alone (mean reduction, 57.2%; P = 0.02 and 0.05, respectively). These data demonstrate that PMP and fluconazole individually reduce the ability of C. albicans to bind to platelets in vitro and that the antiadherence activities of fluconazole are augmented by PMP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berney P., Francioli P. Successful prophylaxis of experimental streptococcal endocarditis with single-dose amoxicillin administered after bacterial challenge. J Infect Dis. 1990 Feb;161(2):281–285. doi: 10.1093/infdis/161.2.281. [DOI] [PubMed] [Google Scholar]
- Calderone R. A., Rotondo M. F., Sande M. A. Candida albicans endocarditis: ultrastructural studies of vegetation formation. Infect Immun. 1978 Apr;20(1):279–289. doi: 10.1128/iai.20.1.279-289.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake T. A., Pang M. Effects of interleukin-1, lipopolysaccharide, and streptococci on procoagulant activity of cultured human cardiac valve endothelial and stromal cells. Infect Immun. 1989 Feb;57(2):507–512. doi: 10.1128/iai.57.2.507-512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum M. A., Filler S. G., Ibrahim A. S., Fu Y., Edwards J. E., Jr Modulation of interactions of Candida albicans and endothelial cells by fluconazole and amphotericin B. Antimicrob Agents Chemother. 1992 Oct;36(10):2239–2244. doi: 10.1128/aac.36.10.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S. A., Harrison J. L., Misra R. P. Aggregated platelets enhance adherence of Candida yeasts to endothelium. J Infect Dis. 1989 Oct;160(4):669–677. doi: 10.1093/infdis/160.4.669. [DOI] [PubMed] [Google Scholar]
- Liss R. H., Letourneau R. J. Fungispecificity of fluconazole against Candida albicans. Mycopathologia. 1989 Dec;108(3):173–178. doi: 10.1007/BF00436222. [DOI] [PubMed] [Google Scholar]
- Maisch P. A., Calderone R. A. Adherence of Candida albicans to a fibrin-platelet matrix formed in vitro. Infect Immun. 1980 Feb;27(2):650–656. doi: 10.1128/iai.27.2.650-656.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch P. A., Calderone R. A. Role of surface mannan in the adherence of Candida albicans to fibrin-platelet clots formed in vitro. Infect Immun. 1981 Apr;32(1):92–97. doi: 10.1128/iai.32.1.92-97.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald P. J., Craig W. A., Kunin C. M. Persistent effect of antibiotics on Staphylococcus aureus after exposure for limited periods of time. J Infect Dis. 1977 Feb;135(2):217–223. doi: 10.1093/infdis/135.2.217. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A., Rinaldi M. G., Galgiani J. N., Bartlett M. S., Body B. A., Espinel-Ingroff A., Fromtling R. A., Hall G. S., Hughes C. E., Odds F. C. Collaborative investigation of variables in susceptibility testing of yeasts. Antimicrob Agents Chemother. 1990 Sep;34(9):1648–1654. doi: 10.1128/aac.34.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raponi G., Ghezzi M. C., Mancini C., Filadoro F. Preincubation of Candida albicans strains with amphotericin B reduces tumor necrosis factor alpha and interleukin-6 release by human monocytes. Antimicrob Agents Chemother. 1993 Sep;37(9):1958–1961. doi: 10.1128/aac.37.9.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerl K. G., Calderone R. A., Sreevalsan T. Platelet interactions with Candida albicans. Infect Immun. 1981 Dec;34(3):938–943. doi: 10.1128/iai.34.3.938-943.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullam P. M., Frank U., Yeaman M. R., Täuber M. G., Bayer A. S., Chambers H. F. Effect of thrombocytopenia on the early course of streptococcal endocarditis. J Infect Dis. 1993 Oct;168(4):910–914. doi: 10.1093/infdis/168.4.910. [DOI] [PubMed] [Google Scholar]
- Sullam P. M., Payan D. G., Dazin P. F., Valone F. H. Binding of viridans group streptococci to human platelets: a quantitative analysis. Infect Immun. 1990 Nov;58(11):3802–3806. doi: 10.1128/iai.58.11.3802-3806.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan G. W., Carper H. T., Mandell G. L. Pentoxifylline modulates activation of human neutrophils by amphotericin B in vitro. Antimicrob Agents Chemother. 1992 Feb;36(2):408–416. doi: 10.1128/aac.36.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M. R., Ibrahim A. S., Edwards J. E., Jr, Bayer A. S., Ghannoum M. A. Thrombin-induced rabbit platelet microbicidal protein is fungicidal in vitro. Antimicrob Agents Chemother. 1993 Mar;37(3):546–553. doi: 10.1128/aac.37.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M. R., Norman D. C., Bayer A. S. Platelet microbicidal protein enhances antibiotic-induced killing of and postantibiotic effect in Staphylococcus aureus. Antimicrob Agents Chemother. 1992 Aug;36(8):1665–1670. doi: 10.1128/aac.36.8.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M. R., Norman D. C., Bayer A. S. Staphylococcus aureus susceptibility to thrombin-induced platelet microbicidal protein is independent of platelet adherence and aggregation in vitro. Infect Immun. 1992 Jun;60(6):2368–2374. doi: 10.1128/iai.60.6.2368-2374.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M. R., Puentes S. M., Norman D. C., Bayer A. S. Partial characterization and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect Immun. 1992 Mar;60(3):1202–1209. doi: 10.1128/iai.60.3.1202-1209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M. R., Sullam P. M., Dazin P. F., Norman D. C., Bayer A. S. Characterization of Staphylococcus aureus-platelet binding by quantitative flow cytometric analysis. J Infect Dis. 1992 Jul;166(1):65–73. doi: 10.1093/infdis/166.1.65. [DOI] [PubMed] [Google Scholar]



