Abstract
Background Zinc supplementation for the treatment of diarrhoea has been shown to decrease the duration and severity of the diarrhoeal episode, diarrhoea hospitalization rates and, in some studies, all-cause mortality. Using multiple outcome measures, we sought to estimate the effect of zinc for the treatment of diarrhoea on diarrhoea mortality and subsequent pneumonia mortality.
Methods We conducted a systematic review of efficacy and effectiveness studies. We used a standardized abstraction and grading format and performed meta-analyses for all outcomes with ≥2 data points. The estimated effect on diarrhoea mortality was determined by applying the standard Child Health Epidemiology Reference Group rules for multiple outcomes.
Results We identified 13 studies for abstraction. Zinc supplementation decreased the proportion of diarrhoeal episodes which lasted beyond 7 days, risk of hospitalization, all-cause mortality and diarrhoea mortality. Using diarrhoea hospitalizations as the closest and most conservative possible proxy for diarrhoea mortality, zinc for the treatment of diarrhoea is estimated to decrease diarrhoea mortality by 23%.
Conclusion Zinc is an effective therapy for diarrhoea and will decrease diarrhoea morbidity and mortality when introduced and scaled-up in low-income countries.
Keywords: Diarrhoea, mortality, treatment, zinc
Background
Zinc for the treatment of diarrhoea has been recommended by the World Health Organization (WHO) and United Nations Children’s Fund (UNICEF) since 2004,1 yet access to this essential treatment remains limited. When given for 10–14 days during and following the diarrhoeal episode, zinc has been shown to decrease the duration and severity of the episode,2 as well as decrease the incidence of diarrhoea and pneumonia episodes in the subsequent 2–3 months.3 Early studies that found a reduction in the duration and severity of the episode were conducted in diarrhoea treatment clinics and inpatient settings.4 In later large-scale studies, investigators randomized entire communities to include zinc in addition to oral rehydration solution (ORS) or ORS alone5,6 and observed similar effects on diarrhoea duration, pneumonia incidence as well as reductions in hospitalizations and mortality that smaller individually randomized controlled trials (RCTs) had not been designed to detect.
Very few studies have been designed or powered to detect differences in all-cause mortality or diarrhoea-specific mortality. Although cause-specific mortality data are the ideal when estimating the possible effect of an intervention on saving lives, for diarrhoea, there are additional outcomes which can serve as adequate proxies, such as hospitalization and prolonged diarrhoea.7,8 Diarrhoea treated promptly in the home to prevent and treat dehydration rarely becomes lethal; therefore, by including outcomes such as diarrhoea hospitalizations and episodes lasting beyond 7 days, we are able to focus on the episodes which are more severe and thus far more likely to result in death.
This systematic review of the effect of zinc for diarrhoea treatment has been designed to meet the needs of the Lives Saved Tool (LiST) and has therefore been designed differently than the previously published traditional systematic reviews.9–11 In LiST, increases in coverage of an intervention result in a reduction of one or more causes of mortality. Therefore, the systematic review and methods presented here, as well as the GRADE process as outlined in this journal supplement,12 were designed to develop estimates of the effect of an intervention in reducing death due to diarrhoea.
Together with low-osmolarity ORS and continued feeding, zinc promises to reduce diarrhoea morbidity and mortality and have additional benefits on pneumonia morbidity and mortality in the 2–3 months following treatment.13,14 Although there have been numerous systematic reviews and meta-analyses summarizing the effect of zinc supplementation,2,3,15,16 none have considered the effect of zinc when given as diarrhoea therapy on diarrhoea mortality. Here we present the evidence supporting this claim as well as the evidence suggesting a reduction in subsequent diarrhoea episodes.
Methods
We systematically reviewed all published literature from 1990 to 2009 to identify studies of zinc supplementation for the treatment of acute and persistent diarrhoea amongst children younger than 5 years of age. As per the Child Health Epidemiology Reference Group (CHERG) systematic review guidelines,12 we searched PubMed, Cochrane Libraries and all WHO regional databases and included publications in every language available in these databases. The initial searches were conducted on 31 January 2009 and updated on 15 October 2009. We used the Medical Subject Heading Terms (MeSH) and keywords-search strategies using various combinations of: zinc, treatment, and diarrhoea. Every effort was made to gather unpublished data when reports were available for full abstraction. Studies were included if data from one of the following outcomes was provided: all-cause mortality, diarrhoea mortality, diarrhoea hospitalizations, pneumonia hospitalizations, prolonged diarrhoea (episode lasting >7 days), diarrhoea and pneumonia incidence in the period up to 3 months following treatment. All outcome measures to be included were determined a priori.
Inclusion/exclusion criteria
We limited the search to RCT studies conducted in low- and middle-income countries (LMICs) where zinc was given as a diarrhoea treatment for ≥7 days to infants and children between 1 and 59 months of age.17 Studies were included if zinc was given alone or in combination with vitamins. Studies that provided iron were excluded because iron is known to interfere with zinc absorption and iron-containing formulations are not recommended for the treatment of diarrhea.18 All included studies contained a placebo or a suitable control group that was identical to the experimental group, except that it did not contain zinc. Studies conducted ‘solely’ in special populations (i.e. only cholera patients, etc.) were excluded. The zinc dose in included studies was between 10 and 40 mg/day which is in line with the WHO 2004 recommendation of 20 mg/day for 6–59 months and 10 mg/day for 1–5 months.1 Acceptable formulations included syrups and tablets. Studies of zinc-fortified ORS were excluded because the zinc dose does not meet WHO guidelines for daily dose or minimal treatment days.
Abstraction, analyses and summary measures
All studies which met final inclusion and exclusion criteria were double-data abstracted into a standardized form for each outcome of interest.12 We abstracted key variables with regard to the study identifiers and context, study design and limitations, intervention specifics and outcome effects. Each study was assessed and graded according to the CHERG adaptation of the GRADE technique.19 Studies received an initial score of high if they were RCTs or cluster-RCTs (cRCTs). The grade was decreased one grade for each study-design limitation. In addition, studies reporting an intent-to-treat analysis or with statistically significant strong levels of association (>80% reduction) received 0.5–1.0 grade increase. Any study with a final grade of very low was excluded on the basis of inadequate study quality.
For any outcome with more than one study we conducted a meta-analysis and reported the Mantel–Haenszel pooled relative risk and corresponding 95% confidence interval (CI) or the DerSimonian–Laird pooled relative risk and corresponding 95% CI where there was unexplained heterogeneity such as major differences in study design.12 All analyses were conducted using STATA 9.0 statistical software.20
We summarized the evidence based on outcome by including assessment of the study quality and quantitative measures according to standard guidelines12 for each outcome. For the outcome of interest, namely the effect of zinc for the treatment of diarrhoea on the reduction of diarrhoea mortality, we applied the CHERG Rules for Evidence Review12 to the collective diarrhoea morbidity and mortality outcomes to generate a final estimate for reduction in diarrhoea mortality and pneumonia mortality.
Results
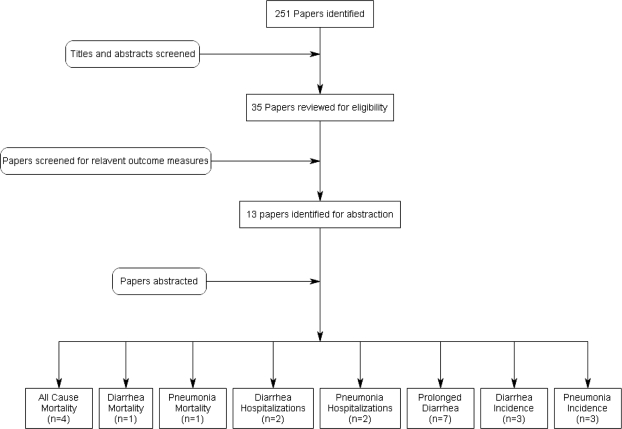
We identified 251 titles from searches conducted in all databases (Figure 1). After initial screening of titles and abstracts we reviewed 35 papers for the identified outcome measures of interest and included 13 papers in the final database. To estimate the effect of zinc for diarrhoea treatment on diarrhoea mortality, we found four studies which reported data on all-cause mortality,4,5,21,22 one study which reported diarrhoea-specific mortality rates,5 two studies which reported diarrhoea hospitalization rates5,6 and seven studies which reported data on prolonged diarrhoea (≥7 days)23–29 (Supplementary Table 1). To estimate the effect of zinc for diarrhoea treatment on diarrhoea incidence, we found three studies which reported data on diarrhoea incidence.5,6,21 To estimate the effect of zinc for diarrhoea treatment on pneumonia morbidity and mortality in the months following treatment, we found one study which reported data on pneumonia mortality,5 two studies which reported data on pneumonia hospitalizations5,6 and three studies which reported data on pneumonia point prevalence.5,6,21 All abstracted studies were either blinded, randomized controlled treatment trials or cluster-randomized intervention trials. There were very few limitations based on study design and execution; one study included infants 1–5 months of age,21,25 one study had <6 clusters per study arm6 and several studies included daily zinc doses less than the WHO recommendation for children ≥6 months.23,24,28,29
Figure 1.
Synthesis of study identification in review of the effects of zinc for the treatment of diarrhoea on all-cause mortality, diarrhoea mortality, diarrhoea hospitalization and prolonged diarrhoea. (Final number of papers reported by outcome; thus one paper may be counted for more than one outcome).
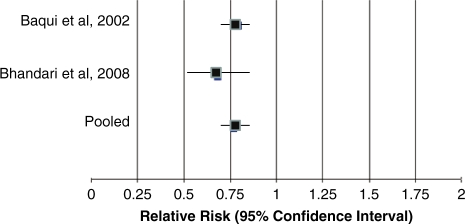
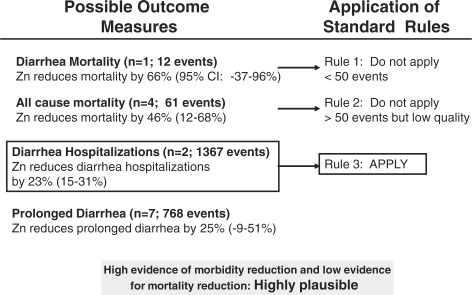
In Table 1 we report the quality assessment of studies by outcome, as well as results from corresponding meta-analyses. Of the four outcomes related to diarrhoea mortality, the effect size ranged from 23% (Figure 2) for diarrhoea hospitalizations to 66% for diarrhoea mortality.5 We applied the CHERG Rules for Evidence Review to these four outcomes. Because diarrhoea mortality data were limited (fewer than 50 deaths) we used a severe morbidity outcome to estimate the effect on mortality. The two large cRCTs reported a 23% difference in hospitalization rates for >300 hospitalizations (Figure 3).
Table 1.
Quality assessment of trials of zinc for the treatment of diarrhea
| Quality assessment |
Summary of findings |
|||||||
|---|---|---|---|---|---|---|---|---|
| Directness |
Number of events |
|||||||
| Number of studies (ref.) | Design | Limitations | Consistency | Generalizability to population of interest | Generalizability to intervention of interest | Intervention | Control | RR (95% CI) |
| Mortality (diarrhoea deaths): moderate/low outcome-specific quality | ||||||||
| 15 | cRCT | None | Not statistically significant (−0.5) | Only 1 study (−0.5) | Cannot separate zinc and ORS (−0.5) | 3 | 9 | 66% (−37, 96%)a |
| Mortality (all cause): low outcome-specific quality | ||||||||
| 44,5,15,16 | RCT | None | Consistent and all 4 studies showing benefit | Mostly Asia (−0.5) | Cannot separate zinc and ORS (−0.5) | 21 | 49 | 46% (12, 68%)b |
| Diarrhea hospitalizations: Moderate outcome-specific quality | ||||||||
| 25,6 | cRCT | None | Consistent and both studies showing benefit | All Asia (−0.5) | Cannot separate zinc and ORS (−0.5) | 583 | 784 | 23% (15, 31%)b |
| Diarrhoea duration (>7 days): moderate/low outcome specific quality | ||||||||
| 723–29 | RCT | 4 of 7 included dose < WHO recommendation (−0.5) | Heterogeneity from meta-analysis; 5 of 7 studies show benefit (−0.5) | 2 studies had specialized populations (−0.5) | 346 | 422 | 25% (−9, 49%)c | |
| Diarrhoea prevalence (1–2 weeks): moderate outcome specific quality | ||||||||
| 35,6,15 | cRCT/RCT | None | Heterogeneity from meta-analysis; 2 of 3 studies show benefit (−0.5) | Mostly Asia (−0.5) | 5261 | 6899 | 19% (−4, 47%)c | |
| Mortality (pneumonia deaths): moderate outcome specific quality | ||||||||
| 15 | cRCT | None | Not statistically significant (−0.5) | Only 1 study (−0.5) | 7 | 10 | 28% (−109, 77%)a | |
| Pneumonia hospitalizations: moderate/low outcome specific quality | ||||||||
| 25,6 | cRCT | None | Heterogeneity from meta-analysis; Both studies show benefit; not statistically significant (−1.0) | Mostly Asia (−0.5) | 428 | 830 | 50% (−39, 82%)c | |
| ALRI prevalence: moderate/low outcome specific quality | ||||||||
| 35,6,15 | cRCT/RCT | None | Heterogeneity from meta-analysis; 2 of 3 studies show benefit; not statistically significant (-1.0) | Mostly Asia (−0.5) | 1786 | 2155 | 23% (−25, 53%)c | |
RCT, randomized controlled trial; RR, relative risk.
aDirectly calculated from study results.
bMH pooled RR.
cD & L pooled RR random effect meta-analysis.
Figure 2.
Forest plot for the effect of zinc for the treatment of diarrhoea on diarrhoea hospitalizations.
Figure 3.
Application of standardized rules for choice of final outcome to estimate effect of zinc on the reduction of diarrhoea mortality.
There are two large-scale studies among children of all ages and one smaller study among infants 1–5 months of age which demonstrated that zinc given as a treatment for diarrhoea may decrease diarrhoea prevalence by 19% and the prevalence of severe acute lower respiratory infection (ALRI)/pneumonia episodes in the months following supplementation by 23%.5,6,21 In addition, the two large-scale effectiveness studies found that the introduction of zinc led to a decrease of pneumonia hospitalizations by 50%. However, these estimates are not statistically significant and thus, at this point in time, it is not possible to conclude that there is an evidence of benefit.
Conclusions
Diarrhoea remains the second leading cause of death among children under 5 in the developing world.30 In our systematic review, 11 of the 13 studies we identified reported results suggesting a benefit of zinc on severe diarrhoea morbidity and mortality outcomes. Applying the CHERG Rules for Evidence Review to reviewed studies and assessment of multiple morbidity and mortality outcomes, we estimate that zinc for the treatment of diarrhoea will reduce diarrhoea mortality by 23%. With >1000 hospitalizations, there is less uncertainty in the effect of zinc on this outcome as compared to the mortality outcomes. In addition, the effect size associated with diarrhoea hospitalizations (23% reduction) is more conservative than the observed reduction in all-cause or cause-specific mortality and prolonged diarrhoea as observed in similar studies.5,6 Because all estimates are consistent, this increases confidence that 23% is a realistic estimate for a reduction in mortality that we would expect to observe when scaling up zinc for the treatment of diarrhoea.
Because this estimate was not derived solely from mortality data, it has some limitations. The two large cRCTs,5,6 which contributed the hospitalization data were effectiveness studies; therefore, coverage was not 100%. Although coverage did reach relatively high levels (>80% in Bangladesh within 7 months), there were no adjustments made to the results to account for the <100% coverage rates which would make the effect estimates conservative. However, it could also be argued that these were still in fact studies and thus countries rolling out programmes in larger communities may not achieve the same effect because of poorer compliance, insufficient training of health-care providers or other implementation obstacles.
One additional limitation is the inability to completely separate the effect of zinc from the effect of ORS in the large-scale effectiveness trials. In these studies the introduction of zinc also increased ORS-use rates in the intervention communities. Although programmatically ideal, it is not possible to separate the effect of zinc from the effect of ORS on diarrhoea hospitalization and mortality. However, in the six studies we reviewed, zinc decreased prolonged diarrhoea by 33%. This estimate is generated from blinded trials where ORS use was constant in the zinc and control groups and thus the zinc effect more accurately represents the added benefit of zinc supplementation. For these reasons the 23% reduction would not appear to overestimate the effect of zinc on diarrhoea mortality.
In addition to the effect on the duration and severity of the treated episode, zinc has been shown to decrease diarrhoea prevalence in both 24-h and 2-week recall surveys. Although in our review we found a 19% reduction in diarrhoea prevalence following the treated episode, the large-scale studies from which the weight of this pooled estimate is derived did not conduct prevalence surveys solely among children receiving zinc vs those not receiving zinc. This effect would logically be greater than that measured simply in all children residing in communities where zinc was available. Thus, the 19% reduction could be a conservative estimate of the preventive benefits on diarrhoea prevalence. Some studies have found that when used as a diarrhoea treatment, zinc also has a preventive effect on future pneumonia morbidity and mortality but these effects are not statistically significant; therefore, definitive conclusions with regard to an effect size cannot be made at this time.
Zinc supplementation has been proven to decrease diarrhoea morbidity and mortality and is currently recommended as an adjunct treatment for all diarrhoea episodes.1 The methods by which we derived a specific effect on diarrhoea mortality are novel, involve multiple outcomes and are inherently based on limitations in available data. Some will challenge the notion that providing an estimate for an effect on mortality based on anything but RCTs with mortality as an outcome should not be done. However, because of the strength of the evidence supporting zinc for diarrhoea treatment, RCTs are no longer ethical; thus the ideal data will likely not be available.
The methods and results we propose in this article are transparent and provide a conservative and comparable estimated effect size of zinc for the treatment of diarrhoea on diarrhoea mortality. Zinc for the treatment of diarrhoea is an important child-survival intervention and, in combination with ORS, is key for a reduction in overall child mortality.
Supplementary data
Supplementary data are available at IJE online.
Funding
This work was supported in part by a grant to the US Fund for UNICEF from the Bill & Melinda Gates Foundation (grant 43386) to “Promote evidence-based decision making in designing maternal, neonatal and child health interventions in low- and middle-income countries”.
KEY MESSAGES.
The evidence supporting zinc for the treatment of diarrhoea includes 12 high-quality randomized efficacy and effectiveness trials with demonstrated reductions in severe morbidity and mortality.
Zinc for the treatment of diarrhoea reduces diarrhoea mortality by 23%.
When given as diarrhoea treatment, zinc supplementation not only decreases the severity of the initial episode, but may prevent future diarrhoeal episodes in the 2–3 months following supplementation.
Acknowledgements
We thank our colleagues at WHO and UNICEF for their review of the manuscript and valuable feedback.
Conflict of interest: None declared.
References
- 1.WHO/UNICEF. Joint Statement: Clinical Management of Acute Diarrhoea (WHO/FCH/CAH/04.07) Geneva and New York: World Health Organization, Department of Child and Adolescent Health and Development, and United Nations Children's Fund, Programme Division; 2004. [Google Scholar]
- 2.Zinc Investigators' Collaborative Group. Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: pooled analysis of randomized controlled trials. Am J Clin Nutr. 2000;72:1516–22. doi: 10.1093/ajcn/72.6.1516. [DOI] [PubMed] [Google Scholar]
- 3.Zinc Investigators' Collaborative Group. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. Zinc Investigators' Collaborative Group. J Pediatr. 1999;135:689–97. doi: 10.1016/s0022-3476(99)70086-7. [DOI] [PubMed] [Google Scholar]
- 4.Roy SK, Tomkins AM, Mahalanabis D, et al. Impact of zinc supplementation on persistent diarrhoea in malnourished Bangladeshi children. Acta Paediatr. 1998;87:1235–39. doi: 10.1080/080352598750030898. [DOI] [PubMed] [Google Scholar]
- 5.Baqui AH, Black RE, el Arifeen S, et al. Effect of zinc supplementation started during diarrhoea on morbidity and mortality in Bangladeshi children: community randomised trial. BMJ. 2002;325:1059. doi: 10.1136/bmj.325.7372.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhandari N, Mazumder S, Taneja S, et al. Effectiveness of zinc supplementation plus oral rehydration salts compared with oral rehydration salts alone as a treatment for acute diarrhea in a primary care setting: a cluster randomized trial. Pediatrics. 2008;121:e1279–85. doi: 10.1542/peds.2007-1939. [DOI] [PubMed] [Google Scholar]
- 7.Bhan MK, Arora NK, Ghai OP, et al. Major factors in diarrhoea related mortality among rural children. Indian J Med Res. 1986;83:9–12. [PubMed] [Google Scholar]
- 8.Griffin PM, Ryan CA, Nyaphisi M, et al. Risk factors for fatal diarrhea: a case-control study of African children. Am J Epidemiol. 1988;128:1322–29. doi: 10.1093/oxfordjournals.aje.a115085. [DOI] [PubMed] [Google Scholar]
- 9.Lazzerini M, Ronfani L. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev. 2008:CD005436. doi: 10.1002/14651858.CD005436.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Patro B, Golicki D, Szajewska H. Meta-analysis: zinc supplementation for acute gastroenteritis in children. Aliment Pharmacol Ther. 2008;28:713–23. doi: 10.1111/j.1365-2036.2008.03787.x. [DOI] [PubMed] [Google Scholar]
- 11.Haider BA, Bhutta ZA. The effect of therapeutic zinc supplementation among young children with selected infections: a review of the evidence. Food Nutr Bull. 2009;30:S41–59. doi: 10.1177/15648265090301S104. [DOI] [PubMed] [Google Scholar]
- 12.Walker N, Fischer Walker CL, Bryce J, et al. Standards for CHERG reviews of intervention effects on child survival. Int J Epidemiol. doi: 10.1093/ije/dyq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones G, Steketee RW, Black RE, et al. How many child deaths can we prevent this year? Lancet. 2003;362:65–71. doi: 10.1016/S0140-6736(03)13811-1. [DOI] [PubMed] [Google Scholar]
- 14.WHO/UNICEF. Expert Consultation on Oral Rehydration Salts (Ors) Formulation. 2001. WHO/UNICEF: New York, 2001. [Google Scholar]
- 15.Fontaine O. Effect of zinc supplementation on clinical course of acute diarrhoea. J Health Popul Nutr. 2001;19:339–46. [PubMed] [Google Scholar]
- 16.Lazzerini M, Ronfani L. Cochrane Database, Systematic Review. 2008. Oral zinc for treating diarrhoea in children (Review) Issue 3:CD005436. [DOI] [PubMed] [Google Scholar]
- 17.World Bank. World Development Report 2004: Equity and Development. Washington, DC: 2006. [Google Scholar]
- 18.WHO. Implementing the New Recommendations on the Clinical Management of Diarrhoea, Guidelines for Policy Makers and Programme Managers. Geneva, 2006. [Google Scholar]
- 19.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.STATA 9.0 Statistical Program. 2005. College Station, TX: STATA Corporation; [Google Scholar]
- 21.Fischer Walker CL, Bhutta ZA, Bhandari N, et al. Zinc during and in convalescence from diarrhea has no demonstrable effect on subsequent morbidity and anthropometric status among infants <6 mo of age. Am J Clin Nutr. 2007;85:887–94. doi: 10.1093/ajcn/85.3.887. [DOI] [PubMed] [Google Scholar]
- 22.Roy SK, Tomkins AM, Akramuzzaman SM, et al. Impact of zinc supplementation on subsequent morbidity and growth in Bangladeshi children with persistent diarrhoea. J Health Popul Nutr. 2007;25:67–74. [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatnagar S, Bahl R, Sharma PK, et al. Zinc with oral rehydration therapy reduces stool output and duration of diarrhea in hospitalized children: a randomized controlled trial. J Pediatr Gastroenterol Nutr. 2004;38:34–40. doi: 10.1097/00005176-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Faruque AS, Mahalanabis D, Haque SS, et al. Double-blind, randomized, controlled trial of zinc or vitamin A supplementation in young children with acute diarrhoea. Acta Paediatr. 1999;88:154–60. doi: 10.1080/08035259950170312. [DOI] [PubMed] [Google Scholar]
- 25.Fischer Walker CL, Bhutta ZA, Bhandari N, et al. Zinc supplementation for the treatment of diarrhea in infants in Pakistan, India and Ethiopia. J Pediatr Gastroenterol Nutr. 2006;43:357–63. doi: 10.1097/01.mpg.0000232018.40907.00. [DOI] [PubMed] [Google Scholar]
- 26.Khatun UH, Malek MA, Black RE, et al. A randomized controlled clinical trial of zinc, vitamin A or both in undernourished children with persistent diarrhea in Bangladesh. Acta Paediatr. 2001;90:376–80. [PubMed] [Google Scholar]
- 27.Sazawal S, Black RE, Bhan MK, et al. Zinc supplementation in young children with acute diarrhea in India. N Engl J Med. 1995;333:839–44. doi: 10.1056/NEJM199509283331304. [DOI] [PubMed] [Google Scholar]
- 28.Strand TA, Chandyo RK, Bahl R, et al. Effectiveness and efficacy of zinc for the treatment of acute diarrhea in young children. Pediatrics. 2002;109:898–903. doi: 10.1542/peds.109.5.898. [DOI] [PubMed] [Google Scholar]
- 29.Patel A, Dibley MJ, Mamtani M, et al. Zinc and copper supplementation in acute diarrhea in children: a double-blind randomized controlled trial. BMC Med. 2009;7:22. doi: 10.1186/1741-7015-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bryce J, Boschi-Pinto C, Shibuya K, et al. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–52. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]