Abstract
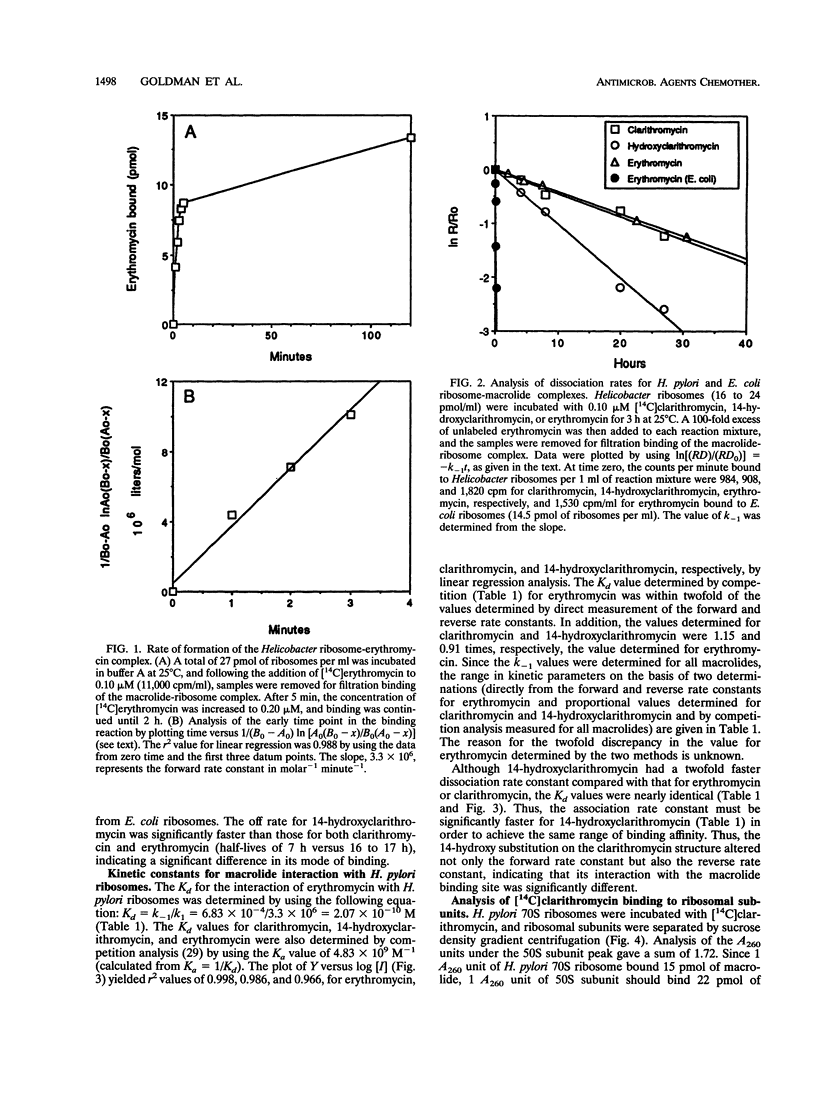
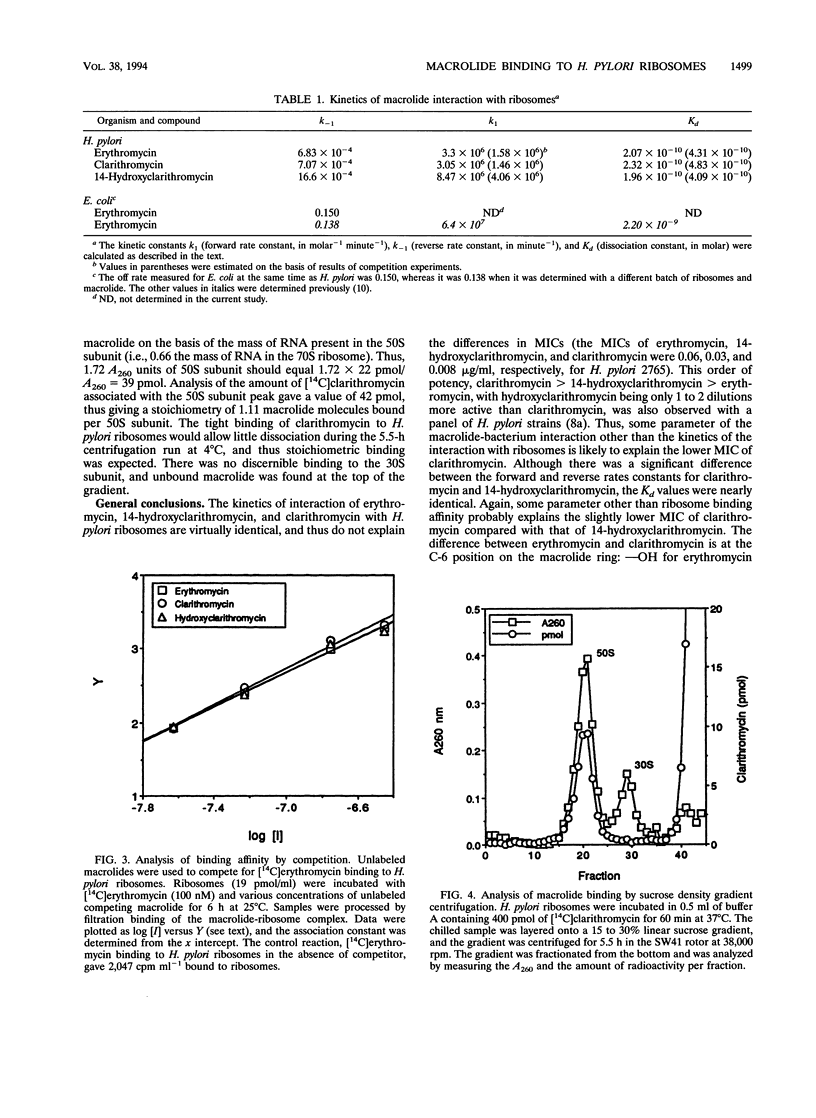
Clarithromycin is a recently approved macrolide with improved pharmacokinetics, antibacterial activity, and efficacy in treating bacterial infections including those caused by Helicobacter pylori, an agent implicated in various forms of gastric disease. We successfully isolated ribosomes from H. pylori and present the results of a study of their interaction with macrolides. Kinetic data were obtained by using 14C-labeled macrolides to probe the ribosomal binding site. Clarithromycin, its parent compound erythromycin, and its 14-(R)-hydroxy metabolite all bound tightly to H. pylori ribosomes. Kd values were in the range of 2 x 10(-10) M, which is the tightest binding interaction observed to date for a macrolide-ribosome complex. This tight binding was due to very slow dissociation rate constants of 7.07 x 10(-4), 6.83 x 10(-4), and 16.6 x 10(-4) min-1 for clarithromycin, erythromycin, and 14-hydroxyclarithromycin, respectively, giving half-times of dissociation ranging from 7 to 16 h, the slowest yet measured for a macrolide-ribosome complex. These dissociation rate constants are 2 orders of magnitude slower than the dissociation rate constants of macrolides from other gram-negative ribosomes. [14C]clarithromycin was bound stoichiometrically to 50S ribosomal subunits following incubation with 70S ribosomes and subsequent separation of the 30S and 50S subunits by sucrose density gradient centrifugation. These data predict that the lower MIC of clarithromycin compared with that of erythromycin for H. pylori is likely due to a faster rate of intracellular accumulation, possibly because of increased hydrophobicity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alder J., Jarvis K., Mitten M., Shipkowitz N. L., Gupta P., Clement J. Clarithromycin therapy of experimental Treponema pallidum infections in hamsters. Antimicrob Agents Chemother. 1993 Apr;37(4):864–867. doi: 10.1128/aac.37.4.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. D., Powell K. U. Eradication of Helicobacter pylori and its effect in peptic ulcer disease. Scand J Gastroenterol Suppl. 1993;196:7–11. doi: 10.3109/00365529309098334. [DOI] [PubMed] [Google Scholar]
- Brown B. A., Wallace R. J., Jr, Onyi G. O. Activities of clarithromycin against eight slowly growing species of nontuberculous mycobacteria, determined by using a broth microdilution MIC system. Antimicrob Agents Chemother. 1992 Sep;36(9):1987–1990. doi: 10.1128/aac.36.9.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giambattista M., Engelborghs Y., Nyssen E., Cocito C. Kinetics of binding of macrolides, lincosamides, and synergimycins to ribosomes. J Biol Chem. 1987 Jun 25;262(18):8591–8597. [PubMed] [Google Scholar]
- Dixon M. Acid, ulcers, and H pylori. Lancet. 1993 Aug 14;342(8868):384–385. doi: 10.1016/0140-6736(93)92808-7. [DOI] [PubMed] [Google Scholar]
- Dooley C. P. Helicobacter pylori: review of research findings. Aliment Pharmacol Ther. 1991;5 (Suppl 1):129–143. doi: 10.1111/j.1365-2036.1991.tb00756.x. [DOI] [PubMed] [Google Scholar]
- Ferrero J. L., Bopp B. A., Marsh K. C., Quigley S. C., Johnson M. J., Anderson D. J., Lamm J. E., Tolman K. G., Sanders S. W., Cavanaugh J. H. Metabolism and disposition of clarithromycin in man. Drug Metab Dispos. 1990 Jul-Aug;18(4):441–446. [PubMed] [Google Scholar]
- Goldman R. C., Fesik S. W., Doran C. C. Role of protonated and neutral forms of macrolides in binding to ribosomes from gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 1990 Mar;34(3):426–431. doi: 10.1128/aac.34.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Kadam S. K. Binding of novel macrolide structures to macrolides-lincosamides-streptogramin B-resistant ribosomes inhibits protein synthesis and bacterial growth. Antimicrob Agents Chemother. 1989 Jul;33(7):1058–1066. doi: 10.1128/aac.33.7.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y., Opekun A. R., Klein P. D. Clarithromycin for the eradication of Helicobacter pylori. J Clin Gastroenterol. 1993 Jun;16(4):292–294. doi: 10.1097/00004836-199306000-00004. [DOI] [PubMed] [Google Scholar]
- Hardy D. J., Guay D. R., Jones R. N. Clarithromycin, a unique macrolide. A pharmacokinetic, microbiological, and clinical overview. Diagn Microbiol Infect Dis. 1992 Jan;15(1):39–53. doi: 10.1016/0732-8893(92)90055-x. [DOI] [PubMed] [Google Scholar]
- Hardy D. J., Hanson C. W., Hensey D. M., Beyer J. M., Fernandes P. B. Susceptibility of Campylobacter pylori to macrolides and fluoroquinolones. J Antimicrob Chemother. 1988 Nov;22(5):631–636. doi: 10.1093/jac/22.5.631. [DOI] [PubMed] [Google Scholar]
- Hardy D. J., Hensey D. M., Beyer J. M., Vojtko C., McDonald E. J., Fernandes P. B. Comparative in vitro activities of new 14-, 15-, and 16-membered macrolides. Antimicrob Agents Chemother. 1988 Nov;32(11):1710–1719. doi: 10.1128/aac.32.11.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirst H. A., Sides G. D. New directions for macrolide antibiotics: pharmacokinetics and clinical efficacy. Antimicrob Agents Chemother. 1989 Sep;33(9):1419–1422. doi: 10.1128/aac.33.9.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebers D. M., Baltch A. L., Smith R. P., Hammer M. C., Conroy J. V., Shayegani M. Comparative in-vitro activities of A-56268 (TE-031) and erythromycin against 306 clinical isolates. J Antimicrob Chemother. 1988 May;21(5):565–570. doi: 10.1093/jac/21.5.565. [DOI] [PubMed] [Google Scholar]
- Malanoski G. J., Eliopoulos G. M., Ferraro M. J., Moellering R. C., Jr Effect of pH variation on the susceptibility of Helicobacter pylori to three macrolide antimicrobial agents and temafloxacin. Eur J Clin Microbiol Infect Dis. 1993 Feb;12(2):131–133. doi: 10.1007/BF01967591. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Putterman M. The intermolecular complex of erythromycin and ribosome. J Mol Biol. 1969 Sep 14;44(2):347–361. doi: 10.1016/0022-2836(69)90180-6. [DOI] [PubMed] [Google Scholar]
- Menninger J. R. Functional consequences of binding macrolides to ribosomes. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):23–34. doi: 10.1093/jac/16.suppl_a.23. [DOI] [PubMed] [Google Scholar]
- Moureau P., Engelborghs Y., Di Giambattista M., Cocito C. Fluorescence stopped flow analysis of the interaction of virginiamycin components and erythromycin with bacterial ribosomes. J Biol Chem. 1983 Dec 10;258(23):14233–14238. [PubMed] [Google Scholar]
- Nagate T., Numata K., Hanada K., Kondo I. The susceptibility of Campylobacter pylori to antiulcer agents and antibiotics. J Clin Gastroenterol. 1990;12 (Suppl 1):S135–S138. doi: 10.1097/00004836-199001001-00023. [DOI] [PubMed] [Google Scholar]
- Oleinick N. L., Corcoran J. W. Two types of binding of erythromycin to ribosomes from antibiotic-sensitive and -resistant Bacillus subtilis 168. J Biol Chem. 1969 Feb 25;244(4):727–735. [PubMed] [Google Scholar]
- Pestka S. Binding of [14C]erythromycin to Escherichia coli ribosomes. Antimicrob Agents Chemother. 1974 Oct;6(4):474–478. doi: 10.1128/aac.6.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson W. L., Graham D. Y., Marshall B., Blaser M. J., Genta R. M., Klein P. D., Stratton C. W., Drnec J., Prokocimer P., Siepman N. Clarithromycin as monotherapy for eradication of Helicobacter pylori: a randomized, double-blind trial. Am J Gastroenterol. 1993 Nov;88(11):1860–1864. [PubMed] [Google Scholar]
- Ruf B., Schürmann D., Mauch H., Jautzke G., Fehrenbach F. J., Pohle H. D. Effectiveness of the macrolide clarithromycin in the treatment of Mycobacterium avium complex infection in HIV-infected patients. Infection. 1992 Sep-Oct;20(5):267–272. doi: 10.1007/BF01710792. [DOI] [PubMed] [Google Scholar]
- Sipponen P. Helicobacter pylori, chronic gastritis and peptic ulcer. Mater Med Pol. 1992 Jul-Sep;24(3):166–168. [PubMed] [Google Scholar]
- Sipponen P., Hyvärinen H. Role of Helicobacter pylori in the pathogenesis of gastritis, peptic ulcer and gastric cancer. Scand J Gastroenterol Suppl. 1993;196:3–6. doi: 10.3109/00365529309098333. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Kubo O., Miyamoto E., Yamana T. Physicochemical properties of beta-lactam antibiotics: oil-water distribution. J Pharm Sci. 1977 Dec;66(12):1675–1679. doi: 10.1002/jps.2600661205. [DOI] [PubMed] [Google Scholar]
- Vince R., Weiss D., Pestka S. Binding of N-substituted erythromycyclamines to ribosomes. Antimicrob Agents Chemother. 1976 Jan;9(1):131–136. doi: 10.1128/aac.9.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]


