Abstract
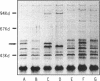
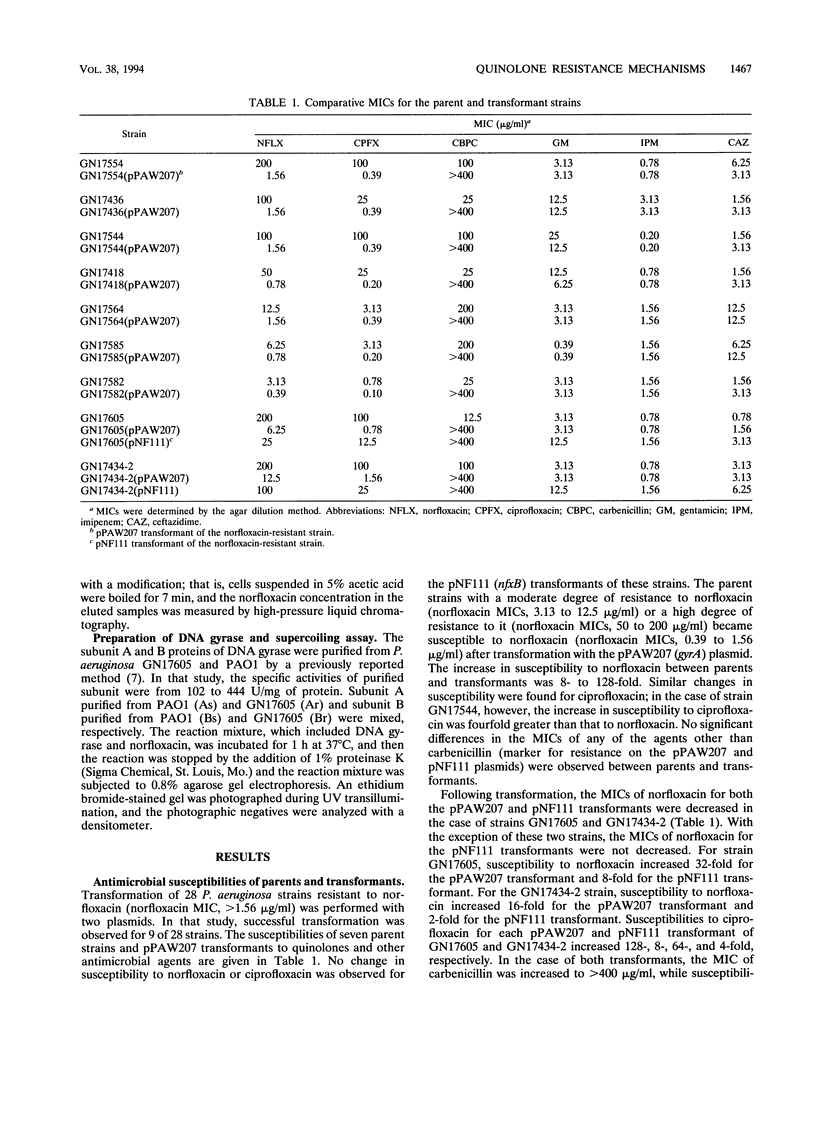
Twenty-eight strains of Pseudomonas aeruginosa with various degrees of norfloxacin resistance were isolated from patients with urinary tract infections. P. aeruginosa strains (norfloxacin MICs, 3.13 to 200 micrograms/ml) were transformed by either pPAW207 or pNF111 plasmid DNA, which included either the gyrA or nfxB gene, respectively. For transformants with pPAW207, norfloxacin MICs decreased 8- to 128-fold. It was suggested that moderate and high degrees of resistance to norfloxacin were expressed as a result of alterations in gyrA. No strain manifesting only an alteration in nfxB permeability was observed. The MICs of norfloxacin (200 micrograms/ml) for two P. aeruginosa strains, GN17605 and GN17434-2, were decreased following transformation not only by pPAW207 but also by pNF111. Analysis of outer membrane proteins disclosed the presence of a 54,000-Da protein in these parent strains that was not expressed in the pNF111 transformants. The level of accumulation of norfloxacin by the pNF111 transformant of GN17605 was higher than that by the parent strain. The norfloxacin susceptibility of DNA gyrase subunit A purified from GN17605 was only 1/35th that of the gyrase containing a subunit A from P. aeruginosa PAO1. These findings suggest that GN17605 is a gyrA-nfxB double mutant and that strain GN17434-2 possesses double mutations in both nfxB and some unknown gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chamberland S., Bayer A. S., Schollaardt T., Wong S. A., Bryan L. E. Characterization of mechanisms of quinolone resistance in Pseudomonas aeruginosa strains isolated in vitro and in vivo during experimental endocarditis. Antimicrob Agents Chemother. 1989 May;33(5):624–634. doi: 10.1128/aac.33.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimaki K., Noumi T., Saikawa I., Inoue M., Mitsuhashi S. In vitro and in vivo antibacterial activities of T-3262, a new fluoroquinolone. Antimicrob Agents Chemother. 1988 Jun;32(6):827–833. doi: 10.1128/aac.32.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H., Hosaka M., Hirai K., Iyobe S. New norfloxacin resistance gene in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1990 Sep;34(9):1757–1761. doi: 10.1128/aac.34.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann L., Williamson R., Moreau N., Kitzis M. D., Collatz E., Acar J. F., Goldstein F. W. Cross-resistance to nalidixic acid, trimethoprim, and chloramphenicol associated with alterations in outer membrane proteins of Klebsiella, Enterobacter, and Serratia. J Infect Dis. 1985 Mar;151(3):501–507. doi: 10.1093/infdis/151.3.501. [DOI] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987 Apr;31(4):582–586. doi: 10.1128/aac.31.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y., Sato K., Fujii T., Hirai K., Inoue M., Iyobe S., Mitsuhashi S. Some properties of subunits of DNA gyrase from Pseudomonas aeruginosa PAO1 and its nalidixic acid-resistant mutant. J Bacteriol. 1987 May;169(5):2322–2325. doi: 10.1128/jb.169.5.2322-2325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakics E. B., Iyobe S., Hirai K., Fukuda H., Hashimoto H. Occurrence of the nfxB type mutation in clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992 Nov;36(11):2562–2565. doi: 10.1128/aac.36.11.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Sato Y., Iyobe S., Mitsuhashi S. Plasmid-mediated gentamicin resistance of Pseudomonas aeruginosa and its lack of expression in Escherichia coli. Antimicrob Agents Chemother. 1982 Sep;22(3):358–363. doi: 10.1128/aac.22.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leying H. J., Büscher K. H., Cullmann W., Then R. L. Lipopolysaccharide alterations responsible for combined quinolone and beta-lactam resistance in Pseudomonas aeruginosa. Chemotherapy. 1992;38(2):82–91. doi: 10.1159/000238946. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Fang W., Gu J. W., Chin N. X. In vitro activity of OPC-17116. Antimicrob Agents Chemother. 1992 Jun;36(6):1310–1315. doi: 10.1128/aac.36.6.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T., Iyobe S., Hashimoto H., Hirai K. Cloning and characterization of a DNA fragment that complements the nfxB mutation in Pseudomonas aeruginosa PAO. FEMS Microbiol Lett. 1991 Mar 15;63(1):31–35. doi: 10.1016/0378-1097(91)90522-c. [DOI] [PubMed] [Google Scholar]
- Robillard N. J. Broad-host-range gyrase A gene probe. Antimicrob Agents Chemother. 1990 Oct;34(10):1889–1894. doi: 10.1128/aac.34.10.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V., Werner V. Selection of multiple antibiotic resistance by quinolones, beta-lactams, and aminoglycosides with special reference to cross-resistance between unrelated drug classes. Antimicrob Agents Chemother. 1984 Dec;26(6):797–801. doi: 10.1128/aac.26.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Brenwald N. The in-vitro activity of Bay y 3118, a new chlorofluoroquinolone. J Antimicrob Chemother. 1993 Jan;31(1):73–80. doi: 10.1093/jac/31.1.73. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Edwards L. J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob Agents Chemother. 1983 Apr;23(4):559–564. doi: 10.1128/aac.23.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura M., Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990 Jun;34(6):1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Nakamura M., Bogaki M., Nakamura S. Proportion of DNA gyrase mutants among quinolone-resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Jun;34(6):1273–1275. doi: 10.1128/aac.34.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]