Abstract
Postmitotic hair-cell regeneration in the inner ear of birds provides an opportunity to study the effect of renewed auditory input on auditory perception, vocal production, and vocal learning in a vertebrate. We used behavioral conditioning to test both perception and vocal production in a small Australian parrot, the budgerigar. Results show that both auditory perception and vocal production are disrupted when hair cells are damaged or lost but that these behaviors return to near normal over time. Precision in vocal production completely recovers well before recovery of full auditory function. These results may have particular relevance for understanding the relation between hearing loss and human speech production especially where there is consideration of an auditory prosthetic device. The present results show, at least for a bird, that even limited recovery of auditory input soon after deafening can support full recovery of vocal precision.
The avian inner ear provides a useful model for the study of hair-cell regeneration and recovery in the vertebrate ear, but the ultimate value of this regenerative capacity depends on whether it results in functional recovery of auditory and vocal behavior (1–3). In response to either acoustic trauma or insult from ototoxic drugs, both young and adult birds show a temporary period of hair-cell loss and regeneration, usually culminating in considerable anatomical, physiological, and behavioral recovery within several weeks (4–12). Behavioral recovery, as typically defined, refers only to a return of absolute auditory sensitivity to near pretrauma levels (13–16). Much less is known about the recovery of more complex auditory behavior, and nothing is known about the effect of hearing loss and recovery on the production or recognition of learned vocalizations. Though evolutionarily distant from humans, birds provide the only animal model for studying hearing restoration by renewed sensory-cell input and for examining the effect of such recovery on learned vocalizations. The question is whether a “new” auditory periphery results in sufficient functional recovery that a bird can again perceive, learn, and produce complex acoustic communication signals. The nature of this recovery bears on fundamental issues in auditory plasticity and sensorimotor interfaces. Moreover, the ability to track the time course of such recovery in a vertebrate auditory system may have particular significance for the effective use of auditory prosthetic devices, such as cochlear implants, for the severely hearing impaired (17).
Budgerigars (domesticated parakeets), learn new vocalizations throughout life, especially in response to changes in their social milieu (18–20). Further, our work, as well as the work of others, has shown that these birds experience hair-cell loss and threshold shift after administration of the ototoxic drug kanamycin followed, within several days or weeks, by hair-cell regeneration and a gradual recovery to within 20 dB of normal auditory sensitivity (21, 22). In these experiments, quantitative measures of hair-cell number were made by using SEM photomicrographic montages from 32 birds (14 behavioral, 18 nonbehavioral) and our results are comparable to those reported by others (21–23). After 6 days of kanamycin injection, virtually all of the hair cells are missing in the basal 40% of the papilla. Hair cells begin to be replaced (regenerated) in the basal 40% during the following 6 days, while hair-cell damage (swelling, stereocilia abnormalities) begins to be seen in the distal one-half of the papilla. Hair-cell number is almost normal within 4 weeks of kanamycin cessation, and by 12 weeks hair-cell number was within normal limits (±1 SD). Abnormalities similar to those reported in chicken hair-cell stereocilia after ototoxic drug administration (24) remain in the basal portion of the papilla, even after 7 months. Fig. 1 shows examples of kanamycin-damaged auditory papillae of the budgerigar.
Figure 1.
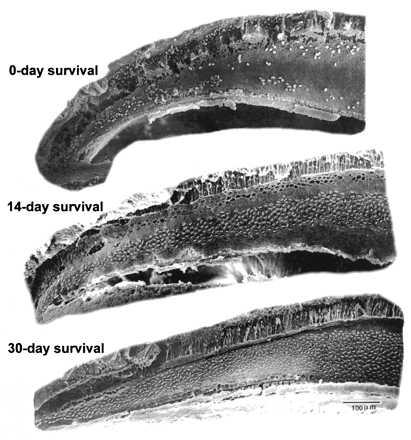
The basal (high-frequency) half of the papillae of budgerigars sacrificed after 8 days of injection with kanamycin (0-day survival), 14-day survival, and 28-day survival. There is an almost complete loss of hair cells after 8 days of kanamycin injections, recovery is well underway by 14 days following the termination of injections, and hair-cell number is back to normal by 28 days following injections but with clear disturbance in the orientation patterns of the hair-cell stereocilia.
In the following experiments we test whether, following hair-cell loss and regeneration, a bird can discriminate among, and reliably classify, complex vocal signals and whether precision in vocal production is permanently affected by such temporary hearing loss. In the first experiment, six budgerigars were trained by operant conditioning with food reward to detect and discriminate pure tones at various frequencies and intensities. All birds showed absolute and differential thresholds that were within normal limits for this species. For testing absolute thresholds, the birds were trained to detect 300 msec pure-tone pulses presented at the rate of two per sec. In difference limen tests with the method of constant stimuli, birds were required to discriminate a change (in frequency or intensity) against a repeating background of tone pulses. Peak sound pressure levels for frequency, intensity, and call discrimination tests were set to a sensation level of 60 dB for normally hearing budgerigars (25–27). The six birds were then injected with the ototoxic drug kanamycin (200 mg/kg/day) for 8–10 days followed by a resumption of psychophysical testing at approximately biweekly intervals for the next 23 weeks. Following kanamycin treatment, three birds were tested only on absolute thresholds at five different frequencies for several months. The other three birds were tested on absolute thresholds measured only at 1.0 and 2.86 kHz. In this way, intensity and frequency difference limens could also be measured concurrently in these birds along with other call discrimination tests.
The three birds were tested on a pair-wise discrimination task to discriminate among five natural, species-typical, contact calls and their synthetic analogs (Fig. 2A) (26, 27). In this test, each call in the set served as both a background and a target a total of 10 times (n = 20 tests on each stimulus pair). Prior to kanamycin injections, discrimination was essentially 100% between contact call types but performance varied considerably when birds were required to discriminate within call types (i.e., discriminate a natural call from its synthetic analog or vice versa).
Figure 2.
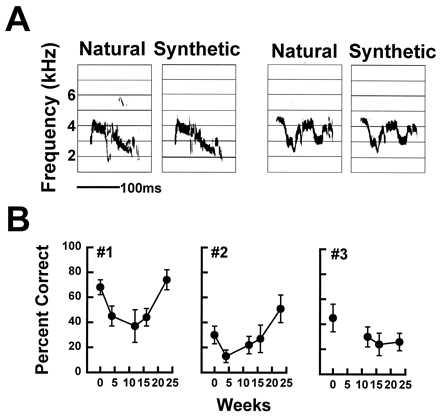
(A) Sonograms of natural contact calls recorded from two different birds and their synthetic analogs. The full test set consisted of 5 natural calls and 5 synthetic analogs presented in random order. (B) The hit rate or percent correct (mean +/− SD) for each of 3 budgerigars discriminating among the five natural contact calls and their synthetic analogs before treatment with kanamycin and at various times during recovery. The false alarm rate remained well below 10% for all three birds and showed no significant change across test sessions. The discrimination performance of two birds eventually returned to preinjection levels or better while one bird was still significantly impaired even after 23 weeks of recovery.
As expected following extensive hair-cell loss, absolute thresholds at all frequencies were elevated immediately following treatment with kanamycin but gradually recovered to within ≈15 and 25 dB of preinjection thresholds at 1.0 and 2.86 kHz, respectively, by 8 weeks. Initial threshold shift was greater at high frequencies (50–60 dB above 2,000 Hz) becoming progressively less for lower frequencies (10–30 dB below 2,000 Hz) with the most rapid recovery occurring for the lowest frequencies. Absolute thresholds for all frequencies reached asymptote by 8 weeks after cessation of kanamycin injections with a return to within 5–15 dB of normal for frequencies below 2,000 Hz and within 20–30 dB of normal above 2,000 Hz. These findings are in general agreement with previous reports of threshold recovery after ototoxicity (13, 14, 21). In spite of elevated absolute thresholds, difference limens for intensity and frequency were within normal limits at 1.0 kHz and only moderately elevated at 2.86 kHz 4 weeks following the cessation of injections. The relatively easy discriminations between contact calls from different birds (e.g., two natural calls of Fig. 2A) were unaffected 4 weeks following kanamycin treatment. In contrast, performance on the more difficult discriminations between a natural call and its synthetic analog (e.g., natural vs. synthetic calls of Fig. 2A) was significantly impaired for several months following treatment with kanamycin but improved to preinjection performance levels for two of the three birds at 23 weeks of recovery (Fig. 2B).
The ability to discriminate among different complex vocalizations is less demanding than the ability to identify previously familiar vocalizations. To test whether previously familiar sounds could be identified after hair-cell loss and regeneration, we trained four birds with a second procedure involving the classification of two different contact call types. In this experiment, birds were trained on a go/no-go task to peck one light-emitting diode (LED) (observation key) to hear one of two contact calls from an overhead loudspeaker. If the “Go” call was presented, the bird was required to peck a second LED (report key) within 2 sec to obtain food. If the no-go call was presented, the bird was required to withhold responding on the report key for 2 sec to avoid punishment, which consisted of extinguishing the chamber lights for 10 seconds and delaying the initiation of the next trial. Daily testing on this call identification task was conducted before, during and after kanamycin injections. The birds’ performance fell to chance levels after several days of kanamycin. Testing at 14 and 28 days following cessation of kanamycin injections showed that performance remained at chance levels. Beginning at 28 days of recovery, the birds were again tested in daily 100-trial sessions. Fig. 3 shows that normal budgerigars given a 28-day pause in testing but no kanamycin, reached criterion after 1 day of testing. The experimental birds, however, had still not returned to preinjection performance levels after 3 days. Additional control experiments with other contact calls showed that kanamycin-treated birds could also learn a new classifications though the acquisition time was variable.
Figure 3.
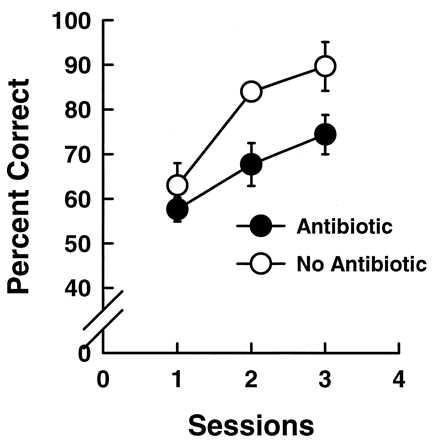
The learning (recovery) curve for 4 budgerigars (mean +/− SD) tested on a call classification task after a 1-month delay which began after a 10-day course of kanamycin (closed circles). The performance of these birds is compared with the learning curve for 4 normal budgerigars tested after a 1 month delay with no kanamycin treatment (○). Untreated budgerigars reach criterion performance again after one session while kanamycin-treated budgerigars were delayed in their reacquisition of classification of familiar calls.
The loss of hearing in humans can have a profound effect on the quality of speech and the quality of acoustic communication. To examine the effect of hair-cell loss and regeneration on vocal production, three male budgerigars were trained by operant conditioning to produce different contact calls to two different LEDs. If the bird’s call matched a spectral template stored in the computer, the bird was rewarded with food. In this task, birds were trained to produce one contact call when the left LED was illuminated and a different contact call when the right LED was illuminated. A Sony Microphone fed to a digital signal processing (DSP) board (National Instruments AT-DSP2200) captured the vocal output at a sampling rate of 24 kHz for a total of 266 msec. Fast Fourier transforms were calculated on the fly so that calculations for the first 10.67 msec were carried out while the second 10.67 msec of data were being stored in memory, and so forth. Each serial power spectrum was normalized to a peak intensity of 1 (28, 29) with a total of 25 successive serial power spectra computed over 266 msec.
Vocal production was shaped by playing typical aviary sounds in the test chamber and reinforcing the bird with food whenever it emitted a call in response to the tape. Once the bird began to emit calls consistently, the background of aviary sounds was omitted and reinforcement was delivered automatically in response to any vocalization. At first, every call was reinforced. Once the bird was responding reliably, a call was reinforced only when the call was sufficiently different (using the similarity index) from the previous call the bird produced. A similarity index was defined as the sum of the overlapping areas of each of the 25 serial spectra of the bird’s call and the stored “template” call. An index of zero means no overlap while an index of one is perfect overlap (i.e., the bird’s call and the template were identical—there was no difference between the instantaneous serial spectra calculated on the bird’s call and those calculated for the stored template). In this way, the bird learned to produce at least two different contact calls to obtain food. After the birds reached an asymptotic level of performance at the most stringent criterion (usually ≈35 sessions), a similarity matrix was constructed of all the calls produced in a test session. This similarity matrix was analyzed by multidimensional scaling (systat for Windows, 5.0), which resulted in two clusters of calls in two-dimensional space. The center call in each cluster was selected and stored as the template-to-be-matched in subsequent experiments with kanamycin.
Fig. 4 shows template matching performance for the two call types before, during, and after an 8-day course of kanamycin in two different birds. Vocal behavior is impaired during kanamycin injections but recovers to previous performance levels within 10–15 days following kanamycin injections. The bird’s ability to produce a vocal match to a stored contact call has recovered to preinjection levels of precision long before auditory recovery reaches asymptote at about 8 weeks. Similar effects are obtained following acoustic overexposure indicating the loss of vocal precision is most likely due to an auditory deficit rather than a generalized effect of kanamycin.
Figure 4.
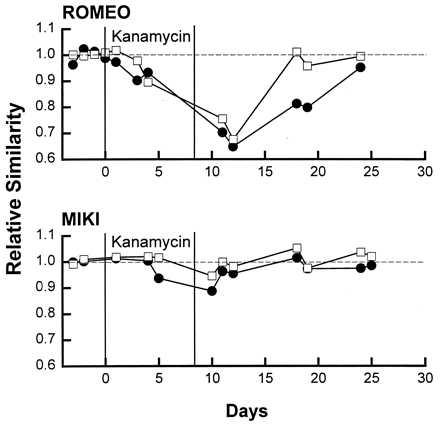
Relative similarity of contact calls produced by two birds to their respective templates before, during and after injections with the ototoxic drug kanamycin. Each bird’s call similarities during and after injections are plotted relative to its average preinjection similarity score which was normalized to 1. Kanamycin causes a decrease in the precision of vocal production in both birds but this precision recovers within 5–15 days following the cessation of injections and well before the return of auditory function as defined by absolute thresholds, difference limens, call discrimination, and call recognition.
These studies are the first to address recovery of the discrimination and perception of vocalizations following hair-cell damage and regeneration and the first to track concomitant changes in the precision of vocal production. Both hearing and vocal production are severely affected when many hair cells are damaged or lost, but both behaviors return to normal or near normal over time. Absolute thresholds as well as frequency and intensity difference limens recover considerably by 4–8 weeks. Discrimination and classification tests with vocal signals reveal instances of severe, prolonged perceptual deficits even with a full complement of new hair cells. But these deficits also recover over time with repeated testing and they can be at least partially offset by raising stimulus level. We conclude that, with sufficient time and training, the mature avian auditory system can accommodate input from a newly regenerated periphery sufficiently to allow for recognition of previously familiar vocalizations and the learning of new complex acoustic classifications.
Interestingly, while the precision of vocal production is initially affected by hearing loss from hair-cell damage, this precision recovers long before the papilla is repopulated with new, functional hair cells. This suggests that, even in the absence of veridical auditory feedback, budgerigars, like humans, can also rely on long-term memory combined perhaps with feedback from other sensory systems to guide vocal production. Because both young and adult budgerigars deafened by cochlear removal show permanent changes in vocal output (20), the questions now should focus on how long the sensorimotor interfaces can do without appropriate sensory feedback before functional recovery in vocal production is no longer possible. The answer to this question, even in a bird, should have relevance for treatment of hearing loss in humans and the timing of auditory prosthetic devices such as cochlear implants.
Acknowledgments
We thank C. Carr, W. Hodos, W. Lippe, C. Moss, A. Popper, and D. Yager for comments on the manuscript. This work was supported by National Institute of Deafness and Other Communication Disorders Grant DC-01372 to R.J.D. and B.M.R., National Institute of Mental Health Grant K05 MH-00982 to R.J.D.
References
- 1.Cotanche D A, Lee K H, Stone J S, Picard D A. Anat Embryol. 1994;189:1–18. doi: 10.1007/BF00193125. [DOI] [PubMed] [Google Scholar]
- 2.Tsue T T, Oesterle E C, Rubel E W. Otolaryngol Head Neck Surg. 1994;111:281–301. doi: 10.1177/01945998941113P118. [DOI] [PubMed] [Google Scholar]
- 3.Corwin J T, Warchol M E. Annu Rev Neurosci. 1991;14:301–333. doi: 10.1146/annurev.ne.14.030191.001505. [DOI] [PubMed] [Google Scholar]
- 4.Corwin J T, Cotanche D A. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- 5.Ryals B M, Rubel E W. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- 6.Girod D A, Tucci D L, Rubel E W. Laryngoscope. 1991;101:1139–1149. doi: 10.1288/00005537-199111000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Hashino E, Sokabe M, Tanaka Y. In: Noise-Induced Hearing Loss. Dancer A L, Henderson D, Salvi R J, Hamernick R P, editors. St. Louis: Mosby Year Book; 1991. pp. 228–236. [Google Scholar]
- 8.Lippe W R, Westbrook E W, Ryals B M. Hear Res. 1991;56:203–210. doi: 10.1016/0378-5955(91)90171-5. [DOI] [PubMed] [Google Scholar]
- 9.McFadden E A, Saunders J C. Hear Res. 1989;41:205–215. doi: 10.1016/0378-5955(89)90012-9. [DOI] [PubMed] [Google Scholar]
- 10.Saunders S S, Adler H, Pugliano F. Exp Neurol. 1992;115:13–17. doi: 10.1016/0014-4886(92)90213-a. [DOI] [PubMed] [Google Scholar]
- 11.Tucci D L, Rubel E W. Otolaryngol Head Neck Surg. 1990;103:443–450. doi: 10.1177/019459989010300317. [DOI] [PubMed] [Google Scholar]
- 12.Saunders J C, Doan D E, Cohen Y E, Adler H J, Poje C P, et al. In: Auditory System Plasticity and Regeneration. Salvi R J, Henderson D, Fiorino F, Colletti V, editors. New York: Thieme; 1996. pp. 62–84. [Google Scholar]
- 13.Niemiec A J, Raphael Y, Moody D B. Hear Res. 1994;79:1–16. doi: 10.1016/0378-5955(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 14.Marean G C, Cunningham D, Burt J M, Beecher M D, Rubel E W, et al. Hear Res. 1995;82:267–276. doi: 10.1016/0378-5955(94)00183-q. [DOI] [PubMed] [Google Scholar]
- 15.Saunders S S, Salvi R J. J Acoust Soc Am. 1993;94:83–90. doi: 10.1121/1.406945. [DOI] [PubMed] [Google Scholar]
- 16.Saunders S S, Salvi R J, Miller K M. J Acoust Soc Am. 1995;97:1150–1164. doi: 10.1121/1.412228. [DOI] [PubMed] [Google Scholar]
- 17.Tyler R S, editor. Cochlear Implants. San Diego: Singular; 1993. [Google Scholar]
- 18.Dooling R J. Exp Biol. 1986;45:195–218. [PubMed] [Google Scholar]
- 19.Farabaugh S M, Linzenbold A, Dooling R J. J Comp Psychol. 1994;108:81–92. doi: 10.1037/0735-7036.108.1.81. [DOI] [PubMed] [Google Scholar]
- 20.Dooling R J, Gephart B F, Price P H, McHale C, Brauth S E. Anim Behav. 1987;35:1264–1266. [Google Scholar]
- 21.Hashino E, Tanaka Y, Salvi R J, Sokabe M. Hear Res. 1992;59:46–58. doi: 10.1016/0378-5955(92)90101-r. [DOI] [PubMed] [Google Scholar]
- 22.Hashino E, Sokabe M. J Acoust Soc Am. 1989;85:289–294. doi: 10.1121/1.397736. [DOI] [PubMed] [Google Scholar]
- 23.Gleich O, Manley G. Hear Res. 1988;34:69–87. doi: 10.1016/0378-5955(88)90052-4. [DOI] [PubMed] [Google Scholar]
- 24.Duckert L G, Rubel E W. J Comp Neurol. 1993;331:75–96. doi: 10.1002/cne.903310105. [DOI] [PubMed] [Google Scholar]
- 25.Dooling R J, Saunders J C. J Comp Phys Psychol. 1975;88:1–20. doi: 10.1037/h0076226. [DOI] [PubMed] [Google Scholar]
- 26.Okanoya K, Dooling R J. J Comp Psychol. 1988;101:7–15. [PubMed] [Google Scholar]
- 27.Dooling R J. In: Comparative Studies of Hearing in Birds. Popper A, Fay R, editors. New York: Springer; 1980. pp. 261–288. [Google Scholar]
- 28.Manabe K, Staddon J E R, Cleveland M J. J Comp Psychol. 1997;111:50–62. [Google Scholar]
- 29.Manabe K, Dooling R J. Behav Proc. 1997;41:117–132. doi: 10.1016/s0376-6357(97)00041-7. [DOI] [PubMed] [Google Scholar]


