Abstract
Objective
Gray matter volume and glucose utilization have been reported to be reduced in the left subgenual cingulate of subjects with familial bipolar or unipolar depression. It is unclear whether these findings are secondary to recurrent illness or are part of a familial/genetic syndrome. The authors’ goal was to clarify these findings.
Method
Volumetric analyses were performed by using magnetic resonance imaging in 41 patients experiencing their first episode of affective disorder or schizophrenia and in 20 normal comparison subjects.
Results
The left subgenual cingulate volume of the patients with affective disorder who had a family history of affective disorder was smaller than that of patients with affective disorder with no family history of the illness and the normal comparison subjects. Patients with schizophrenia did not differ from comparison subjects in left subgenual cingulate volume.
Conclusions
Left subgenual cingulate abnormalities are present at first hospitalization for psychotic affective disorder in patients who have a family history of affective disorder.
Magnetic resonance imaging (MRI) studies have reported volume reduction in several brain regions in affective disorder, albeit inconsistently (see reference 1 for review). Positron emission tomography (PET) studies reported lower levels of activity in the left prefrontal and anterior cingulate cortex (2, 3). Drevets and coworkers (4) found that patients with chronic affective disorder who had a family history of affective disorder showed both smaller gray matter volume and lower levels of PET activity in the left subgenual cingulate, below the corpus callosum genu. These authors did not determine, however, whether the volume reduction resulted from chronic illness or was specific to affective disorder.
We obtained 1.5-mm contiguous magnetic resonance (MR) images from patients experiencing their first episode of affective disorder or schizophrenia and from normal comparison subjects to determine whether volume reduction of the subgenual cingulate would be present at first hospitalization and be specific to patients with affective disorder who had a family history of affective disorder compared with patients with affective disorder who had no family history of the disorder and patients with schizophrenia. Specific planned comparisons were conducted separately for left-sided and right-sided structures because Drevets et al. (4) reported significant volume reduction only in the left subgenual cingulate in patients with affective disorder who had a family history of affective disorder.
METHOD
Forty-one psychotic patients were tested during their first hospitalization (N=37) or in the 8 months following their first hospitalization (N=4). All patients were right-handed, and all had an IQ above 75, no history of seizures, no head trauma with loss of consciousness, no neurological disorder, no alcohol or drug dependence, and no substance abuse within the past 5 years. Twenty-four of the patients were experiencing their first episode of affective disorder; their mean age was 23.7 years (SD=5.1), six were women, 21 had manic bipolar disorder, and three had unipolar disorder. Seventeen of the psychotic patients were experiencing their first episode of schizophrenia; their mean age was 27.2 (SD=7.4), and three were women. Fourteen patients with affective disorder (five women) had a family history of affective disorder, as determined by direct query about psychiatric illnesses in parents, siblings, grandparents, aunts, uncles, and cousins and by chart review. Charts included a social worker’s family history constructed from interviews with the patient, family members, and outside caregivers.
For the patients with affective disorder and the patients with schizophrenia, respectively, the mean neuroleptic dose was 189.0 and 270.0 mg/day in chlorpromazine equivalents and the mean age at first medication was 23.5 and 27.1. Median duration of medication before first admission was only 0.0 months for patients with affective disorder (range=0.0–18.8) and 1.8 months for patients with schizophrenia (range=0.0–18.8). Neither illness duration nor medication dose was significantly correlated with subgenual cingulate volume. Diagnoses were confirmed at follow-up approximately 12 months later.
Twenty psychiatrically well comparison subjects who met the same inclusion criteria as patients but who had no axis I disorder in themselves or their first-degree relatives were recruited through advertisements. The mean age of the normal comparison subjects was 24.0 (SD=4.3); two were women.
Socioeconomic status and parental socioeconomic status (5) were ascertained for all subjects. Symptoms were assessed by using the Brief Psychiatric Rating Scale (BPRS) (6). After a description of the study, all subjects gave written informed consent. Subjects were paid for participation.
Two MRI acquisition protocols were used on a 1.5-T Scanner (GE Medical Systems, Milwaukee) (7). The three-dimensional Fourier transform spoiled gradient-recalled acquisition sequence yielding contiguous coronal images throughout the brain (124 slices, 1.5-mm thick) was used for manually delineating and measuring the subgenual cingulate gray matter according to the criteria of Drevets et al. (4); reformatted sagittal images were used for gyral boundaries. The second protocol, which yielded double-echo axial proton density and T2-weighted images (interleaved 3-mm slices), was used to compute intracranial contents volume. All analyses were performed blind to subjects’ identity, age, and diagnosis. The average intraclass correlation coefficient for three raters (Y.H., J-S.K., and I.A.F.) on ratings of 10 subjects was 0.87 (F=3.29, df=2, 29) for the left subgenual cingulate and 0.92 (F=4.10, df=2, 29) for the right subgenual cingulate.
One-way analysis of variance was used to test subjects’ socioeconomic status, parental socioeconomic status, clinical measures, and medication doses. Group differences in intracranial contents were tested with one-way analysis of covariance (ANCOVA), covarying for age. To test the hereditary effect of affective disorder on subgenual cingulate volume, we performed a mixed-model ANCOVA with group (patients with affective disorder who had a family history of affective disorder, patients with affective disorder who had no family history of the disorder, patients with schizophrenia, and comparison subjects) as the between-subjects factor and side (left versus right) as the within-subjects factor, covarying for age and intracranial contents. In the case of significant group differences in the main ANCOVA, each pair of groups was compared. In the case of significant group differences in the decomposed ANCOVAs, planned comparisons were conducted separately for the left and right subgenual cingulate. Results of p<0.05 were considered significant.
RESULTS
There were no significant group differences in age or parental socioeconomic status. Patients with schizophrenia had significantly lower socioeconomic status than comparison subjects (F=7.11, df=2, 58, p=0.02) but not patients with affective disorder. There were no significant differences among patient groups on BPRS scores or neuroleptic dose. None of the demographic or clinical measures differed between patients with affective disorder who did or did not have a family history of affective disorder.
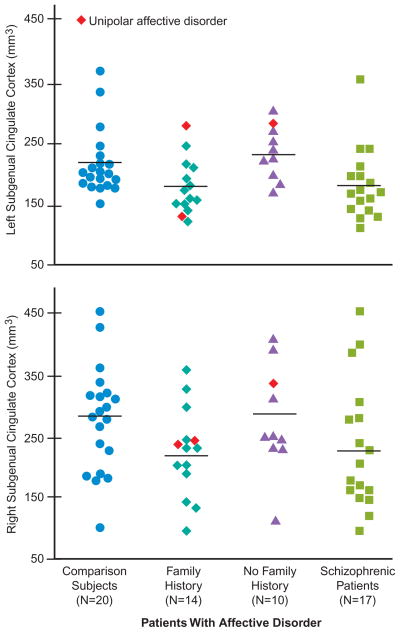
Intracranial contents volume did not differ significantly among groups (mean=1533 ml, SD=166, for comparison subjects; mean=1449 ml, SD=149, for patients with affective disorder who had a family history of affective disorder; mean=1433 ml, SD=133, for patients with affective disorder who had no family history of the disorder; mean=1510 ml, SD=101, for patients with schizophrenia). Subgenual cingulate volume was significantly different among groups (F= 3.00, df=3, 55, p=0.04) (figure 1). Follow-up ANCOVAs revealed that the subgenual cingulate volume of patients with affective disorder who had a family history of affective disorder was significantly smaller than that of normal comparison subjects (F=4.97, df=1, 30, p=0.03) and patients with affective disorder who had no family history of the disorder (F=6.22, df=1, 20, p= 0.02). There was a trend for the subgenual cingulate volume of patients with schizophrenia to be smaller than that of patients with affective disorder who had no family history of the disorder (F=3.25, df=1, 23, p= 0.08). Planned-comparisons analysis revealed that the patients with affective disorder who had a family history of affective disorder had significantly smaller left subgenual cingulate volume than normal comparison subjects (t=2.67, df=32, p=0.01) and patients with affective disorder who had no family history of the disorder (t=3.16, df=22, p<0.01). There were no significant group differences in the right subgenual cingulate.
FIGURE 1. Absolute Volumes of the Subgenual Cingulate Cortex in Patients Experiencing Their First Episode of Affective Disorder or Schizophrenia and Normal Comparison Subjectsa.
aHorizontal lines represent means. The mean left subgenual cingulate volume for patients with affective disorder who had a family history of affective disorder was 181 mm3 (SD=45); for patients with affective disorder who had no family history of the disorder, mean=239 mm3 (SD=43); for patients with schizophrenia, mean= 184 mm3 (SD=59); for normal comparison subjects, mean=226 mm3 (SD=51). The mean right subgenual cingulate volume for patients with affective disorder who had a family history of affective disorder was 225 mm3 (SD=73); for patients with affective disorder who had no family history of the disorder, mean=277 mm3 (SD= 89); for patients with schizophrenia, mean=232 mm3 (SD=105); for normal comparison subjects, mean=280 mm3 (SD=87).
DISCUSSION
The left subgenual cingulate volume of patients with affective disorder who had a family history of affective disorder was 24% smaller than that of patients with affective disorder who had no family history of the disorder and 20% smaller than that of nonpsychiatrically ill comparison subjects, but there were no volume differences among these groups in the right subgenual cingulate. These findings are consistent with those of Drevets et al. (4).
Reduced left subgenual cingulate volume at first hospitalization suggests that this abnormality is not a product of chronicity. Subgenual cingulate abnormalities may be associated with abnormal processing of emotion, in contrast to attention-related processing in the dorsal cingulate (8). Further studies are required to clarify the functions of bilateral subgenual cingulate and whether the subgenual cingulate volume reduction in patients with affective disorder who had a family history of affective disorder is associated with other brain abnormalities. A limitation of the present study is the absence of structured, direct interview of relatives. However, detection of the reduction without this more sensitive procedure suggests its robustness.
Patients with schizophrenia did not differ from normal comparison subjects, but their subgenual cingulate was smaller than that of the patients with affective disorder who had no family history of the disorder; this difference reached trend-level statistical significance. Five patients with schizophrenia had a family history of affective disorder. The mean left subgenual cingulate volume of these patients (mean=217 mm3, SD=86) was nonsignificantly larger than that of patients with affective disorder who had no family history of the disorder (mean=170 mm3, SD=40). Therefore, we conclude that volume reduction of the subgenual cingulate in patients with schizophrenia was not associated with a family history of affective disorder.
Acknowledgments
Supported by NIMH grants MH-01110 and MH-50747 (Dr. Shenton) and MH-40799 (Dr. McCarley), by awards from the Department of Veterans Affairs Medical Research Service and the Brockton VA Center for Clinical and Basic Neuroscience Studies of Schizophrenia (Dr. McCarley), by the Commonwealth of Massachusetts Research Center (Dr. McCarley), by the McDonnell-Pew Program in Cognitive Neuroscience and the National Alliance for Research on Schizophrenia and Depression (Dr. Salisbury), by NIH/NCRR grant RR-13218 (Dr. Jolesz), and by NSF grant RR-11747 (Dr. Kikinis).
References
- 1.Dougherty D, Rauch SL. Neuroimaging and neurobiological models of depression. Harv Rev Psychiatry. 1997;5:138–159. doi: 10.3109/10673229709000299. [DOI] [PubMed] [Google Scholar]
- 2.Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RS, Dolan RJ. The anatomy of melancholia—focal abnormalities of cerebral blood flow in major depression. Psychol Med. 1992;22:607–615. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- 3.Ågren H, Reibring L. PET studies of presynaptic monoamine metabolism in depressed patients and healthy volunteers. Pharmacopsychiatry. 1994;27:2–6. doi: 10.1055/s-2007-1014265. [DOI] [PubMed] [Google Scholar]
- 4.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 5.Hollingshead AB. Two-Factor Index of Social Position. New Haven: Conn, Yale University; 1965. [Google Scholar]
- 6.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 7.Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW. Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- 8.Neafsey EJ, Terreberry RR, Hurley KM, Ruit KG, Frysztak RJ. Anterior cingulate cortex in rodents: connections, visceral control functions, and implications for emotion. In: Vogt BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Boston: Birkhauser; 1993. pp. 206–223. [Google Scholar]