Abstract
OBJECTIVE:
The aim of this study was to describe blood pressure responses during resistance exercise in hypertensive subjects and to determine whether an exercise protocol alters these responses.
INTRODUCTION:
Resistance exercise has been recommended as a complement for aerobic exercise for hypertensive patients. However, blood pressure changes during this kind of exercise have been poorly investigated in hypertensives, despite multiple studies of normotensives demonstrating significant increases in blood pressure.
METHODS:
Ten hypertensive and ten normotensive subjects performed, in random order, two different exercise protocols, composed by three sets of the knee extension exercise conducted to exhaustion: 40% of the 1-repetition maximum (1RM) with a 45-s rest between sets, and 80% of 1RM with a 90-s rest between sets. Radial intra-arterial blood pressure was measured before and throughout each protocol.
RESULTS:
Compared with normotensives, hypertensives displayed greater increases in systolic BP during exercise at 80% (+80±3 vs. +62±2 mmHg, P<0.05) and at 40% of 1RM (+75±3 vs. +67±3 mmHg, P<0.05). In both exercise protocols, systolic blood pressure returned to baseline during the rest periods between sets in the normotensives; however, in the hypertensives, BP remained slightly elevated at 40% of 1RM. During rest periods, diastolic blood pressure returned to baseline in hypertensives and dropped below baseline in normotensives.
CONCLUSION:
Resistance exercise increased systolic blood pressure considerably more in hypertensives than in normotensives, and this increase was greater when lower-intensity exercise was performed to the point of exhaustion.
Keywords: Strength exercise, Exercise intensity, Hypertension, Resistive exercise, Autonomic nervous system
INTRODUCTION
Dynamic resistance exercise has been recommended as a component of exercise programs for health maintenance in patients with cardiovascular disease.1 Similarly, it has also been prescribed for hypertensive patients in conjunction with aerobic training.2–6 Nevertheless, cardiovascular responses during this kind of exercise are poorly understood, especially in individuals with hypertension.
Previous studies involving healthy subjects have suggested that resistance exercise causes a pronounced increase in systolic (SBP) and diastolic (DBP) blood pressures,7–13 which can reach values as high as 360/234 mmHg.13 In individuals with hypertension, responses might be even greater because, in addition to higher resting BP levels, such individuals typically display greater elevation of BP during stressful situations, including aerobic exercise.14, 15 However, only one study measured intra-arterial BP during resistance exercise in hypertensive subjects.12 The authors of that study reported a maximum value of 345/245 mmHg; however, only three patients were included in that study. Harris and Holly16 also measured BP responses to this kind of exercise; however, they measured auscultatory BP immediately after exercise cessation and found a maximum value of 155/87 mmHg, which they considered safe for these patients.
Resistance training protocols vary depending on the objective of the program.17 To increase muscle resistance, training should involve one to three sets of exercises performed at low intensity, defined as 40–60% of the 1-repetition maximum (1RM), with a high number of repetitions12–20 and short rest periods (30–60s) between sets. To increase muscle strength and produce muscle hypertrophy, training should involve one to three sets of exercises performed at high intensity (>70% of 1RM), with a low number of repetitions (6–12) and long rest periods (90–180 s) between sets. The effects of these two kinds of training on BP in individuals with hypertension are unknown.
In studies of BP response during resistance exercise, various methods of assessing BP have been employed. Some authors used the indirect auscultatory technique, taking measurements in the resting arm during or immediately after exercise.16, 18, 19 However, Wiecek et al.18 demonstrated that these measurements underestimate intra-arterial SBP by more than 15% and 30%, respectively. Although finger photo-plethysmographic measurement has also been employed during resistance exercise,20, 21 its validity has not been fully established. Therefore, intra-arterial BP measurement is currently the gold standard method for assessing BP during resistance exercise.
In light of these facts, the aim of the present study was to describe the intra-arterial BP responses to low- and high-intensity resistance exercises performed to exhaustion in hypertensive patients and to compare such responses with those in normotensive subjects. We hypothesized that the increase in BP during resistance exercise would be greater in hypertensive subjects, and that, when such exercises were performed to exhaustion, the increase during low-intensity exercise would be similar to that occurring during high-intensity exercise.
MATERIAL AND METHODS
Subjects
Ten subjects with mild essential hypertension (six males and four females) and ten normotensive subjects (three males and seven females) participated in this study. Hypertensive subjects were selected from those treated in the Hypertension Unit of the Nephrology Department at the University of São Paulo School of Medicine Hospital das Clínicas, located in São Paulo, Brazil. Normotensive subjects were recruited from members of the hospital housekeeping and security staff. The study was approved by the Ethics in Research Committee of the University of São Paulo School of Medicine Hospital das Clínicas. All participants gave written informed consent.
None of the subjects presented any other cardiovascular diseases, cardiovascular risk factors or target organ damage. Cardiovascular diseases were ruled out by resting and exercise electrocardiogram (ECG). Cardiovascular risk factors such as diabetes and hypercholesterolemia were excluded by self-reported data in both groups and were verified by measuring fasting glucose and total cholesterol in the hypertensives. Moreover, weight and height were measured, and obese subjects were excluded from the study. Obesity was excluded when subjects’ body mass index was greater or equal to 30 kg/m2. Target organ damage was excluded in the hypertensive cohort by the routine exams suggested by the Guidelines for Hypertension Treatment.19 Hypertensive subjects did not take anti-hypertensive medications during the study, and a washout period of at least 4 weeks was observed prior to study outset for patients receiving previous treatment.
Using auscultation, we measured the BP six times for each subject (three times during each of two visits to the laboratory), and the mean of each set of three measurements was used in the analysis. The first and fifth Korotkoff sounds were used to determine SBP and DBP, respectively. For inclusion in the study, normotensive subjects were required to have BP lower than 140/90 mmHg, whereas hypertensive subjects were required to have BP between 140/90 and 159/99 mmHg.22
Experiments
Prior to the experiment, all subjects attended two familiarization sessions to be instructed in the correct execution of the knee extension exercise. During each session, they performed 10 repetitions of the exercise at the lowest workload allowed by the machine. In addition, the 1RM for each subject was established one week before the experiment commenced, following the Kraemer & Fry protocol.23
During each experiment, intra-arterial BP was measured in the radial artery of the non-dominant arm. After subcutaneous administration of a local anesthetic (2% lidocaine without vasoconstrictor), a 20-gauge catheter (BD-Insyte; Becton Dickinson, Franklin Lakes, NJ, USA) was inserted into the radial artery. The catheter was connected to a transducer kit (model PX-260; Edwards Life Sciences, Irvine, CA, USA) positioned at the level of the fourth intercostal space. A signal amplifier was used (KS3800; Gould Instrument Systems, Valley View, OH, USA), and the signal was acquired on a computer at a sampling frequency of 500 Hz using a data acquisition system (WinDaq DI-720; DataQ Instruments Inc, Akron, OH, USA).
Subjects were asked to sit at the knee extension machine and rest for 10 min, at which point the baseline measurements were taken. The subjects then performed to exhaustion, in random order, two knee extension exercise protocols at different intensities: three sets at a workload corresponding to 40% of 1RM with a 45-s rest period between sets, and three sets at a workload corresponding to 80% of 1RM with a 90-s rest period between the sets. A resting period of at least 10 min and long enough to ensure that BP would return to the baseline value, was allowed between the protocols.
In each protocol, intra-arterial BP was measured continuously: for three min prior to beginning exercise (pre-exercise), throughout the set (S1, S2, and S3), and throughout the rest periods between sets (R1 and R2).
Data processing and statistical analysis
For each group and each exercise protocol, the pre-exercise BP value was calculated as the average of the three-min data, and compared against the highest values achieved during S1, S2, and S3, and against the lowest values achieved at R1 and R2, using a one-way analysis of variance (ANOVA) for repeated measures (Statistica for Windows 4.3; Statsoft Inc., Tulsa, OK, USA).
As the objective of the study was to compare BP responses during exercises, the responses were calculated as differences between pre-exercise values and those obtained during the sets. These differences were compared using a three-way ANOVA for repeated measures (Statistica for Windows 4.3; Statsoft Inc), establishing group (hypertensive and normotensive) as a between-groups main factor, and intensity (40% of 1RM and 80% of 1RM), and set (S1, S2, and S3) as within-subjects main factors. Moreover, these analyses were repeated using gender and age as covariates, with no change in results.
Considering a power of 90%, an alpha error of 5%, and a standard deviation of 3 mmHg, the minimal sample size necessary to detect a difference of 4 mmHg in systolic BP was calculated to be six subjects.
The Newman-Keuls post-hoc test was employed when necessary. Values of P<0.05 were accepted as significant. Data are shown as mean±standard error (SE).
RESULTS
Sample characteristics
Subject characteristics are shown in Table 1. Hypertensive subjects were, on average, older than normotensive subjects. As expected, hypertensive subjects had higher baseline BP levels than did normotensive subjects. Weight, body mass index, and resting heart rate were similar between the two groups.
Table 1.
Physical and cardiovascular characteristics of nor-motensive and hypertensive subjects
| Characteristic | Normotensive (n=10) | Hypertensive (n=10) |
|---|---|---|
| Age (yr) | 39±2 | 46±3 † |
| Weight (kg) | 69.0±3.2 | 68.0±3.1 |
| Height (cm) | 165±3 | 164±2 |
| Body mass index (kg/m2) | 25.2±0.7 | 25.4±1.0 |
| Baseline SBP (mmHg) | 116±4 | 141±3 † |
| Baseline MAP (mmHg) | 91±3 | 112±2 † |
| Baseline DBP (mmHg) | 78±3 | 97±1 † |
| Resting heart rate (bpm) | 78±8 | 80±4 |
| 1RM | 55±6 | 60±5 |
Abbreviations: 1RM, 1-repetition maximum.
†, P<0.05 vs. normotensive subjects.
Exercise characteristics
In the hypertensive subjects, exercise at 80% of 1RM was performed with a mean load of 48±3 kg, and the mean number of repetitions to exhaustion was similar among the three sets (S1=8±1, S2=7±1, and S3=6±1 repetitions). Exercise at 40% of 1RM was performed with a mean load of 24±2 kg, and the mean number of repetitions to exhaustion decreased significantly over the course of the sets (S1=19±1 vs. S2=13±1 vs. S3=11±1, P<0.05 for all). In the normotensive subjects, exercise at 80% of 1RM was performed with a mean load of 44±5 kg, and the mean number of repetitions to exhaustion was also similar among sets (S1=10±1, S2=8±1, and S3=7±1). Exercise at 40% of 1RM was performed with a mean load of 22±3 kg, and the mean number of repetitions to exhaustion was significantly greater in set 1 than in sets 2 and 3 (19±2 vs. 13±1 and 11±1, P<0.05 for both). The workload and number of repetitions to exhaustion in each exercise protocol were similar between the two groups.
Absolute responses
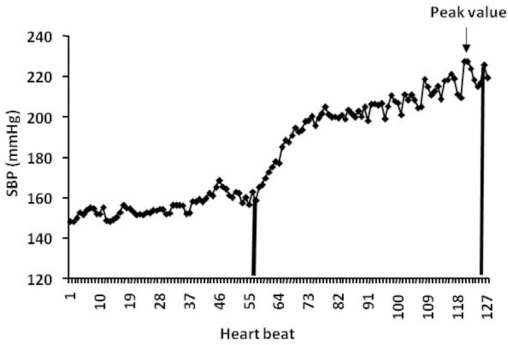
A representative tracing of SBP observed in a single hypertensive patient during an exercise set is shown in Figure 1. During each exercise set, SBP and DBP increased progressively, reaching their greatest values at the end of the set.
Figure 1.
Systolic blood pressure (SBP) measured during each heart beat in a representative hypertensive subject performing a set of leg extension exercises until exhaustion at 40% of 1RM (repetition maximum).
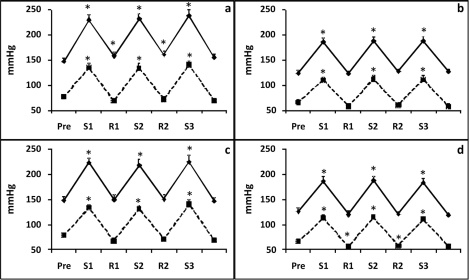
The highest and lowest values of SBP and DBP achieved during the sets and the rest periods in each exercise protocol are shown for each cohort in Figure 2. As expected, in all exercise protocols and in both groups, SBP and DBP increased significantly during the exercise sets and decreased during the rest periods. However, during exercises at 40% of 1RM, SBP returned to pre-exercise levels during the rest periods in the normotensive subjects but not in the hypertensive subjects. In addition, during exercises at 80% of 1RM, DBP dropped below pre-exercise levels during the rest periods in the normotensive subjects but not in the hypertensive subjects.
Figure 2.
Systolic blood pressure (SBP, solid lines) and diastolic blood pressure (DBP, dashed lines) measured before (Pre), during sets (S), and during rest periods (R) of knee extension exercises performed at 40% of the 1-repetition maximum (1RM, panels a and b) and 80% of 1RM (panels c and d) in hypertensive subjects (panels a and c) and normotensive subjects (panels b and d). *, P<0.05 vs. Pre.
Comparison of BP variations during exercise
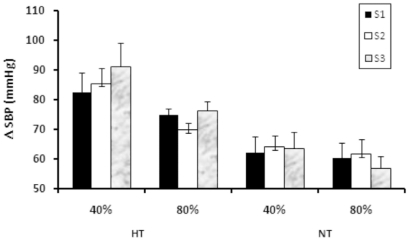
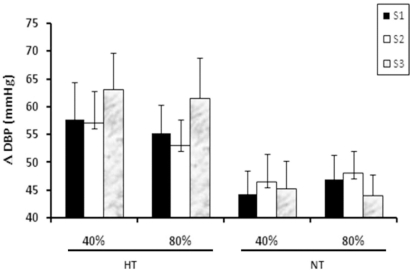
Variations in SBP and DBP measured during both exercise protocols are in Figures 3 and 4.
Figure 3.
Systolic blood pressure (SBP) responses, expressed as the value during a set minus the pre-exercise value (ΔSBP), during each of three sets (S1, S2, and S3) of knee extension resistance exercises performed to exhaustion at 40% and 80% of 1RM (repetition maximum) in hypertensive (HT) and normotensive (NT) subjects. †, P<0.05 vs. normotensive subjects; ‡, P<0.05 vs. 80% of 1RM.
Figure 4.
Diastolic blood pressure (DBP) responses, expressed as the value during a set minus the pre-exercise value (ΔDBP), during each of three sets (S1, S2, and S3) of knee extension resistance exercises performed to exhaustion at 40% and 80% of 1RM (repetition maximum) in hypertensive (HT) subjects and normotensive (NT) subjects.
The ANOVA revealed no significant interactions between the main factors for SBP. However, significant main effects were detected for the factors group and intensity. Thus, the mean increase in SBP during exercise sets was significantly greater in hypertensive vs. normotensive subjects (40% of 1RM, +86.4±3.7 vs. +63.3±2.7 mmHg and 80% of 1RM = +73.7± 4.3 vs. +59.7±2.7 mmHg, respectively, P<0.05). In addition, the mean increase in SBP was greater during exercise performed at 40% of 1RM than at 80% of 1RM (hypertensives: +86.4±3.7 vs. +73.7± 4.3 mmHg; normotensives: +63.3±2.7 vs. +59.7±2.7 mmHg; P<0.05).
No significant interaction or main effect was observed in the ANOVA for changes in DBP. The increases in DBP during exercise sets did not differ significantly between groups or exercise protocols, although the mean increase in DBP tended to be slightly greater in hypertensive subjects than in normotensive subjects (40% of 1RM: +59.3±3.6 vs. +45.4±2.6 mmHg and 80% of 1RM: +56.6±3.3 vs. +46.3±2.2 mmHg, respectively, P=0.068).
DISCUSSION
The principal contribution of the present study is the first description of intra-arterial BP responses to resistance exercise of varying intensity in hypertensive subjects. The most important findings were as follows: the increase in SBP during resistance exercise was greater in hypertensive vs. normotensive subjects; in hypertensive and normotensive subjects alike, low-intensity resistance exercise, when performed to exhaustion, induced an increase in SBP greater than that induced by high-intensity resistance exercise; and BP recovery during the rest periods between exercise sets was impaired in hypertensive subjects.
To our knowledge, this is the first study to describe BP response over three sets of resistance exercises of varying intensities in hypertensive subjects. The only other study to have investigated this issue focused on cardiac patients20, whose cardiovascular responses might have differed from those of our subjects, none of whom suffered from cardiovascular disease. In all of our subjects, mean SBP and DBP increased considerably during exercise, reaching values as high as 238±12 and 140±8 mmHg, respectively. Many previous studies have demonstrated BP increases of this magnitude during resistance exercise7–13, 18, 24; however, only one such study included hypertensive patients12. In that report, only three patients were studied, and BP was measured during just one set of exercises.
Due to increased intramuscular pressure, resistance exercise promotes significant impedance to blood flow to the muscles,25–28 resulting in accumulation of metabolites, thereby triggering the metabolic reflex and activating the sympathetic nervous system.27 The resulting systemic vasoconstriction increases peripheral vascular resistance. In addition, the heart rate increases, which, in conjunction with an increased stroke volume during the release of contractions, increases cardiac output.29 Therefore, the elevation in BP that occurs during resistance exercise is attributable not only to greater vascular resistance but also to greater cardiac output. It is worth noting, however, that previous studies,16, 18, 19 one of which involved hypertensive patients,16 demonstrated only a mild increase in BP during resistance exercise. However, in those studies, BP was measured using the auscultatory method, applied during or immediately after the end of exercise; this method might account for the different findings with respect to our study, as this method of BP measurement underestimates intra-arterial values during resistance exercise.18
The increase in SBP was significantly greater in the hypertensive subjects than in the normotensive subjects. In addition, there was a tendency toward a significant difference between the two groups in terms of the increase in DBP, which was also greater in the hypertensive subjects. It has previously been shown that BP responses to stimuli such as aerobic exercise, mental stress, and other laboratory tests are greater in hypertensive subjects than in normotensive subjects.14, 15 This greater responsiveness might be linked to the fact that hypertensive patients, in addition to presenting greater sympathetic activity at rest,30 also present greater sympathetic activation during stress and greater hemodynamic responsiveness to sympathetic activation,15 Other mechanisms, such as the renin-angiotensin system, are also enhanced in hypertension and might therefore increase BP responsiveness.31
Aging also increases cardiovascular reactivity to stress.32 In the present study, the hypertensive subjects were, on average, older than the normotensive subjects. Therefore, in a complementary analysis, age was set as a covariate. This analysis revealed that the influence of age was not significant. Moreover, gender distribution was also not the same between normotensive and hypertensive subjects, which might affect the results because females present lower exercise capacity than males. However, cardiovascular responses to resistance exercise are mainly determined by relative rather than absolute workload,6 and exercise was established based on relative intensity in the present study. Moreover, in another complementary analysis that employed gender as a covariate, the results were the same. Therefore, the differences observed between normotensive and hypertensive groups cannot be explained by differences in age or gender distribution.
As the objective of this study was to describe BP responses during resistance exercise in hypertensive subjects, data were collected for patients who were not receiving any pharmacological treatment. The results would undoubtedly be different in patients receiving medication, an issue that could be addressed in further studies.
The drop in BP during the rest periods also differed between normotensive and hypertensive subjects. During low-intensity exercise, a 45-s rest between sets was sufficient for SBP to return to pre-exercise values in normotensive but not in hypertensive subjects. During the high-intensity exercise protocol, a 90-s rest between sets was sufficient for DBP to drop below pre-exercise values between sets in normotensive subjects, although it returned only to pre-exercise values in hypertensive subjects. These results show that in hypertensive subjects, BP recovery during the rest periods between sets of resistance exercises is impaired. After one set of resistance exercises, the reactive hyperemia induced by the accumulation of vasodilator substances causes a decrease in BP.33 Our findings suggest that hypertension is accompanied by blunted reactive hyperemia, which agrees with findings from other studies showing that individuals with hypertension have endothelial dysfunction.34
The exercise protocols employed in the present study were designed to produce a total force production during exercise (intensity × repetitions) that was similar for both protocols. In the 40% of 1RM protocol, subjects performed twice the number of repetitions with half of the load used in the 80% of 1RM protocol. The increase in SBP was greater in the low-intensity protocol than in the high-intensity protocol, suggesting that the number of repetitions was more important than intensity in determining BP increase during exercise. However, it should be noted that in order to simulate clinical practice,35 the rest period between sets was shorter during the low-intensity protocol than during the high-intensity protocol. Therefore, the total workload (including intensity, number of repetitions, and rest period duration) was greater in the low-intensity protocol than in the high-intensity protocol, due to the shorter recovery times. This greater total workload might explain the greater BP increase in the low-intensity protocol. These results suggest that the rest period, as well as the intensity and number of repetitions, are important for determining the degree of BP increase during resistance exercise.
The results of the present study may have practical implications for the prescription of resistance exercise for hypertensive patients. We have demonstrated that resistance exercise produces a pronounced increase in SBP and that this increase is obtained in a matter of seconds. This increased SBP may represent a risk for hypertensive subjects, who are more prone to aneurysms than are normotensive subjects36, 37 because an abrupt increase in BP can cause an aneurysm to rupture.38 In fact, Haykowsky et al.39 reported three cases of subarachnoid hemorrhage due to aneurysm rupture during resistance exercise in hypertensive patients. Therefore, it is important to minimize BP increases during resistance exercise in hypertensive patients. Although this study was not designed to compare different aspects of exercise protocol (intensity or interval duration alone), the results suggest that total workload may be the determinant of BP increase, and that each of its components (intensity, number of repetitions, and rest period duration) can be adjusted to reduce cardiovascular load. Thus, to minimize BP increase during resistance exercise, it is possible to suggest that exercises should be of low intensity, repetitions should be few, and the rest periods between sets should be long. These recommendations have already been made by some institutions for resistance training for cardiovascular patients.1 Nevertheless, specific studies aimed at examining each of these exercise variables should be conducted in the future to validate these suggestions.
REFERENCES
- 1.Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116:572–84. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong L, Balady GJ, Berry MJ, Davis SE, Davy BM, Davy KP, et al. Other Clinical Conditions Influencing Exercise Prescription. In: Whaley MH, Brubaker PH, Otto RM, editors. ACSM’s Guidelines for Exercise Testing & Prescription. 7th ed. Baltimore: Lippincott, Williams & Wilkins, Inc.; 2006. pp. 205–36. [Google Scholar]
- 3.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–53. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 4.Ciolac EG, Guimaraes GV, D’Avila VM, Bortolotto LA, Doria EL, Bocchi EA. Acute aerobic exercise reduces 24-h ambulatory blood pressure levels in long-term-treated hypertensive patients. Clinics. 2008;63:753–8. doi: 10.1590/S1807-59322008000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camargo VM, Martins Bdo C, Jardim C, Fernandes CJ, Hovnanian A, Souza R. Validation of a treadmill six-minute walk test protocol for the evaluation of patients with pulmonary arterial hypertension. J Bras Pneumol. 2009;35:423–30. doi: 10.1590/s1806-37132009000500006. [DOI] [PubMed] [Google Scholar]
- 6.Barreto SS. Assessment of exercise capacity in pulmonary hypertension. J Bras Pneumol. 2009;35:401–3. doi: 10.1590/s1806-37132009000500002. [DOI] [PubMed] [Google Scholar]
- 7.Fleck SJ, Dean LS. Resistance-training experience and the pressor response during resistance exercise. J Appl Physiol. 1987;63:116–20. doi: 10.1152/jappl.1987.63.1.116. [DOI] [PubMed] [Google Scholar]
- 8.Lentini AC, McKelvie RS, McCartney N, Tomlinson CW, MacDougall JD. Left ventricular response in healthy young men during heavy-intensity weight-lifting exercise. J Appl Physiol. 1993;75:2703–10. doi: 10.1152/jappl.1993.75.6.2703. [DOI] [PubMed] [Google Scholar]
- 9.MacDougall JD, McKelvie RS, Moroz DE, Sale DG, McCartney N, Buick F. Factors affecting blood pressure during heavy weight lifting and static contractions. J Appl Physiol. 1992;73:1590–7. doi: 10.1152/jappl.1992.73.4.1590. [DOI] [PubMed] [Google Scholar]
- 10.MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol. 1985;58:785–790. doi: 10.1152/jappl.1985.58.3.785. [DOI] [PubMed] [Google Scholar]
- 11.McCartney N, McKelvie RS, Martin J, Sale DG, MacDougall JD. Weight-training-induced attenuation of the circulatory response of older males to weight lifting. J Appl Physiol. 1993;74:1056–60. doi: 10.1152/jappl.1993.74.3.1056. [DOI] [PubMed] [Google Scholar]
- 12.Palatini P, Mos L, Munari L, Valle F, Del Torre M, Rossi A, et al. Blood pressure changes during heavy-resistance exercise. J Hypertens Suppl. 1989;7:S72–73. doi: 10.1097/00004872-198900076-00032. [DOI] [PubMed] [Google Scholar]
- 13.Sale DG, Moroz DE, McKelvie RS, MacDougall JD, McCartney N. Effect of training on the blood pressure response to weight lifting. Can J Appl Physiol. 1994;19:60–74. doi: 10.1139/h94-004. [DOI] [PubMed] [Google Scholar]
- 14.Jern S, Bergbrant A, Hedner T, Hansson L. Enhanced pressor responses to experimental and daily-life stress in borderline hypertension. J Hypertens. 1995;13:69–79. [PubMed] [Google Scholar]
- 15.Kaushik RM, Mahajan SK, Rajesh V, Kaushik R. Stress profile in essential hypertension. Hypertens Res. 2004;27:619–624. doi: 10.1291/hypres.27.619. [DOI] [PubMed] [Google Scholar]
- 16.Harris KA, Holly RG. Physiological response to circuit weight training in borderline hypertensive subjects. Med Sci Sports Exerc. 1987;19:246–52. [PubMed] [Google Scholar]
- 17.Fleck SJ, Kraemer WJ. Basic Principles of Resistance Training and Exercise Prescription. In: Fleck SJ, Kraemer WJ, editors. eds Designing Resistance Training Programs. 3th ed. Champaign: Human Kinetics; 2004. pp. 3–12. [Google Scholar]
- 18.Wiecek EM, McCartney N, McKelvie RS. Comparison of direct and indirect measures of systemic arterial pressure during weightlifting in coronary artery disease. Am J Cardiol. 1990;66:1065–9. doi: 10.1016/0002-9149(90)90506-v. [DOI] [PubMed] [Google Scholar]
- 19.Westcott W, Howes B. Blood pressure response during weight training exercise. National Strenght and Conditioning Association Journal. 1983;5:67–71. [Google Scholar]
- 20.Lamotte M, Niset G, van de Borne P. The effect of different intensity modalities of resistance training on beat-to-beat blood pressure in cardiac patients. Eur J Cardiovasc Prev Rehabil. 2005;12:12–7. [PubMed] [Google Scholar]
- 21.Polito MD, Farinatti PT, Lira VA, Nobrega AC. Blood pressure assessment during resistance exercise: comparison between auscultation and Finapres. Blood Press Monit. 2007;12:81–6. doi: 10.1097/MBP.0b013e32809ef9f1. [DOI] [PubMed] [Google Scholar]
- 22.Committee JN. The sixth report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–46. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 23.Kraemer WJ, Fry AC. Strength testing: development and evaluation of methodology. In: Maud PJ, Foster C, editors. Physiological assessement of human fitness. Champaign: Human Kinetics; 1995. pp. 115–38. [Google Scholar]
- 24.Haslam DRS, McCartney N, McKelvie RS, MacDougall JD. Direct Measurements of Arterial Blood Pressure During Formal Weightlifting in Cardiac Patients. J Cardiop Rehabil. 1988;8:213–225. [Google Scholar]
- 25.Asmussen E. Similarities and dissimilarities between static and dynamic exercise. Circ Res. 1981;48(6 Pt 2):I3–10. [PubMed] [Google Scholar]
- 26.Mitchell JH, Payne FC, Saltin B, Schibye B. The role of muscle mass in the cardiovascular response to static contractions. J Physiol. 1980;309:45–54. doi: 10.1113/jphysiol.1980.sp013492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seals DR, Chase PB, Taylor JA. Autonomic mediation of the pressor responses to isometric exercise in humans. J Appl Physiol. 1988;64:2190–6. doi: 10.1152/jappl.1988.64.5.2190. [DOI] [PubMed] [Google Scholar]
- 28.Sejersted OM, Hargens AR, Kardel KR, Blom P, Jensen O, Hermansen L. Intramuscular fluid pressure during isometric contraction of human skeletal muscle. J Appl Physiol. 1984;56:287–95. doi: 10.1152/jappl.1984.56.2.287. [DOI] [PubMed] [Google Scholar]
- 29.Oliver D, Pflugfelder PW, McCartney N, McKelvie RS, Suskin N, Kostuk WJ. Acute cardiovascular responses to leg-press resistance exercise in heart transplant recipients. Int J Cardiol. 2001;81:61–74. doi: 10.1016/s0167-5273(01)00529-0. [DOI] [PubMed] [Google Scholar]
- 30.Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, et al. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension. 2004;43:169–75. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- 31.Shim CY, Ha JW, Park S, Choi EY, Choi D, Rim SJ, et al. Exaggerated blood pressure response to exercise is associated with augmented rise of angiotensin II during exercise. J Am Coll Cardiol. 2008;52:287–92. doi: 10.1016/j.jacc.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 32.Meier A, Gubelin U, Weidmann P, Grimm M, Keusch G, Gluck Z, et al. Age-rated profile of cardiovascular reactivity to norepinephrine and angiotensin II in normal and hypertensive man. Klin Wochenschr. 1980;58:1183–8. doi: 10.1007/BF01478874. [DOI] [PubMed] [Google Scholar]
- 33.Sparks HV. Mechanism of vasodilation during and after ischemic exercise. Fed Proc. 1980;39:1487–1490. [PubMed] [Google Scholar]
- 34.Guazzi M, Lenatti L, Tumminello G, Guazzi MD. Effects of orthostatic stress on forearm endothelial function in normal subjects and in patients with hypertension, diabetes, or both diseases. Am J Hypertens. 2005;18:986–94. doi: 10.1016/j.amjhyper.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 35.Fleck SJ, Kraemer WJ. Developing the Individualized Resistance Training Workout. In: Fleck SJ, Kraemer WJ, editors. Designing Resistance Training Programs. 3th ed. Champaign: Human Kinetics; 2004. pp. 151–86. [Google Scholar]
- 36.Isaksen J, Egge A, Waterloo K, Romner B, Ingebrigtsen T. Risk factors for aneurysmal subarachnoid haemorrhage: the Tromso study. J Neurol Neurosurg Psychiatry. 2002;73:185–7. doi: 10.1136/jnnp.73.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuda M, Watanabe K, Saito A, Matsumura K, Ichikawa M. Circumstances, activities, and events precipitating aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2007;16:25–9. doi: 10.1016/j.jstrokecerebrovasdis.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Vermeer SE, Rinkel GJ, Algra A. Circadian fluctuations in onset of subarachnoid hemorrhage. New data on aneurysmal and perimesencephalic hemorrhage and a systematic review. Stroke. 1997;28:805–8. doi: 10.1161/01.str.28.4.805. [DOI] [PubMed] [Google Scholar]
- 39.Haykowsky MJ, Findlay JM, Ignaszewski AP. Aneurysmal subarachnoid hemorrhage associated with weight training: three case reports. Clin J Sport Med. 1996;6:52–5. doi: 10.1097/00042752-199601000-00011. [DOI] [PubMed] [Google Scholar]