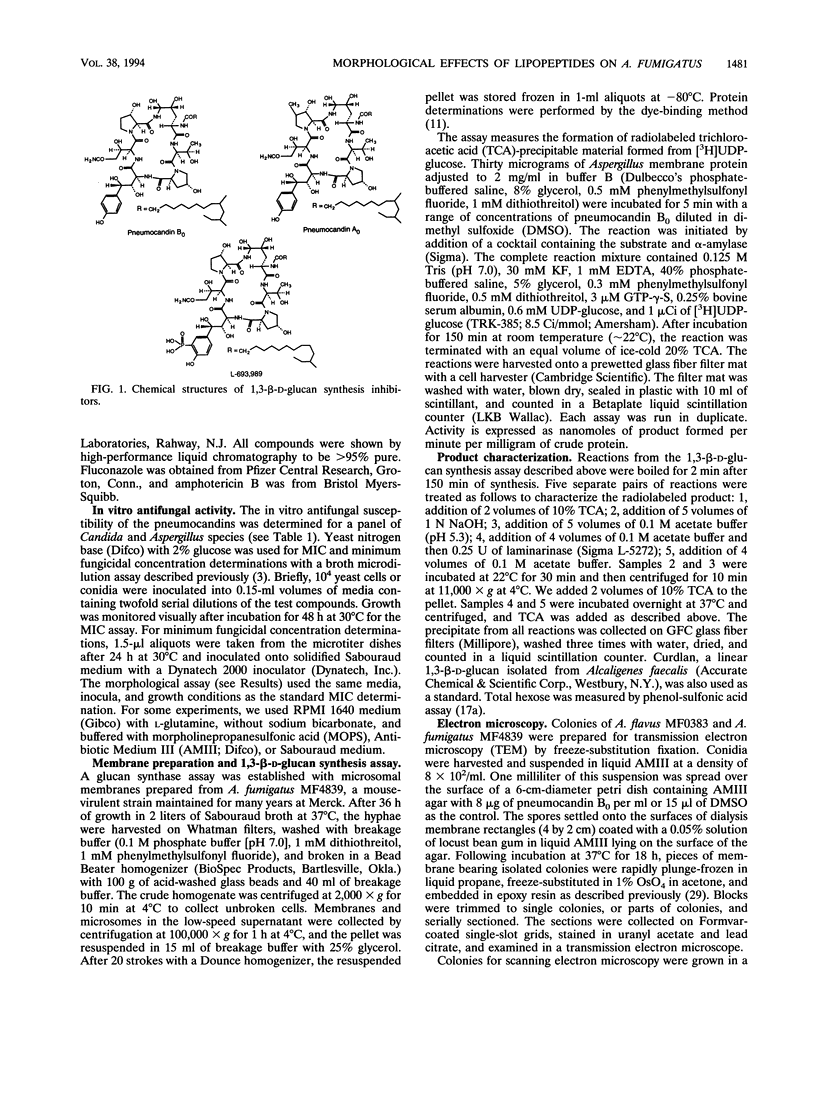
Abstract
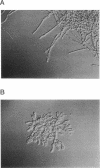
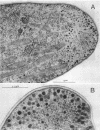
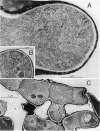
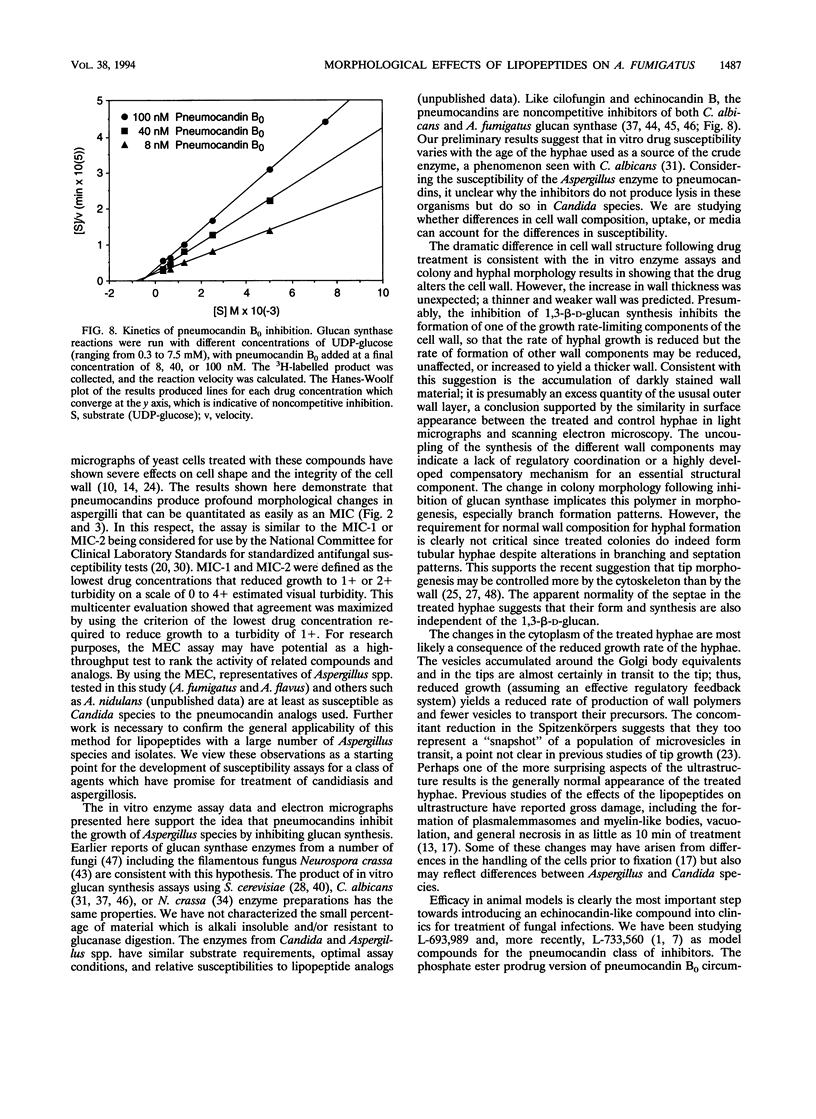
The lipopeptide antifungal agents, echinocandins, papulacandins, and pneumocandins, kill Candida albicans by inhibiting glucan synthesis. For this fungus, there is a good correlation of in vitro enzyme inhibition with in vitro assays of MICs. Semisynthetic lipopeptides such as cilofungin, LY303366, L-693,989, and L-733,560 have activity in vivo against Aspergillus infections but appear to be inactive in broth dilution in vitro tests (MICs, > 128 micrograms/ml). To understand how compounds which lack activity in vitro can have good in vivo activity, we monitored the effect of pneumocandins on the morphology of Aspergillus fumigatus and A, flavus strains by light microscopy and electron microscopy and related the changes in growth to inhibition of glucan synthesis. Pneumocandin B0 caused profound changes in hyphal growth; light micrographs showed abnormally swollen germ tubes, highly branched hyphal tips, and many cells with distended balloon shapes. Aspergillus electron micrographs confirmed that lipopeptides produce changes in cell walls; drug-treated germlings showed very stubby growth with thick walls and a conspicuous dark outer layer which was much thicker in the subapical regions. The rest of the hyphal tip ultrastructure was unaffected by the drug, indicating considerable specificity for the primary target. The drug-induced growth alteration produced very compact clumps in broth dilution wells, making it possible to score the morphological effect macroscopically. The morphological changes could be assayed quantitatively by using conventional broth microdilution susceptibility assay conditions. We defined the endpoint as the lowest concentration required to produce the morphological effect and called it the minimum effective concentration to distinguish it from the no-growth endpoints used in MIC determinations. The minimum effective concentration assay was related to inhibition of glucan synthase activity in vitro and may provide a starting point for development of susceptibility testing methods for lipopeptides.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartizal K., Abruzzo G., Trainor C., Krupa D., Nollstadt K., Schmatz D., Schwartz R., Hammond M., Balkovec J., Vanmiddlesworth F. In vitro antifungal activities and in vivo efficacies of 1,3-beta-D-glucan synthesis inhibitors L-671,329, L-646,991, tetrahydroechinocandin B, and L-687,781, a papulacandin. Antimicrob Agents Chemother. 1992 Aug;36(8):1648–1657. doi: 10.1128/aac.36.8.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Beaulieu D., Tang J., Zeckner D. J., Parr T. R., Jr Correlation of cilofungin in vivo efficacy with its activity against Aspergillus fumigatus (1,3)-beta-D-glucan synthase. FEMS Microbiol Lett. 1993 Apr 1;108(2):133–137. doi: 10.1111/j.1574-6968.1993.tb06088.x. [DOI] [PubMed] [Google Scholar]
- Beauvais A., Drake R., Ng K., Diaquin M., Latgé J. P. Characterization of the 1,3-beta-glucan synthase of Aspergillus fumigatus. J Gen Microbiol. 1993 Dec;139(12):3071–3078. doi: 10.1099/00221287-139-12-3071. [DOI] [PubMed] [Google Scholar]
- Borgia P. T., Dodge C. L. Characterization of Aspergillus nidulans mutants deficient in cell wall chitin or glucan. J Bacteriol. 1992 Jan;174(2):377–383. doi: 10.1128/jb.174.2.377-383.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouffard F. A., Zambias R. A., Dropinski J. F., Balkovec J. M., Hammond M. L., Abruzzo G. K., Bartizal K. F., Marrinan J. A., Kurtz M. B., McFadden D. C. Synthesis and antifungal activity of novel cationic pneumocandin B(o) derivatives. J Med Chem. 1994 Jan 21;37(2):222–225. doi: 10.1021/jm00028a003. [DOI] [PubMed] [Google Scholar]
- Bozzola J. J., Mehta R. J., Nisbet L. J., Valenta J. R. The effect of aculeacin A and papulacandin B on morphology and cell wall ultrastructure in Candida albicans. Can J Microbiol. 1984 Jun;30(6):857–863. doi: 10.1139/m84-133. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cabib E., Kang M. S. Fungal 1,3-beta-glucan synthase. Methods Enzymol. 1987;138:637–642. doi: 10.1016/0076-6879(87)38057-7. [DOI] [PubMed] [Google Scholar]
- Cassone A., Mason R. E., Kerridge D. Lysis of growing yeast-form cells of Candida albicans by echinocandin: a cytological study. Sabouraudia. 1981 Jun;19(2):97–110. [PubMed] [Google Scholar]
- Denning D. W., Stevens D. A. Efficacy of cilofungin alone and in combination with amphotericin B in a murine model of disseminated aspergillosis. Antimicrob Agents Chemother. 1991 Jul;35(7):1329–1333. doi: 10.1128/aac.35.7.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouhet E., Dupont B., Improvisi L., Lesourd M., Prevost M. C. Activity of cilofungin (LY 121019), a new lipopeptide antibiotic, on the cell wall and cytoplasmic membrane of Candida albicans. Structural modifications in scanning and transmission electron microscopy. J Med Vet Mycol. 1990;28(6):425–436. doi: 10.1080/02681219080000541. [DOI] [PubMed] [Google Scholar]
- Dávila T., San-Blas G., San-Blas F. Effect of papulacandin B on glucan synthesis in Paracoccidioides brasiliensis. J Med Vet Mycol. 1986 Jun;24(3):193–202. [PubMed] [Google Scholar]
- Espinel-Ingroff A., Kish C. W., Jr, Kerkering T. M., Fromtling R. A., Bartizal K., Galgiani J. N., Villareal K., Pfaller M. A., Gerarden T., Rinaldi M. G. Collaborative comparison of broth macrodilution and microdilution antifungal susceptibility tests. J Clin Microbiol. 1992 Dec;30(12):3138–3145. doi: 10.1128/jcm.30.12.3138-3145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromtling R. A., Galgiani J. N., Pfaller M. A., Espinel-Ingroff A., Bartizal K. F., Bartlett M. S., Body B. A., Frey C., Hall G., Roberts G. D. Multicenter evaluation of a broth macrodilution antifungal susceptibility test for yeasts. Antimicrob Agents Chemother. 1993 Jan;37(1):39–45. doi: 10.1128/aac.37.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordee R. S., Zeckner D. J., Ellis L. F., Thakkar A. L., Howard L. C. In vitro and in vivo anti-Candida activity and toxicology of LY121019. J Antibiot (Tokyo) 1984 Sep;37(9):1054–1065. doi: 10.7164/antibiotics.37.1054. [DOI] [PubMed] [Google Scholar]
- Gordee R. S., Zeckner D. J., Howard L. C., Alborn W. E., Jr, Debono M. Anti-Candida activity and toxicology of LY121019, a novel semisynthetic polypeptide antifungal antibiotic. Ann N Y Acad Sci. 1988;544:294–309. doi: 10.1111/j.1749-6632.1988.tb40415.x. [DOI] [PubMed] [Google Scholar]
- Grove S. N., Bracker C. E. Protoplasmic organization of hyphal tips among fungi: vesicles and Spitzenkörper. J Bacteriol. 1970 Nov;104(2):989–1009. doi: 10.1128/jb.104.2.989-1009.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A., Edwards F., Bernard E. M., Armstrong D., Schmitt H. J. In vitro activity of the new semi-synthetic polypeptide cilofungin (LY121019) against Aspergillus and Candida species. Eur J Clin Microbiol Infect Dis. 1990 Sep;9(9):697–699. doi: 10.1007/BF01964276. [DOI] [PubMed] [Google Scholar]
- Meyer S. L., Kaminskyj S. G., Heath I. B. Nuclear migration in a nud mutant of Aspergillus nidulans is inhibited in the presence of a quantitatively normal population of cytoplasmic microtubules. J Cell Biol. 1988 Mar;106(3):773–778. doi: 10.1083/jcb.106.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlean P. A. (1,3)-beta-D-Glucan synthase from budding and filamentous cultures of the dimorphic fungus Candida albicans. Eur J Biochem. 1982 Oct;127(2):397–403. doi: 10.1111/j.1432-1033.1982.tb06885.x. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A., Gerarden T., Yu M., Wenzel R. P. Influence of in vitro susceptibility testing conditions on the anti-candidal activity of LY121019. Diagn Microbiol Infect Dis. 1988 Sep;11(1):1–9. doi: 10.1016/0732-8893(88)90067-3. [DOI] [PubMed] [Google Scholar]
- Ruiz-Herrera J., Sentandreu R. Fungal cell wall synthesis and assembly. Curr Top Med Mycol. 1989;3:168–217. doi: 10.1007/978-1-4612-3624-5_8. [DOI] [PubMed] [Google Scholar]
- Saag M. S., Powderly W. G., Cloud G. A., Robinson P., Grieco M. H., Sharkey P. K., Thompson S. E., Sugar A. M., Tuazon C. U., Fisher J. F. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. N Engl J Med. 1992 Jan 9;326(2):83–89. doi: 10.1056/NEJM199201093260202. [DOI] [PubMed] [Google Scholar]
- Sawistowska-Schröder E. T., Kerridge D., Perry H. Echinocandin inhibition of 1,3-beta-D-glucan synthase from Candida albicans. FEBS Lett. 1984 Jul 23;173(1):134–138. doi: 10.1016/0014-5793(84)81032-7. [DOI] [PubMed] [Google Scholar]
- Schmatz D. M., Abruzzo G., Powles M. A., McFadden D. C., Balkovec J. M., Black R. M., Nollstadt K., Bartizal K. Pneumocandins from Zalerion arboricola. IV. Biological evaluation of natural and semisynthetic pneumocandins for activity against Pneumocystis carinii and Candida species. J Antibiot (Tokyo) 1992 Dec;45(12):1886–1891. doi: 10.7164/antibiotics.45.1886. [DOI] [PubMed] [Google Scholar]
- Schmatz D. M., Powles M. A., McFadden D. C., Pittarelli L., Balkovec J., Hammond M., Zambias R., Liberator P., Anderson J. Antipneumocystis activity of water-soluble lipopeptide L-693,989 in rats. Antimicrob Agents Chemother. 1992 Sep;36(9):1964–1970. doi: 10.1128/aac.36.9.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmatz D. M., Romancheck M. A., Pittarelli L. A., Schwartz R. E., Fromtling R. A., Nollstadt K. H., Vanmiddlesworth F. L., Wilson K. E., Turner M. J. Treatment of Pneumocystis carinii pneumonia with 1,3-beta-glucan synthesis inhibitors. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5950–5954. doi: 10.1073/pnas.87.15.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shematek E. M., Braatz J. A., Cabib E. Biosynthesis of the yeast cell wall. I. Preparation and properties of beta-(1 leads to 3)glucan synthetase. J Biol Chem. 1980 Feb 10;255(3):888–894. [PubMed] [Google Scholar]
- Shematek E. M., Cabib E. Biosynthesis of the yeast cell wall. II. Regulation of beta-(1 leads to 3)glucan synthetase by ATP and GTP. J Biol Chem. 1980 Feb 10;255(3):895–902. [PubMed] [Google Scholar]
- Szaniszlo P. J., Kang M. S., Cabib E. Stimulation of beta(1----3)glucan synthetase of various fungi by nucleoside triphosphates: generalized regulatory mechanism for cell wall biosynthesis. J Bacteriol. 1985 Mar;161(3):1188–1194. doi: 10.1128/jb.161.3.1188-1194.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taft C. S., Selitrennikoff C. P. LY121019 inhibits Neurospora crassa growth and (1-3)-beta-D-glucan synthase. J Antibiot (Tokyo) 1988 May;41(5):697–701. doi: 10.7164/antibiotics.41.697. [DOI] [PubMed] [Google Scholar]
- Taft C. S., Stark T., Selitrennikoff C. P. Cilofungin (LY121019) inhibits Candida albicans (1-3)-beta-D-glucan synthase activity. Antimicrob Agents Chemother. 1988 Dec;32(12):1901–1903. doi: 10.1128/aac.32.12.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Parr T. R., Jr W-1 solubilization and kinetics of inhibition by cilofungin of Candida albicans (1,3)-beta-D-glucan synthase. Antimicrob Agents Chemother. 1991 Jan;35(1):99–103. doi: 10.1128/aac.35.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann C. F., Liesch J. M., Schwartz R. E. L-671,329, a new antifungal agent. II. Structure determination. J Antibiot (Tokyo) 1989 Feb;42(2):168–173. doi: 10.7164/antibiotics.42.168. [DOI] [PubMed] [Google Scholar]