Abstract
HCV infection continues to spread at an alarming rate among IDU populations. The available evidence suggests that HCV is acquired relatively quickly following onset of injection. However, there are few prospective studies of HCV acquisition, particularly among IDU populations in resource-poor settings. A sample of young male heroin injectors with recent onset of injection (<4 years) was recruited in Hanoi, Vietnam for a prospective assessment of the early course of injection (n = 179). Both behavioral and biological assessments (including detailed retrospective assessment of injection initiation) were conducted at baseline and repeated at 6-month intervals for a period of 16 months. Variables associated with HCV infection (p value < 0.05) in bivariate analyses were considered for inclusion in logistic regression models to identify risk factors independently associated with HCV infection. HCV incidence was calculated by using the incidence density approach and was expressed in terms of person-years of observation. The baseline of prevalence of HCV was 46%. HCV significantly increased in relation to time since first injection, from 30% in subjects with ≤10 months of injection risk to 70% in subjects with ≥30 months injection risk (p value = 0.0005). In multivariate logistic regression analysis, increasing age, incarceration in a drug detention facility (OR = 2.54; 95%CI 1.05, 6.15), and time since first injection remained significantly associated with HCV infection. Use of injection as primary mode of administration (OR = 2.56; 95%CI 0.98, 6.69) achieved marginal significance. After 16 months of follow-up, the incidence rate of HCV was 23.35 per 100 person-years and the mean time between first injection and first positive HCV test was 1.2 years. HCV is acquired much more rapidly among new injector populations than previously recognized, demonstrating the need for early behavioral intervention among new heroin-user populations. Particularly critical are interventions that target new heroin user populations, including interventions that improve understanding of viral transmission dynamics, that promote alternative strategies for drug sharing, and that delay initiation of injection.
Keywords: Hepatitis C prevalence, HCV incidence, Onset and initiation of heroin injection, IDU's, Harm reduction, Vietnam
Introduction
Serial reuse of syringes and injection paraphernalia (e.g., cookers, cottons, rinse water, etc.) have been associated with exposure to multiple blood-borne pathogens, including hepatitis B, hepatitis C, and HIV-11–3. Both HBV and HCV infection confer high risk for liver cirrhosis and hepatocellular carcinoma. Although the advent of the HAART therapy has significantly improved both longevity and the quality of life of persons with HIV infection, co-infection with HBV or HCV increases risk for poor outcomes and HCV co-infection may also limit HIV-treatment options4. Although serial syringe-sharing has declined among IDUs and contributed to reductions in new HIV infections, HCV infection continues to be spread at an alarming rate among IDUs, notably among younger age cohorts and those with relatively recent onset of injection risk5–10. While several explanations for this pattern have been offered, much of the available information about HCV among young IDUs is based on retrospective analysis of somewhat older IDU samples (notably, with relatively lengthy intervals from first injection) or from studies of high-risk youth populations in which injection risk was prevalent but in which there was limited assessment of early injection risk practices. Although both types of studies have highlighted the importance of understanding early acquisition of HCV infection, there is little prospective assessment of HCV acquisition and a general dearth of specific information about risk factors in the early course of injection11.
With the overall goal of further elaborating our understanding of unique risks associated with the early course of injection, particularly acquisition of HCV, this paper describes data from a prospective, ethno-epidemiological study of behavioral risk in a sample of young male heroin users in Hanoi, Vietnam. Included in this report are data on the baseline prevalence, behavioral correlates of HCV infection, and a preliminary analysis of the incidence of HCV during the first 16 months of follow-up.
Methods
Study Design and Research Procedures
Between October 2005 and December 2006, a sample of active young heroin injectors was recruited for participation in a prospective study of the early course of injection risk (N = 236). Participation was limited to men, age 16 to 29, who self-reported current heroin use (last 30 days) and whose first use of heroin was within 4 years since time of study enrollment. Mobile teams of interviewers employed targeted and chain referral sampling strategies in targeted areas of all nine inner-city districts of metropolitan Hanoi, concentrating on settings and venues which had been identified in prior ethnographic research as having high- or medium-density activity associated with heroin distribution and injection. Owing to concerns about efficiency and confidentiality, areas with low-density heroin activity were excluded. Participation was relatively high, with the majority (80.55%) of eligible subjects agreeing to participate.
Following written informed consent, subjects completed a formal, face to face behavioral interview conducted at a private research office. Domains included demographic characteristics, lifetime exposure and current use of alcohol, tobacco and a wide range of illegal or illicit substances, onset and sequence of drug initiation, mode of administration at first use, current patterns of heroin use (last 30 days), onset of sexual activity, current sexual profile (including practices, frequency, and partnering), concurrency of drug and sexual risk, knowledge of HIV transmission risks, and history of drug detention.
Following completion of the interview, subjects were asked to donate a blood specimen for research purposes. Subjects were given pre-test counseling as well as information about how to obtain test results. Subjects were also provided post-test counseling. Those who tested positive for an active STD were offered free STD treatment and those who test HIV positive were offered a referral and escort to an HIV care facility. Additionally, those who tested negative for HBV infection were offered free HBV vaccination. Blood specimens were screened for HIV-1, HBV, and HCV infection. HIV infection was determined by positive enzyme immunoassay screening (SFD, Bio-Rad of France) and two enzyme-linked immunosorbent assays (Abbott, USA and Biomerieux, France). HCV antibodies were detected with ELISA, using the third-generation UBI HCV EIA 3.0 (Biomerieux, France).
Subjects were paid the Vietnamese equivalent of $7 in compensation for their time.
All study procedures and instruments were reviewed and approved by a U.S. Institutional Review Board as well as by the Institutional Review Board of Hanoi Medical University.
Measures and Analyses
Since the purpose of this analysis is to assess injection risk and early acquisition of HCV infection, the current analysis is limited to data from subjects with a history of injection (n = 179). Measures included a baseline interview and repeated assessments at 4-month intervals for a period of 24 months. This report describes the incidence of HCV following 16 months of follow-up. Time since first injection (calculated in months) was computed by subtracting the age at first injection from age at the baseline interview. For bivariate and multivariate analyses, time since first injection is described in 10-month intervals.
Frequency distributions and descriptive statistics were used to characterize the sample at baseline. Overall seroprevalence estimates and 95% Confidence Intervals (CI) for HCV, HBV, and HIV were calculated. Chi-square analysis or Fisher exact tests were used to evaluate differences in categorical outcome measures, and independent t test or one-way analysis of variance (ANOVA) were used to evaluate the relationship between HCV infection and continuous outcomes. Risk factors significantly associated with HCV seroprevalence (p < 0.05) in bivariate analysis were included in multivariate logistic regression models to identify risk factors independently associated with HCV seropositivity.
We evaluated possible multicollinearity between covariates included in the final model for indicators such as age (two indicator variables [20–24 years, 25+], for which age 15–19 was the reference category) and length of injection (three indicator variables [11–20 months, 21–30, and 31-43], for which 1–10 months was the reference category) by correlation analysis and collinearity statistics test, as suggested by Allison12. There was no significant collinearity between any of the covariates included in the final model.
HCV incidence was calculated by using the incidence density approach13 and is expressed in terms of person-years of observation. Confidence intervals for the HCV incidence rate (95%CI) were based on the Poisson distribution. Incidence rates were calculated as the number of incident cases divided by person-years at risk. The date of HCV seroconversion was estimated as the midpoint between the last negative and the first positive visit. To identify the mean time from age of first injection to seroconversion, the estimated age at first positive HCV test was subtracted from the age of injection initiation.
All statistical analyses were performed using the statistical package SAS (Version 9.1, Cary, NC).
Results
Participant Characteristics
Demographic and individual risk characteristics are shown in Table 1. The mean age of the cohort was 21.25 years (range 15–27 years). Most (88.27%) were born in Hanoi. Most were unmarried and had, on average, completed only 9 years of primary education. Only a third had formal employment. Nearly 9% of the participants had a history of criminal imprisonment and more than half (56.98%) had a history of forced incarceration in a drug detention facility.
Table 1.
Demographic and risk related characteristics of the 179 young men aged 15–27 in Hanoi, Vietnam 2005–2006
| Characteristic | Number of subjects | Frequency (%) |
|---|---|---|
| Sociodemographic characteristics | ||
| Age at recruitment | ||
| 15–19 | 60 | 33.52 |
| 20–24 | 99 | 55.31 |
| 25+ | 20 | 11.17 |
| Place of birth | ||
| Hanoi | 158 | 88.27 |
| Outside of Hanoia | 21 | 11.73 |
| Marital status | ||
| Single | 168 | 93.85 |
| Married/separated/divorced | 11 | 6.15 |
| Years of education, mean (SD) | 9.42 (2.82) | |
| Have a paid job | ||
| Yes | 55 | 30.73 |
| No | 124 | 69.27 |
| Ever imprisoned | ||
| Yes | 16 | 8.94 |
| No | 163 | 91.06 |
| Ever in treatment facility | ||
| Yes | 102 | 56.98 |
| No | 77 | 43.02 |
| Know someone who has been diagnosed with HIV infection or AIDS | ||
| Yes | 83 | 46.63 |
| No | 95 | 53.37 |
| Age of heroin use, mean (std) | 16.66 (2.78) | |
| Method of administration first time | ||
| Smoked (lung inhalation) | 175 | 97.77 |
| Injection | 4 | 2.23 |
| Since that first time that you use heroin, how long was it before you used heroin again? | ||
| Within one week or less | 112 | 62.57 |
| Within one month | 32 | 17.88 |
| Within 1 year/more than one year later | 35 | 19.55 |
| Injecting risk behavior | ||
| Age of first injection use, mean (STD) | 19.69 | 2.97 |
| How did you know ways to prepare and to get heroin into your body through injection | ||
| Self experimenting | 7 | 3.91 |
| Observed another inject/another person gave verbal instructions | 8 | 4.47 |
| Another person provide assistance directly | 164 | 91.62 |
| Age of person who provided assistance, mean (SD) | 28.43 | 21.79 |
| Did you heat heroin solution the first time of injection? | ||
| Yes | 14 | 7.82 |
| No | 164 | 91.62 |
| In the last 30 days, how many days have you used heroin at least once | ||
| Less than daily | 78 | 43.58 |
| Daily | 101 | 56.42 |
| In the last 30 days, did you use heroin with a fixed group of people | ||
| I participated in a group that used heroin together and the group was closed (fixed) | 75 | 56.82 |
| I used heroin with other people and those people change by circumstances and or days | 57 | 43.18 |
| Sharing syringes in the last 30 days | ||
| Yes | 16 | 8.94 |
| No | 163 | 91.06 |
| In the last 30 days what other ways have you bought Heroin into your body | ||
| Only though injection | 127 | 70.95 |
| Both injection and smoking | 43 | 24.02 |
| Only smoking | 9 | 5.03 |
| How long before you can inject heroin by yourself? | ||
| Less than 1 month | 77 | 43.26 |
| 2–5 months | 47 | 26.40 |
| 6 months or more | 25 | 14.04 |
| Never but still injecting | 29 | 16.29 |
| Who prepared solution for you first time | ||
| Myself | 10 | 5.59 |
| The person who provided direct assistance | 155 | 86.59 |
| Another injector who was present | 14 | 7.82 |
aPlace of birth outside Hanoi: Bac Giang, Ha Nam, Ha Tay, Hai Phong, Hung Yen, Lang Son, Lao Cai, Nam Dinh, Nghe An, Phy Tho. Quang Ninh, Son La, Tuyen Quang, Vihh Phuc, Yen Bai
Patterns of Heroin Initiation
Mean age of first use of heroin was 16 years (range 11–23 years), the vast majority (97.7%) employed smoking as their first mode of administration. Mean age of first injection was 19.69 years (range 13–26). At the time of their first injection, the vast majority (91.62%) received some form of technical assistance from another person (typically a somewhat older injector) in preparing a heroin solution and injecting it. Consistent with the easily soluble form of powder heroin available in Vietnam, most reported simply mixing heroin with water in order to dissolve it into liquid form for injection and only a minority (7.82%) applied heat as an aide to dissolving heroin into solution form.
Current Heroin Use and Injection Risk (Last 30 Days)
As an artifact of study criteria, all subjects reported the use of heroin within 30 days prior to the interview. More than half reported injecting heroin on a daily basis (56.42%) during this time period, with a mean number of 2.43 injections per day. Use of a wide range of CNS depressants was also prevalent, including novocaine (91.62%) and promethazine hydrochloride (marketed locally as Pipolphen; 76.54%). In earlier ethnographic studies, heroin users described frequent use of these substances as a temporary substitute for heroin, as a means of augmenting the effects of heroin, and as means of forestalling onset of heroin withdrawal14.
Two inter-related indicators of injection risk are noteworthy: first, nearly half (43.18%) indicated that most of their current drug-sharing partners came from “open,” situationally defined injection groups with whom they engaged in cooperative activities related to pooling resources for the joint purchase of drugs. Once a shared-drug purchase had been made, strategies for dividing the shared-drug solution included direct needle-sharing (8.94%) and “frontloading”—a practice in which the heroin is mixed with water in a donor syringe and then injected into the front end of each recipient syringe; 87.65%).
Prevalence and Correlates of Blood-Borne Infections
At baseline, 9.1% of the participants had antibodies to HBV core antigen (95% CI [5.29%, 14.34%]). 46.37% tested positive for HCV infection (95% CI, [38.90%, 53.96%]); 12.29% tested positive for HIV infection (95% CI [7.87%, 18.01%]). A high HIV/HCV co-infection prevalence was observed in this young IDU sample. All subjects who had HIV infection (n = 22) were also HCV-positive.
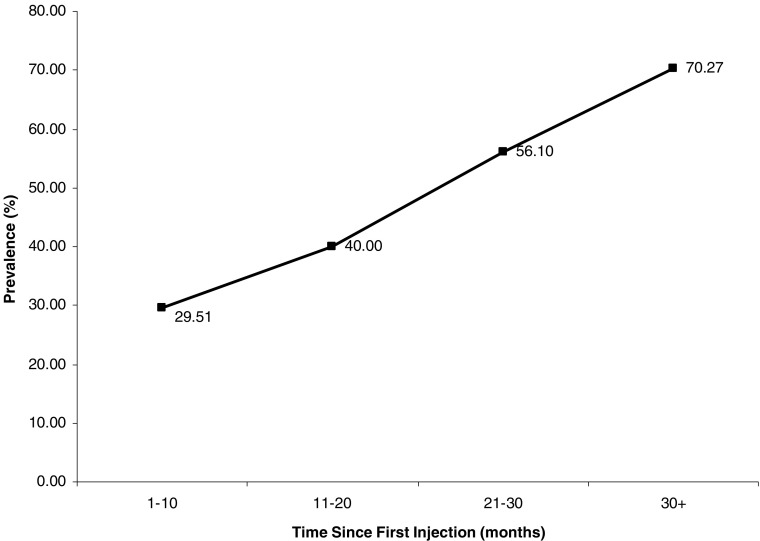
Figure 1 shows the prevalence of HCV by time since first injection. HCV prevalence was significantly increasing over time since first injection, rising from 30% in subjects with 10 months or less of injection risk to 70% in subjects with 30 or more months (p value = 0.0005).
FIGURE 1.
HCV seroprevalence by time since first injection among young injection drug users in Hanoi, Vietnam.
Table 2 shows the association between demographic characteristics, behavioral risks, and HCV status. HCV prevalence was significantly higher (p < 0.05) for participants with the following characteristics: older age, ever been in a treatment facility, knowing someone who has been diagnosed with HIV infection or AIDS, participating in an open-injection group and administering heroin exclusively by injection in the last 30 days.
Table 2.
Seroprevalence of HCV infection in relationship to demographic and risk related characteristics among young Heroin injectors in Hanoi, Vietnam, 2005–2006
| Characteristic | HCV-negative % (n) | HCV-positive % (n) | p value |
|---|---|---|---|
| Age at recruitment | |||
| 15–19 | 71.67 (43) | 28.33 (17) | 0.0004 |
| 20–24 | 48.48 (48) | 51.52 (51) | |
| 25+ | 25.00 (15) | 75.00 (15) | |
| Place of birth | |||
| Hanoi | 51.90 (82) | 48.10 (76) | 0.2023 |
| Outside of Hanoi | 66.67 (14) | 33.33 (7) | |
| Marital status | |||
| Single | 54.17 (91) | 45.83 (77) | 0.5746 |
| Married/separated/divorced | 45.45 (5) | 54.55 (6) | |
| Have a paid job | |||
| Yes | 52.73 (29) | 47.27 (26) | 0.8717 |
| No | 54.03 (67) | 45.97 (57) | |
| Ever imprisoned | |||
| Yes | 37.50 (6) | 62.50 (10) | 0.1751 |
| No | 55.21 (90) | 44.79 (73) | |
| Ever in treatment facility | |||
| Yes | 44.12 (45) | 55.88 (57) | 0.0033 |
| No | 66.23 (51) | 33.77 (26) | |
| Know someone who has been diagnosed with HIV infection or AIDS | |||
| Yes | 44.58 (37) | 55.42 (46) | 0.0193 |
| No | 62.11 (59) | 37.89 (36) | |
| Onset of heroin use | |||
| Since that first time that you use heroin, how long was it before you used heroin again? | |||
| Within one week or less | 58.04 (65) | 41.96 (47) | 0.2622 |
| Within one month | 50.00 (16) | 50.00 (16) | |
| Within 1 year/more than one year later | 42.86 (15) | 57.14 (20) | |
| How did you know ways to prepare and to get heroin into your body through injection? | |||
| Self experimenting | 85.17 (6) | 14.29 (1) | 0.1563 |
| Observed another inject/another person gave verbal instructions | 37.50 (3) | 62.50 (5) | |
| Another person provide assistance directly | 53.05 (87) | 46.95 (77) | |
| How long after before you can inject heroin by yourself? | |||
| Less than 1 month | 58.44 (45) | 41.56 (32) | 0.2359 |
| 2–5 months | 51.06 (24) | 48.94 (23) | |
| 6 months or more | 36.00 (9) | 64.00 (16) | |
| Never but still injecting | 58.62 (17) | 41.38 (12) | |
| Did you heat heroin solution the first time of injection? | |||
| Yes | 78.57 (11) | 21.43 (3) | 0.0909 |
| No | 51.83 (85) | 48.17 (79) | |
| Who prepared solution for you first time | |||
| Myself | 60.00 (6) | 40.00 (4) | 0.6314 |
| The person who provided direct assistance | 52.26 (81) | 47.74 (74) | |
| Another injector who was present | 64.29 (9) | 35.71 (5) | |
| In the last 30 days what other ways have you bought Heroin into your body | |||
| Only though injection | 46.46 (59) | 53.54 (68) | 0.0344 |
| Both injection and smoking | 65.12 (28) | 34.88 (15) | |
| In the last 30 days, how many days have you used heroin at least once | |||
| Less than daily | 56.41 (44) | 43.59 (34) | 0.5123 |
| Daily | 51.49 (52) | 48.51 (49) | |
| Sharing syringes in the last 30 days | |||
| Yes | 8.33 (8) | 9.64 (8) | 0.7602 |
| No | 91.67 (88) | 90.36 (75) | |
| In the last 30 days, did you use heroin with a fixed group of people | |||
| I participated in a group that used heroin together and the group was closed (fixed) | 65.33 (49) | 34.67 (26) | 0.0386 |
| I used heroin with other people and those people change by circumstances and or days | 47.37 (27) | 52.63 (30) |
Table 3 shows the results of the univariate and multivariate logistic regression models evaluating the relationship between significantly associated risk factors and the likelihood of hepatitis C infection. A number of inter-related factors indicative of chronic heroin use were strongly associated with increased risk for HCV in unadjusted analysis: IDUs with a history of drug detention were approximately 2.5 times more likely to have HCV infection; injectors who used injection as their primary mode of administration were 2.15 times more likely to have HCV infection; injectors who knew someone with HIV/AIDS were more than twice as likely to be HCV-infected; injectors who reported that most of their current drug-sharing partners came from an open, situationally derived injection group were twice as likely to have HCV infection (compared to those who primarily used closed, drug-sharing groups). A higher probability of HCV infection was also observed among those with more than 21 months of injection risk. No other sociodemographic or injection risk factors recorded at baseline were significantly associated with HCV infection.
Table 3.
Logistic regression analysis of sociodemographic and injection drug use practices associated with HCV seropositivity among young short-term injecting drug users in Hanoi, Vietnam, 2005–2006
| Variable | Unadjusteda OR (95% CI) | Adjusted ORb (95% CI) | p value c |
|---|---|---|---|
| Age group | |||
| 15–19 | 1.00 | 1.00 | |
| 20–24 | 2.69 (1.35, 5.34) | 3.65 (1.38, 9.67) | 0.0091 |
| 25+ | 7.59 (2.39, 24.14) | 7.51 (1.66, 34.05) | 0.0089 |
| Ever in treatment facility | |||
| Yes | 2.48 (1.35, 4.59) | 2.54 (1.05, 6.15) | 0.0383 |
| No | 1.00 | 1.00 | |
| Heroin administration in the last 30 days | |||
| Heroin injection only | 2.15 (1.05, 4.41) | 2.56 (0.98, 6.69) | 0.0547 |
| Heroin and Smoking | 1.00 | 1.00 | |
| Know someone who has been diagnosed with HIV infection or AIDS | |||
| Yes | 2.04 (1.12, 3.71) | 1.24 (0.53, 2.93) | 0.6247 |
| No | 1.00 | 1.00 | |
| Group of people for injection in the last 30 days | |||
| Fixed | 1.00 | 1.00 | |
| Change with circumstances | 2.09 (1.04, 4.24) | 1.20 (0.51, 2.82) | 0.6793 |
| Duration of heroin injection use (months) | |||
| 1–10 | 1.00 | 1.00 | |
| 11–20 | 1.59 (0.69, 3.68) | 1.52 (0.48, 4.83) | 0.4757 |
| 21–30 | 3.05 (1.34, 6.98) | 3.06 (0.99, 9.53) | 0.0529 |
| 30–43 | 5.65 (2.31, 13.81) | 3.27 (1.04, 10.39) | 0.0431 |
aUnadjusted OR were estimated by a simple logistic regression models
bORs in the full model included adjustment for all the other listed variables
cp values from Wald test obtained in the multiple logistic regression models
In adjusted multivariate models, those with a history of incarceration in a drug detention facility, use of injection as a primary current mode of drug administration, and length of time since first injection remained positively associated with HCV infection.
HCV Incidence
For those participants who were HCV-negative at baseline, the follow-up rates were 54.17% in month 4, 43.75% in month 8, 36.46% in month 12, and 35.42% in month 16. Participants lost to follow-up were similar to those who remained in the study with respect to most sociodemographic characteristics and duration of heroin use (p > 0.05). However, participants lost to follow-up were significantly more likely to use heroin on a daily basis and more likely to participate in “open” injection groups (p ≤ 0.05). Reasons for loss to follow-up included criminal imprisonment or drug detention, (30.58%), migration (9.78%), and death (1.68%). 22.56% were lost to follow-up for unknown reasons.
During a median follow-up of 16 months, 11 participants became HCV seropositive after 47.11 person-years of follow-up. The overall incidence of HCV infection was 23.35 cases per 100 person-years (95% CI [11.65, 41.78]). The mean time from age of first injection to HCV seroconversion was 1.2 years (SD 0.7). No incident cases of HIV and only one case of HBV infection were observed during the same time period.
Discussion
Few epidemiological studies of IDU populations in Vietnam include assessment of HCV infection and information about the prevalence of HCV is limited. The prevalence of HCV infection in this sample was 46%, similar to others studies of IDU’s outside Vietnam with a comparable median time since first injection15,16. Risk factors for HCV resemble those reported in the general IDU literature, including older age and longer duration of drug injection6,16–18. However, all subjects who were HIV+ at baseline also had HCV infection, reflecting a considerably higher rate of co-infection than reported in prior studies19–24.
Of particular importance, however, is the high rate of HCV seroconversion (23.35/100 person-years) within only 16 months of observation. Although previous studies have suggested that HCV is acquired relatively early following onset of injection, most of the available evidence derives from retrospective studies of older, long-term IDU populations in which a precise evaluation of the interval between onset of injection risk and acquisition of HCV was not possible. In examining this question prospectively in this study, we calculated time to seroconversion for each incident case using the interval between age at first injection and date of seroconversion. For IDUs injecting 4 years or less at baseline, the mean time to HCV seroconversion from onset of injecting was 1.2 years, earlier than detected in previously HCV studies25,26 and indicating a significantly narrower window of opportunity for behavioral interventions for new heroin-using populations27. Three general arenas should be targeted for early intervention:
High-risk drug-sharing practices: the majority of the sample reported recent frontloading, probably significantly under-representing the actual frequency of drug sharing. It is noteworthy in this context that we abandoned a formal assessment of the frequency of drug sharing because formative research indicated that the practice was so commonplace that it would be difficult to capture sufficient variability so as make the frequency data analytically useful. However, high rates of frontloading associated with drug sharing may partially account for the high rates of HCV infection observed. Although awareness of HIV risk from direct needle-sharing was generally high, injectors had virtually no knowledge of HCV. Moreover, understanding of transmission risks other than syringe-sharing was also limited; although many recognized the importance of using a sterile “donor syringe” in sharing drug solutions, there was relatively little understanding of how viral transmission is associated with serial reuse of cookers, cotton, and water sources in the course of preparing and dividing shared-drug solutions. Few were aware of the protective effects of heating drug solutions28. Thus, although there is a general understanding of risk from needle-sharing, young injectors generally lack a complete and detailed understanding of viral transmission risks and of safer alternatives for preparing and distributing shared-drug solutions.
High-risk injection groups: there is limited research on the history, composition, and stability of injection groups in Vietnam. As noted above, initial formative ethnographic research in this study indicated that IDU’s recognized an “emic” distinction between “open” and “closed” injection groups. “Open” groups were described as forming on an ad hoc, situational basis, typically without any abiding prior social association, undifferentiated membership, and opportunistically defined roles in cooperative activities associated with drug acquisition, preparation, and disposition of shared-drug solutions. Though quite frequent, participation in open-injection groups was nevertheless typically described in the context of situational “emergencies,” particularly, insufficient money to secure heroin in the context of impending heroin withdrawal. Although many of the same individuals may re-group on a repeated basis, injectors indicated that there was no expectation of an abiding affinity amongst the individuals and no expectation that the cooperative activities would extend beyond those required for that particular injection event. At a social level, interactions within these groups were described as in terms that reflected a brittle quality to interactions in these groups, with high levels of suspicion and high risk for interpersonal conflict.
In contrast, “closed groups” were described as having a more elastic quality, having often been formed on the basis of some kind of prior social affinity, and supporting a more complex set of social and economic functions beyond the exigencies of drug sharing itself. This included expectations of mutual support and friendship in which expectations of reward or repayment related to drug sharing might be substituted or deferred. Importantly, for this discussion, injectors explicitly described the advantages of closed groups in relation to reduced for HIV risk since acceptance into these types of groups is often predicated upon demonstration that an individual is HIV negative and also upon a promise that the individual will not share syringes outside the group. Injectors also described this context as one in which they might be less vigilant about sharing injection paraphernalia, in part because of the assurances that others in the group are HIV− and in part because of the additional pressure to share drugs that is derived from the social foundation upon which these groups are predicated.
In summary, the situation is one in which the high cost of drugs and high risk for arrest both create drug scarcity (at least at the level of the individual user). Strategies for managing this scarcity, including frontloading and participation in multiple concurrent injection groups, may serve to increase risk for exposure to viral pathogens because they increase the number of partners with whom an injector engages in potential behavioral risk. Injectors appear to recognize the protective utility of delimiting their drug-sharing partners, although externally derived circumstances associated with supply and cost often constrain decisions about injection groups. However, interventions should build upon this foundation with the goal of better informing injectors about alternative options for dividing shared drugs and potential transmission risk in drug preparation paraphernalia, perhaps using the very ad hoc composition of injection groups themselves as a tool for broadly disseminating and modeling safer injection knowledge and skills acquisition.
-
3.
High-risk environments: although Vietnam has a long history of opiate use, in the last two decades it has become increasingly urbanized. While somewhat different than the types of “shooting galleries” described in North America and Europe, heroin injection was also often situated in public or semi-public environments in which serial reuse of syringes was commonplace29,30. More recently, however, significant police pressure has been exerted on drug “copping” areas and public injection settings, significantly reducing the availability of shooting galleries. However, this significantly increased risk for arrest associated with drug possession, and thus placed a premium on rapid injection, including adoption of drug-sharing strategies and drug-injection practices that contribute to increased risk for exposure to viral pathogens14.
Additionally, increased police pressure also resulted in significant increases in drug-related arrest, including extended detention in “O6 centers.” During initial formative ethnographic research in this study, drug users with a history of drug detention described high rates of drug use within the centers. Moreover, owing to the scarcity of both drugs and injection equipment within the centers, high-risk injection practices were also common. Thus, drug detention centers may have inadvertently replicated many of the same high-risk ecological conditions that had given rise to high rates of HIV transmission in “shooting galleries,” including the presence of a large and generally older population of heroin injectors (contributing to age cohort mixing) and an environment characterized by extraordinary external pressures (including scarcity of drugs and sterile injection equipment).
Study limitations include a high number of subjects who were lost to follow-up. However, analysis showed that loss to follow-up did not differ significantly on age, time since first injection, history of drug detention, and administration of heroin only through injection (p > 0.05) when compared to subjects who remained in the study. While differential loss to follow-up may still be operant, it is likely that this would result in an under-estimation of HCV incidence. Finally, we cannot exclude the possibility of residual confounding due to unmeasured covariates associated with HCV infection such as tattooing and future studies should address these questions.
In summary, these data indicate that HCV infection is acquired more rapidly following onset of injection than previously recognized. There is a critical need for targeted behavioral interventions among new heroin user populations that both delay onset of injection and also accelerate acquisition of knowledge and skills related to safe injection practices. Additionally, structural interventions and policy reforms should be undertaken to reduce ecologically based sources of risk, including policing activities and drug detention environments that increase risk for drug-related harm.
Acknowledgments
Research was supported by Grant Number DA016188 from the U.S. National Institute on Drug Abuse, Michael Clatts, Ph.D., Principal Investigator. Additional support was provided by Grant Number 325 (03-050) from the World AIDS Foundation. We would like to thank the many young men who participated in the study. Finally, we acknowledge the significant contributions of the staff from the Department of Epidemiology, particularly, guidance and support from Dr. Nguyen Tran Hien, M.D., Ph.D.
References
- 1.Day C, Dolan K. Correlates of hepatitis C testing among heroin injectors in Sydney. Health Promot J Austr. 2006;17(1):70–72. doi: 10.1071/he06070. [DOI] [PubMed] [Google Scholar]
- 2.Garfield J, Drucker E. Fatal overdose trends in major US cities: 1990–1997. Addictions Research and Theory. 2001;9(5):425–436. doi: 10.3109/16066350109141762. [DOI] [Google Scholar]
- 3.Wood E, Kerr T, Stoltz J, et al. Prevalence and correlates of hepatitis C infection among users of North America's first medically supervised safer injection facility. Public Health. 2005;119(12):1111–1115. doi: 10.1016/j.puhe.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Sulkowski M. Viral hepatitis and HIV coinfection. J Hepatol. 2008;48(2):353–367. doi: 10.1016/j.jhep.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Doherty MC, Garfein RS, Monterroso E, Brown D, Vlahov D. Correlates of HIV infection among young adult short-term injection drug users. AIDS. 2000;14(6):717–726. doi: 10.1097/00002030-200004140-00011. [DOI] [PubMed] [Google Scholar]
- 6.Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health. 1996;86(5):655–661. doi: 10.2105/AJPH.86.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagan H, Thiede H, Des Jarlais DC. Hepatitis C virus infection among injection drug users: survival analysis of time to seroconversion. Epidemiology. 2004;15(5):543–549. doi: 10.1097/01.ede.0000135170.54913.9d. [DOI] [PubMed] [Google Scholar]
- 8.Hagan H, Des Jarlais DC, Stern R, et al. HCV synthesis project: preliminary analyses of HCV prevalence in relation to age and duration of injection. Int J Drug Policy. 2007;18(5):341–351. doi: 10.1016/j.drugpo.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Roy E, Haley N, Leclerc P, Cédras L, Boivin JF. Drug injection among street youth: the first time. Addiction. 2002;97(8):1003–1009. doi: 10.1046/j.1360-0443.2002.00161.x. [DOI] [PubMed] [Google Scholar]
- 10.van Ameijden EJ, van den Hoek JA, Hartgers C, Coutinho RA. Risk factors for the transition from noninjection to injection drug use and accompanying AIDS risk behavior in a cohort of drug users. Am J Epidemiol. 1994;139(12):1153–1163. doi: 10.1093/oxfordjournals.aje.a116962. [DOI] [PubMed] [Google Scholar]
- 11.Clatts MC, Goldsamt LA, Yi H. Drug and sexual risk in four men who have sex with men populations: evidence for a sustained HIV epidemic in New York City. J Urban Health. 2005;82(1 Suppl 1):i9–i17. doi: 10.1093/jurban/jti019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allison PD. Logistic Regression Using the SAS System: theory and Application. Cary, NC; 1999.
- 13.Szklo M, Nieto FJ. Epidemiology: beyond the Basics. 2nd ed. Gaithersburg, Maryland; 2004.
- 14.Clatts MC, Giang LM, Goldsamt LA, Yi H. Novel heroin injection practices: implications for transmission of HIV and other bloodborne pathogens. Am J Prev Med. 2007;32(6 Suppl):S226–S233. doi: 10.1016/j.amepre.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn JA, Page-Shafer K, Lum PJ, Ochoa K, Moss AR. Hepatitis C virus infection and needle exchange use among young injection drug users in San Francisco. Hepatology. 2001;34(1):180–187. doi: 10.1053/jhep.2001.25759. [DOI] [PubMed] [Google Scholar]
- 16.Miller CL, Johnston C, Spittal PM, et al. Opportunities for prevention: hepatitis C prevalence and incidence in a cohort of young injection drug users. Hepatology. 2002;36(3):737–742. doi: 10.1053/jhep.2002.35065. [DOI] [PubMed] [Google Scholar]
- 17.Thorpe LE, Ouellet LJ, Levy JR, Williams IT, Monterroso ER. Hepatitis C virus infection: prevalence, risk factors, and prevention opportunities among young injection drug users in Chicago, 1997–1999. J Infect Dis. 2000;182(6):1588–1594. doi: 10.1086/317607. [DOI] [PubMed] [Google Scholar]
- 18.Garfein RS, Doherty MC, Monterroso ER, Thomas DL, Nelson KE, Vlahov D. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(Suppl 1):S11–S19. doi: 10.1097/00042560-199802001-00004. [DOI] [PubMed] [Google Scholar]
- 19.Amin J, Kaye M, Skidmore S, Pillay D, Cooper DA, Dore GJ. HIV and hepatitis C coinfection within the CAESAR study. HIV Med. 2004;5(3):174–179. doi: 10.1111/j.1468-1293.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 20.Klein MB, Lalonde RG, Suissa S. The impact of hepatitis C virus coinfection on HIV progression before and after highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2003;33(3):365–372. doi: 10.1097/00126334-200307010-00011. [DOI] [PubMed] [Google Scholar]
- 21.Greub G, Ledergerber B, Battegay M, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356(9244):1800–1805. doi: 10.1016/S0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 22.Fainboim H, González J, Fassio E, et al. Prevalence of hepatitis viruses in an anti-human immunodeficiency virus-positive population from Argentina. A multicentre study. J Viral Hepat. 1999;6(1):53–57. doi: 10.1046/j.1365-2893.1999.t01-1-6120135.x. [DOI] [PubMed] [Google Scholar]
- 23.Solomon SS, Srikrishnan AK, Mehta SH, et al. High prevalence of HIV, HIV/hepatitis C virus coinfection, and risk behaviors among injection drug users in Chennai, India: a cause for concern. J Acquir Immune Defic Syndr. 2008;49(3):327–332. doi: 10.1097/QAI.0b013e3181831e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reyes JC, Robles RR, Colon HM, et al. HIV and HCV co-infection among street-recruited injection drug users in San Juan, Puerto Rico. Int Conf AIDS. 2004 Jul 11–16; 15: abstract no. C12400. http://gateway.nlm.nih.gov/MeetingAbstracts/ma?f=102277577.html. Accessed December 3, 2008.
- 25.Maher L, Jalaludin B, Chant KG, et al. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in Australia. Addiction. 2006;101(10):1499–1508. doi: 10.1111/j.1360-0443.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- 26.Roy E, Boudreau JF, Boivin JF. Hepatitis C virus incidence among young street-involved IDUs in relation to injection experience. Drug Alcohol Depend. 2009;102(1–3):158–161. doi: 10.1016/j.drugalcdep.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Kapadia F, Latka MH, Hagan H, et al. Design and feasibility of a randomized behavioral intervention to reduce distributive injection risk and improve health-care access among hepatitis C virus positive injection drug users: the Study to Reduce Intravenous Exposures (STRIVE) J Urban Health. 2007;84(1):99–115. doi: 10.1007/s11524-006-9133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clatts MC, Heimer R, Abdala N, et al. HIV-1 transmission in injection paraphernalia: heating drug solutions may inactivate HIV-1. J Acquir Immune Defic Syndr. 1999;22(2):194–199. doi: 10.1097/00126334-199910010-00013. [DOI] [PubMed] [Google Scholar]
- 29.Vinh D. A qualitative study on HIV risk among injecting drug users in Vietnam: reasons for sharing syringes and needles. 2002. Int Conf AIDS. 2002 Jul 7-12; 14: abstract no. WeOrE1361.http://gateway.nlm.nih.gov/MeetingAbstracts/ma?f=102251200.html. Accessed December 11, 2008.
- 30.Doussantousse S, Hoa NT. The life and times of the Hanoi drug users: some recent insights from field research. A research report to the UNDCP, UNAIDS, Vietnam. 2001. http://www.ahrn.net/library_upload/uploadfile/HanoiDrug.pdf. Accessed November 13, 2008.