Abstract
Background Approximately 39% of the global diarrhoea deaths in children aged 5 years may be attributable to rotavirus infection. Two rotavirus vaccines were recently introduced to the market, with evidence of efficacy in the USA, Europe and Latin America. We sought to estimate the effectiveness of these vaccines against rotavirus morbidity and mortality.
Methods We conducted a systematic review of published efficacy and effectiveness trials of rotavirus vaccines. Study descriptors and outcome measures were abstracted into standardized tables and the quality of each study was graded. We performed meta-analyses for any outcome with two or more data points, and used child health epidemiology reference group (CHERG) Rules for Evidence Review to estimate the effect of the vaccine on rotavirus mortality.
Results We identified six papers for abstraction, reporting results from four studies. No studies reported diarrhoea or rotavirus deaths, but all studies showed reductions in hospitalizations due to rotavirus or diarrhoea of any aetiology, severe and any rotavirus infections and diarrhoea episodes of any aetiology in children who received rotavirus vaccine compared with placebo. Effectiveness against very severe rotavirus infection best approximated effectiveness against the fraction of diarrhoea deaths attributable to rotavirus, and was estimated to be 74% (95% confidence interval: 35–90%).
Conclusions Rotavirus vaccines are efficacious against rotavirus morbidity and mortality and have the potential to substantially reduce child mortality in low-income countries if implemented appropriately.
Keywords: Rotavirus vaccine, diarrhoea, child, systematic review, meta-analysis
Background
Rotavirus is a leading cause of watery diarrhoea in children, estimated to account for ∼39% of diarrhoea hospitalizations in young children.1 Applying this fraction to the 1.7 million diarrhoea deaths among children aged <5 years,2 Parashar et al.1 estimated that rotavirus is responsible for 611 000 diarrhoea deaths annually. Rotavirus vaccines represent an important preventive approach to reducing rotavirus infections and, along with therapeutic interventions such as oral rehydration solution and zinc supplementation, present an opportunity to decrease diarrhoea morbidity and mortality.
Efforts to develop a rotavirus vaccine began in 1980s and led in 1998 to the licensing and introduction of a live attenuated rhesus rotavirus vaccine in the US market. However, it was voluntarily withdrawn from the market in 1999 after reports of increased risk of intussusception among vaccinated infants. Despite this setback, development of other candidate rotavirus vaccines continued. In 2006, two new oral rotavirus vaccines were licensed and introduced in the USA following large-scale safety and efficacy studies in Europe and North and Latin America.3,4 Studies are ongoing in Asia and Africa to assess the safety and efficacy of the vaccines in these populations.
In 2004, a Cochrane review of rotavirus vaccines reported pooled efficacy estimates against severe rotavirus ranging from 58% to 72%, depending on the vaccine virus strain.5 This review, however, included efficacy data for any rotavirus vaccine, including the withdrawn rhesus rotavirus vaccine and other vaccines never licensed or marketed.5 Also, it did not include Phase III efficacy data on the two currently marketed rotavirus vaccines, as those efficacy trials had not yet been completed. In recent years, numerous reviews of the two new rotavirus vaccines have been published.6–17 To our knowledge, however, there have been no systematic reviews or meta-analyses assessing the effect of currently marketed vaccines on severe rotavirus morbidity and mortality. Here, we report the results of a systematic review and meta-analyses assessing the estimated effect of rotavirus vaccine on moderate to severe morbidity, which we extrapolated to estimate the effect of the vaccine on diarrhoea mortality attributable to rotavirus.
This review was prompted and shaped by the needs of the computer-based Lives Saved Tool (LiST), which combines effectiveness estimates for maternal, neonatal and child health (MNCH) interventions with information about cause of death, coverage of interventions and population structure to estimate the impact of different intervention packages and coverage levels on child mortality in selected geographic areas over time. Evidence-based estimates of the effectiveness of MNCH interventions against mortality are a critical aspect of the LiST model. In that model, an increase in coverage levels for an intervention results in a reduction of deaths from one or more causes, or in reduction of the prevalence of a risk factor such as stunting. Therefore, the LiST reviews, including this study, and the grading process used were designed to provide estimates of the effect of an intervention in reducing either a risk factor or a death due to specific cause. For more details of the review methods, the adapted GRADE approach, or the LiST model, see other articles in this supplement.
Methods
According to child health epidemiology reference group (CHERG) guidelines,18 we searched published literature from PubMed, the Cochrane Libraries and all World Health Organization Regional Databases to identify studies examining the effect of rotavirus vaccine on diarrhoea morbidity and mortality in children aged <5 years. We used various combinations of rotavirus vaccine, efficacy and effectiveness, and included publications of any language. We also contacted subject matter experts to ascertain the status of ongoing efficacy and effectiveness studies in Asia, Africa and Latin America and included data from these studies where full manuscripts and author permissions were available.
Inclusion and exclusion criteria
We included Phase III efficacy trials and post-market efficacy or effectiveness studies of currently marketed rotavirus vaccines that reported one or more of the following outcomes in children aged <5 years: all-cause mortality; diarrhoea- or rotavirus-specific mortality; diarrhoea- or rotavirus-specific hospitalizations; or incidence or risk of rotavirus or diarrhoeal disease. Pre-clinical, Phase I and Phase II trials were excluded, as were trials of vaccines were not marketed as of January 2009. Thus, our review excluded trials of the withdrawn rhesus rotavirus vaccine. The included studies evaluated two vaccines: a pentavalent human-bovine reassortant rotavirus vaccine and a monovalent attenuated human rotavirus vaccine.
Abstraction and analysis
Studies meeting our inclusion/exclusion criteria were categorized according to outcome, and key variables from each were abstracted into standardized tables. These variables were used to grade each study according to the CHERG adaptation of the GRADE technique.18
In a separate standardized table, we summarized the evidence by outcome and study design, including study quality, generalizability and summary outcome measures. For each outcome/study design category with more than one study, we performed both fixed and random effects meta-analyses and reported the Mantel–Haenszel pooled relative risk and corresponding 95% confidence interval (CI) or, if there was evidence of heterogeneity, the DerSimonian–Laird pooled relative risk and 95% CI. The meta-analyses of efficacy trials included only efficacy estimates based on at least 2 years or two rotavirus seasons of follow-up. For efficacy trials that reported both intent-to-treat and per protocol analyses, we used only the per protocol results in the meta-analysis, as this approach allowed us to include the greatest number of studies. However, we used the results of intent-to-treat analysis for the effectiveness data, as this approach provided a better measure of the potential impact of the vaccine when implemented under routine conditions. We did not perform a meta-analysis for the effectiveness studies, as the settings and circulating rotavirus strains were too different to assume a common underlying effect. All analyses were performed using STATA 10.1 statistical software.19
Results
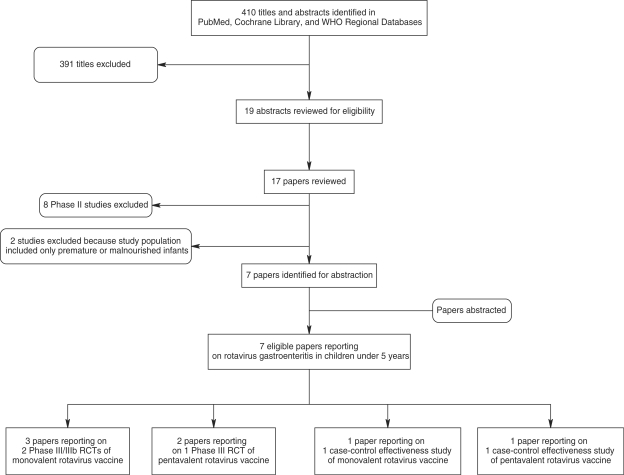
We screened 410 titles and abstracts identified through literature searches and contacts with subject area experts. Of these, we reviewed 17 papers and included seven in our final database (Figure 1). The included three Phase III clinical trials, of which two used the monovalent vaccine and one used the pentavalent vaccine, and two case–control effectiveness studies. The Phase III trials included European and Latin American sites for both vaccines, as well as US (including Navajo and Apache populations) and Taiwanese sites for the pentavalent vaccine. The effectiveness studies of the monovalent and pentavalent vaccines were conducted in northern Australia and Nicaragua, respectively. We identified studies reporting rotavirus hospitalizations (n = 5); diarrhoea hospitalizations (n = 4); severe rotavirus gastroenteritis (n = 4); severe diarrhoea (n = 2); and rotavirus gastroenteritis of any severity (n = 2) (Supplementary table 1). No studies reported any diarrhoea deaths.
Figure 1.
Rotavirus search process
Table 1 presents the summary characteristics and meta-analysis results for each outcome. The efficacy of the vaccine against serious rotavirus morbidity in vaccinated infants, compared with placebo, ranged from 89% (78–95%) for severe rotavirus to 93% (77–98%) for rotavirus hospitalization. The effectiveness of the pentavalent vaccine in Latin America was somewhat reduced compared with the efficacy estimates; the vaccine was 74% (35–90%) effective against very severe rotavirus, 61% (38–75%) effective against severe rotavirus, and 47% (22–64%) effective against rotavirus hospitalizations. Similarly, the monovalent vaccine was 57% (<0–83%) effective against rotavirus hospitalizations in a predominantly indigenous population in northern Australia.20 In accordance with the CHERG Rules, in the absence of mortality data, we used effectiveness against serious morbidity, i.e. very severe rotavirus, which is based on a clinical scoring system to ascertain the most severe cases and those most likely to result in death, to estimate the effect of rotavirus vaccine on the fraction of diarrhoea mortality attributable to rotavirus. Although this estimate comes from one study, because it is an effectiveness study, it is believed that it would more closely represent the results that might be observed when rotavirus is scaled up in a community setting. The estimated effect of the vaccine on rotavirus mortality in children aged <5 years was thus 74% (35–90%) (Figure 1).
Table 1.
Quality assessment of rotavirus vaccine trials
| Quality assessment |
Summary of findings |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Directness |
No. of events |
Effect | |||||||
| No. of studies | Design | Limitations | Consistency | Generalizability to population of interest | Generalizability to intervention of interest | Intervention | Control | Relative reduction (95% CI) | Comments |
| Effectiveness against very severe rotavirus infection (moderate/low outcome-specific quality) | |||||||||
| One29 | Matched case control | Hospital-based surveillance for cases (−0.5) | NA | Urban and peri-urban hospitals in Nicaragua (−0.5) | Pentavalent vaccine (−0.5) | 43 | 255 | 74% (35–90%) | |
| Effectiveness against severe rotavirus infection (moderate/low outcome-specific quality) | |||||||||
| One29 | Matched case control | Hospital-based surveillance for cases (−0.5) | NA | Urban and peri-urban hospitals in Nicaragua (−0.5) | Pentavalent vaccine (−0.5) | 155 | 926 | 61% (38–75%) | |
| Effectiveness against rotavirus hospitalizations (moderate outcome-specific quality) | |||||||||
| One29 | Matched case control | None | NA | Urban and peri-urban hospitals in Nicaragua (−0.5) | Pentavalent vaccine (−0.5) | 216 | 1250 | 47% (22–64%) | |
| One20 | Matched case control | None | NA | Rural hospital in the Northern Territory of Australia | Monovalent vaccine (−0.5) | 10 | 58 | 57% (<0–83%) | |
| Efficacy against severe rotavirus infection (moderate outcome-specific quality) | |||||||||
| Three4,30,31 | RCT | None | Heterogeneity from meta-analysis (−0.5); all studies show benefit | USA, Europe and Latin America | Two of three studies used monovalent vaccine. No co-interventions with potential to impact rotavirus outcomes | 59 | 358 | 89.1% (77.9–94.6%) | Random effects meta-analysis |
| Efficacy against severe GI infection (moderate outcome-specific quality) | |||||||||
| Two30,31 | RCT | None | Borderline heterogeneity from meta-analysis (P = 0.07); all studies show benefit | Europe and Latin America | All studies used monovalent vaccine. No co-interventions with potential to impact rotavirus outcomes | 598 | 808 | 44.2% (32.8–53.7%) | Random effects meta-analysis |
| Efficacy against rotavirus hospitalizations (moderate outcome-specific quality) | |||||||||
| Three4,30,31 | RCT | None | Heterogeneity from meta-analysis (−0.5); all studies show benefit | USA, Europe and Latin America | Two of three studies used monovalent vaccine; one used pentavalent. No co-interventions with potential to impact rotavirus outcomes | 30 | 290 | 92.7% (77.2–97.6%) | Random effects meta-analysis |
| Efficacy against GI hospitalizations (moderate outcome-specific quality) | |||||||||
| Three4,30,31 | RCT | None | Heterogeneity from meta-analysis (−0.5); all studies show benefit | USA, Europe and Latin America | Two of three studies used monovalent vaccine; one used pentavalent. No co-interventions with potential to impact rotavirus outcomes | >292 | >477 | 56% (39–73%) | Random effects meta-analysis |
| Efficacy against any rotavirus (moderate outcome-specific quality) | |||||||||
| Two4,31 | RCT | None | Heterogeneity from meta-analysis (−0.5); all studies show benefit | USA, Europe and Latin America | One of two studies used monovalent vaccine. No co-interventions with potential to impact rotavirus outcomes | 203 | 607 | 74.4% (63.2–82.2%) | Random effects meta-analysis |
RCT: randomized controlled trial.
Discussion
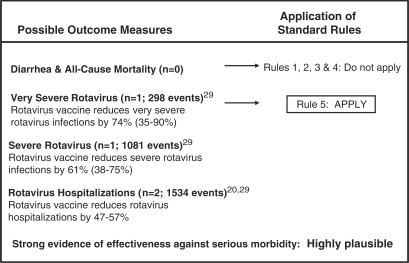
Diarrhoeal diseases continue to kill ∼1.7 million children aged <5 years annually,2 despite the existence of highly efficacious interventions for the treatment of diarrhoea.21–23 We estimated that currently marketed rotavirus vaccines could prevent 74% (95% CI: 35–90%) of rotavirus deaths and 47–57% of rotavirus hospitalizations (Figure 2).
Figure 2.
Application of standardized rules for choice of final outcome to estimate the effect of rotavirus vaccine on rotavirus-specific mortality
We based our estimate on effectiveness rather than efficacy data because this approach provides a better measure of the potential impact of the vaccine on rotavirus deaths when implemented under routine conditions. We used very severe rotavirus rather than rotavirus hospitalizations as a proxy for mortality because hospitalizations include both severe and non-severe cases, and rotavirus severity is based on a well-defined scale that can be applied consistently across settings, whereas rotavirus hospitalization rates may vary according to local clinical guidelines. However, because effectiveness against severe rotavirus has been reported only for pentavalent vaccine in Nicaragua, the generalizability of this estimate is limited.
Evidence suggests that rotavirus vaccine efficacy may vary by setting, due to regional differences in circulating rotavirus vaccine strains,24 and reduced efficacy of oral vaccines in settings with a high prevalence of malnutrition and gastrointestinal infections.25,26 Additional efficacy trials of both vaccines are underway in low-income African and Asian settings where malnutrition and gastrointestinal infections are highly prevalent. Preliminary efficacy data from Phase III trials of the monovalent rotavirus vaccine in Malawi and South Africa support some regional differences in vaccine efficacy, showing 77% vaccine efficacy against severe rotavirus infection in South Africa and 50% in Malawi.27 These estimates are somewhat lower than those seen in earlier trials of monovalent vaccine in developed countries and Latin America.27 Peter et al.27 have suggested that rotavirus vaccine efficacy data may be extrapolated to settings with similar under-five mortality rates. Given much higher under-five mortality rates in most sub-Saharan and South Asian countries compared with the middle- and high-income settings in which the earlier studies were conducted, it may not be appropriate to apply our estimated mortality effect to these regions; separate analyses and estimates will be conducted for these high-mortality populations when the complete results are published.
Our estimated effect size for rotavirus deaths is based on effectiveness data for the pentavalent rotavirus vaccine only. The monovalent and pentavalent vaccines differ in important ways, including their origins and valencies, which could limit the generalizability of our effectiveness estimate to the monovalent vaccine. An unpublished effectiveness study of the monovalent vaccine in El Salvador, however, reported identical effectiveness against severe rotavirus (74%),28 suggesting that our effectiveness estimate may be more broadly applicable to both vaccines in this region.
Taken together, the effectiveness and efficacy data for both rotavirus vaccines provide sufficient evidence to conclude that rotavirus vaccines are highly efficacious and effective in preventing severe rotavirus episodes and rotavirus deaths in children aged <5 years in developed countries and Latin America. Rotavirus vaccines, thus, have the potential to greatly reduce the fraction of diarrhoea deaths attributable to rotavirus if high coverage levels are achieved for the recommended number of doses. Although it would not be appropriate to suggest that the effect size observed in Latin America mirrors what we can anticipate observing in Africa and Asia, some countries may chose to use this estimate as a proxy until regional specific data become available. Additional data from efficacy trials in Asia and Africa, further effectiveness studies and impact evaluations for rotavirus vaccine introduction in Latin America will be critical in strengthening the evidence base for rotavirus vaccine, clarifying regional differences in vaccine efficacy and confirming its effectiveness globally.
Supplementary data
Supplementary data are available at IJE online.
Funding
US Fund for UNICEF from the Bill and Melinda Gates Foundation (grant 43386 to ‘Promote evidence-based decision making in designing maternal, neonatal and child health interventions in low- and middle-income countries’). MKM is supported by a training grant from the U.S. National Institutes of Health (grant T32HD046405 for ‘International Maternal and Child Health’)
Acknowledgements
We thank Drs Manish Patel and Umesh Parashar (Centers for Disease Control and Prevention) for sharing with us a copy of their manuscript pre-publication, conducting additional data analyses and reviewing the draft manuscript. We are also grateful to colleagues at WHO and UNICEF for their review of the manuscript and valuable feedback.
Conflict of interest: None declared.
KEY MESSAGES.
Five large-scale high-quality studies provide evidence of the efficacy and effectiveness of rotavirus vaccines against severe rotavirus infection and hospitalization.
Rotavirus vaccines may prevent ∼74% of rotavirus deaths.
Studies are underway in high-mortality, low-income countries to further validate this estimate in these settings.
References
- 1.Parashar UD, Gibson CJ, Bresse JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–6. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–52. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 4.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 5.Soares-Weiser K, Goldberg E, Tamimi G, Pitan OC, Leibovici L. Rotavirus vaccine for preventing diarrhoea. Cochrane Database Syst Rev. 2004;(1):CD002848. doi: 10.1002/14651858.CD002848.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein DI. Live attenuated human rotavirus vaccine, Rotarix. Semin Pediatr Infect Dis. 2006;17:188–94. doi: 10.1053/j.spid.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Caple J. Pentavalent human-bovine reassortant rotavirus vaccine: a review of its efficacy and safety in preventing acute rotavirus gastroenteritis in healthy infants. Drugs Today. 2006;42:313–19. doi: 10.1358/dot.2006.42.5.973591. [DOI] [PubMed] [Google Scholar]
- 8.Clark HF, Offit PA, Plotkin SA, Heaton PM. The new pentavalent rotavirus vaccine composed of bovine (strain WC3)-human rotavirus reassortants. Pediatr Infect Dis J. 2006;25:577–83. doi: 10.1097/01.inf.0000220283.58039.b6. [DOI] [PubMed] [Google Scholar]
- 9.Keating GM. Rotavirus vaccine RIX4414 (Rotarix) Paediatr Drugs. 2006;8:389–95. doi: 10.2165/00148581-200608060-00006. discussion 396–97. [DOI] [PubMed] [Google Scholar]
- 10.Keating GM. Rotavirus vaccine (RotaTeq) Paediatr Drugs. 2006;8:197–202. doi: 10.2165/00148581-200608030-00008. discussion 203–4. [DOI] [PubMed] [Google Scholar]
- 11.Matson DO. The pentavalent rotavirus vaccine, RotaTeq. Semin Pediatr Infect Dis. 2006;17:195–99. doi: 10.1053/j.spid.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 12.McCormack PL, Keam SJ, Bernstein DI, et al. Rotavirus vaccine RIX4414 (Rotarix): a review of its use in the prevention of rotavirus gastroenteritis. Paediatr Drugs. 2009;11:75–88. doi: 10.2165/0148581-200911010-00025. [DOI] [PubMed] [Google Scholar]
- 13.Offit PA, Clark HF. RotaTeq: a pentavalent bovine–human reassortant rotavirus vaccine. Pediatr Ann. 2006;35:29–34. doi: 10.3928/0090-4481-20060101-11. [DOI] [PubMed] [Google Scholar]
- 14.O’Ryan M. Rotarix (RIX4414): an oral human rotavirus vaccine. Expert Rev Vaccines. 2007;6:11–19. doi: 10.1586/14760584.6.1.11. [DOI] [PubMed] [Google Scholar]
- 15.Tom-Revzon C. Rotavirus live, oral, pentavalent vaccine. Clin Ther. 2007;29:2724–37. doi: 10.1016/j.clinthera.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Vesikari T. Rotavirus vaccines. Scand J Infect Dis. 2008;40:691–95. doi: 10.1080/00365540802040570. [DOI] [PubMed] [Google Scholar]
- 17.Ward RL, Bernstein DI. Rotarix: a rotavirus vaccine for the world. Clin Infect Dis. 2009;48:222–28. doi: 10.1086/595702. [DOI] [PubMed] [Google Scholar]
- 18.Walker N, Fischer Walker CL, Bahl R, et al. Standards for CHERG reviews of intervention effects on child survival. Int J Epidemiol. 2010;39(Suppl 1):i21–31. doi: 10.1093/ije/dyq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- 20.Snelling TL, Schultz R, Graham J, et al. Rotavirus and the indigenous children of the Australian outback: monovalent vaccine effective in a high-burden setting. Clin Infect Dis. 2009;49:428–31. doi: 10.1086/600395. [DOI] [PubMed] [Google Scholar]
- 21.Fischer Walker CL, Black RE. Zinc for the treatment of diarrhea: effect on diarrhea mortality, severe morbidity and diarrhea incidence. Int J Epidemiol. 2010;39(Suppl 1):i63–69. doi: 10.1093/ije/dyq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munos MK, Fischer Walker CL, Black RE. The effect of oral rehydration solution and recommended home fluids on diarrhea mortality. Int J Epidemiol. 2010;39(Suppl 1):i75–87. doi: 10.1093/ije/dyq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Traa BS, Fischer Walker CL, Munos M, Black RE. Antibiotics for the treatment of dysentery in children. Int J Epidemiol. 2010;39(Suppl 1):i70–74. doi: 10.1093/ije/dyq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gentsch JR, Laird AR, Bielfelt B, et al. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. J Infect Dis. 2005;192(Suppl 1):S146–59. doi: 10.1086/431499. [DOI] [PubMed] [Google Scholar]
- 25.Bresee JS, Parashar UD, Widdowson MA, et al. Update on rotavirus vaccines. Pediatr Infect Dis J. 2005;24:947–52. doi: 10.1097/01.inf.0000186295.18969.e6. [DOI] [PubMed] [Google Scholar]
- 26.Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. 1991;13:926–39. doi: 10.1093/clinids/13.5.926. [DOI] [PubMed] [Google Scholar]
- 27.Peter G, Aguado T, Bhutta Z, et al. Detailed Review Paper on Rotavirus Vaccines. Presented to the WHO Strategic Advisory Group of Experts (SAGE) on Immunization, April 2009. [Google Scholar]
- 28.Meeting of the immunization Strategic Advisory Group of Experts. April 2009–conclusions and recommendations. Wkly Epidemiol Rec. 2009;84:220–36. [PubMed] [Google Scholar]
- 29.Patel M, Pedreira C, De Oliveira LH, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. J Am Med Assoc. 2009;301:2243–51. doi: 10.1001/jama.2009.756. [DOI] [PubMed] [Google Scholar]
- 30.Linhares AC, Velazquez FR, Perez-Schael I, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–89. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- 31.Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370:1757–63. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]