Abstract
Background In high-income countries, administration of antenatal steroids is standard care for women with anticipated preterm labour. However, although >1 million deaths due to preterm birth occur annually, antenatal steroids are not routine practice in low-income countries where most of these deaths occur.
Objectives To review the evidence for and estimate the effect on cause-specific neonatal mortality of administration of antenatal steroids to women with anticipated preterm labour, with additional analysis for the effect in low- and middle-income countries.
Methods We conducted systematic reviews using standardized abstraction forms. Quality of evidence was assessed using an adapted GRADE approach. Existing meta-analyses were reviewed for relevance to low/middle-income countries, and new meta-analysis was performed.
Results We identified 44 studies, including 18 randomised control trials (RCTs) (14 in high-income countries) in a Cochrane meta-analysis, which suggested that antenatal steroids decrease neonatal mortality among preterm infants (<36 weeks gestation) by 31% [relative risk (RR) = 0.69; 95% confidence interval (CI) 0.58–0.81]. Our new meta-analysis of four RCTs from middle-income countries suggests 53% mortality reduction (RR = 0.47; 95% CI 0.35–0.64) and 37% morbidity reduction (RR = 0.63; 95% CI 0.49–0.81). Observational study mortality data were consistent. The control group in these equivalent studies was routine care (ventilation and, in many cases, surfactant). In low-income countries, many preterm babies currently receive little or no medical care. It is plausible that antenatal steroids may be of even greater effect when tested in these settings.
Conclusions Based on high-grade evidence, antenatal steroid therapy is very effective in preventing neonatal mortality and morbidity, yet remains at low coverage in low/middle-income countries. If fully scaled up, this intervention could save up to 500 000 neonatal lives annually.
Keywords: Neonatal mortality, newborn care, preterm births, prematurity, low birth weight, antenatal steroids, respiratory distress syndrome, bethamethasone, dexamethasone
Background
Every year, ∼4 million newborns die, and the leading cause of death is direct preterm complications accounting for >1 million deaths.1 The most common cause of deaths among preterm babies is respiratory distress syndrome (RDS), an acute lung disease related to immaturity of the lungs and, specifically, surfactant deficiency.2 The incidence and severity of RDS show an inverse relationship with gestational age.3–6 In high-income countries, most babies >25 weeks gestation now survive.7 In many low-income countries, even moderately preterm babies have high mortality rates—for example, in a hospital in Dhaka, capital of Bangladesh, babies born under 32 weeks of gestation had a 79% mortality rate.8 We use the World Bank definition of high-, low- and middle-income countries.9
Antenatal steroid treatment for women who are at risk of preterm delivery has emerged as the most effective intervention for the prevention of RDS, reducing early neonatal mortality and morbidity.10 Most glucocorticoid hormones, natural and artificial, are capable of crossing the placenta and trigger the maturational process that leads to the production and release of surfactant into the alveoli of the foetal lung.11–13 In 1969, Liggins14 employed β-methasone in the first clinical trial.
Given the evidence of benefit, antenatal steroid treatment is now considered standard practice, and in high-income countries, litigation is likely if antenatal steroids are not given when indicated.15 In high-income countries, coverage remained low for a decade or so after clear evidence of effectiveness was available, but following the NIH Consensus Statement in 1994,15 there has been almost universal uptake in North America and Western Europe. As a result, the profile of RDS incidence and severity in high-income countries has altered allowing wider use of non-invasive ventilation and continuous airway pressure ventilation, reducing damage to the lungs.16
Antenatal steroid administration has been identified as an essential and feasible intervention that could beof enormous public health benefit in low/middle-income countries where preterm birth is more common, yet adequate neonatal care is often unavailable. For over half of the world's births,ventilator support for RDS is very unlikely. To date, no systematic reviews have focused on the potential benefit of antenatal steroid therapy in these settings.
Objective
The objective of the present study was to review the evidence globally for and estimate the effect on neonatal mortality due to preterm birth complications of antenatal steroid administration to women before anticipated preterm labour, compared with placebo or no treatment, with specific focus on variation on the effect size in low- and middle-income countries.
Methods
Searches
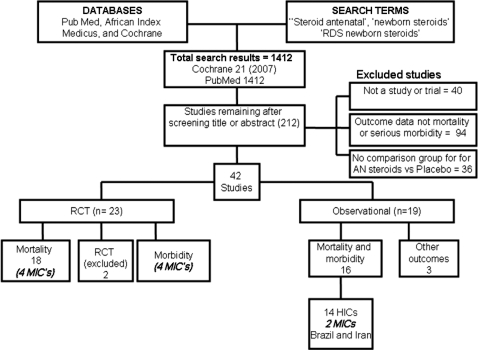
Systematic searches of electronic databases were undertaken including Cochrane Libraries, PubMed, LILACS, African Medicus, EMRO, all World Health Organization Regional Databases and publications in any language (Figure 1). Online searches of major conference proceedings were also conducted in order to identify unpublished literature. The key search terms were Steroid antenatal, newborn steroids, RDS newborn steroids, Antenatal steroids, newborn steroids, RDS newborn steroids and prenatal steroids. The systematic searches were for studies published between January 1970 and September 2009. After initial screening of titles and abstracts, we reviewed full-text publications of possible studies.
Figure 1.
Synthesis of study identification in review of the effects of antenatal corticosteroids for the treatment of RDS morbidity and mortality in preterm labour. Bold/italic text shows new meta-analysis undertaken until May 2009
Inclusion/exclusion criteria, abstraction
We applied the PICO format (Patient, Intervention, Comparison and Outcome) to define the studies to be included as follows. The population of interest was neonates, and the intervention being studied was administration of corticosteroids to women in pretem labour. We included randomized controlled trials or observational studies, where antenatal steroids were given as therapy in premature labour and where birth occurred between 24 h and 7 days after treatment. All included studies incorporated a placebo or a suitable control group that was similar to the experimental group except that it did not receive antenatal steroids. Studies were included if antenatal steroids were given alone or in combination with antibiotics and surfactants. In trials including women with multiple pregnancies, the number of babies was used as the denominator for neonatal outcomes. We sought to identify randomized controlled trials, but due to lack of such studies, especially in low-income settings, we also reviewed observational studies fitting the above criteria.
The outcomes of interest were (i) neonatal mortality due to complications of preterm birth as used in International Classification of Disease (ICD) version 10 and for global estimates for neonatal mortality; and (ii) serious neonatal morbidity related to prematurity (RDS and necrotizing enterocolitis). Preterm birth (<37 weeks completed gestational age) is not considered a cause of death in ICD. Deaths are classified as due to preterm birth if subsequent to specific complications of preterm birth (such as RDS) or ‘extreme prematurity’ (<32 weeks gestation).
All studies, which met the inclusion criteria, were abstracted onto a standardized form. We abstracted key variables with regard to the study identifiers and context, study design and limitations, intervention specifics and outcome effects (Supplementary Table 1). We assessed the quality of each of these studies using a standard approach developed by the Child Health Epidemiology Reference Group (CHERG) based on an adaptation of the GRADE approach.17
Analysis and summary measures
We planned a priori to conduct three meta-analyses, two for mortality outcomes (one with RCT as input and one with observational studies) and one for morbidity outcomes (RCT only). We also planned to undertake additional sensitivity analysis to examine bias that may be introduced by excluding certain studies not meeting our criteria. We conducted all meta-analysis using STATA version 10.0 statistical software18 and report the Mantel–Haenszel pooled relative risk and corresponding 95% confidence interval (CI). Heterogeneity between studies was summarized using the I2 statistic. If this statistic was <10%, then a random effects analysis was performed as opposed to fixed effects. We summarized the overall quality of evidence for each outcome and each data input type using an adapted version of the GRADE protocol table.17
We conducted meta-analyses restricted to studies performed in middle-income countries to assess the effect of antenatal steroid administration on neonatal mortality and morbidity in such settings. We also reviewed the Cochrane neonatal mortality meta-analysis for high-income settings taking account of time period. In addition, we conducted sensitivity meta-analyses for the studies included in the Cochrane but including two of the studies that had been excluded.
Results
We identified 1412 titles for screening (Figure 1) and reviewed the full text of 212 papers. Of these, 40 were not studies, 36 had no comparison group and 94 did not report on mortality or serious morbidity. A total of 42 papers were included in the final database (Supplementary Table). We identified 20 RCTs that reported data on cause-specific mortality of which 1814,19–35 had been included in the Cochrane review. We excluded two studies36,37 for similar reasons to those given in the recent Cochrane, i.e. >20% post randomization exclusions. The remaining trials had few limitations. The majority (13) were placebo-controlled, usually with normal saline injection. For the remainder, the control group received management according to the local standard practice. In around half of the studies (eight), randomization was well described and was adequately concealed, but for 10 studies, the randomization methods were not clearly described. In web table, we summarize the details of these studies and assess the quality of each.
Although 18 RCTs were identified, only four were from middle-income countries: Brazil,32 South Africa,33 Jordan34 and Tunisia.35 As we were specifically interested in evidence from low- and middle-income countries, we also sought observational studies from such settings. Two observational studies, reporting the effect of antenatal steroids on neonatal deaths among preterm infants, were identified from Iran38 (RR = 0.39; 95% CI 0.18–0.84; 282 babies) and Brazil39 (RR = 0.61; 95% CI 0.43–0.85; 410 babies). No studies were identified from low-income countries. The only African study was from South Africa, and no studies were identified form South Asia, where around half of newborn deaths are found.
Definition of the intervention
Who for?
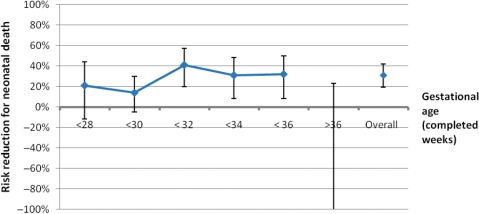
This intervention is for women in preterm labour or at high risk of preterm labour (e.g. with pre-eclampsia and planning preterm caesarean section). Studies included the gestational age at administration, ranging from 23 weeks to 36 weeks, and women with both spontaneous preterm labour or planned preterm delivery. Figure 2 shows the variation of effect on neonatal mortality according to gestational age. Between 31 weeks and 36 weeks of gestational age, there is >30% reduction in mortality. Before 30 weeks, the evidence for an effect is weaker and the benefit may be smaller. After 36 weeks of gestation, there is no evidence of a mortality benefit. Hence, this intervention has an effect only on preterm babies, is mediated through a reduction in preterm specific respiratory complications and can be assumed to relate to the category of direct preterm deaths used in ICD and in LiST.
Figure 2.
The variation of mortality effect according to gestational age of administration of antenatal steroids to women in preterm labour compared with placebo. Figure created using data from10 showing distinct risk by gestational age bands (i.e. not cumulative risk). For gestational age of >36 weeks, the 95% CI is not shown (RR = 2.2; 0.79, 8.96; 896 babies)
Treatment and dose
Dexamethasone is administered in four doses of 6 mg at 12-h intervals, whereas β-methasone is administered as two doses of 12 mg at 12-h interval.15 Most of the RCTs (14 studies) involved the administration of β-methasone. Six of the previous studies21,23,30,31,33,34 used dexamethasone. According to a Cochrane review, β-methasone resulted in a greater reduction in RDS (RR = 0.56; 95% CI 0.48–0.65; 14 studies; 2563 infants) than dexamethasone treatment (RR = 0.80; 95% CI 0.68–0.93; six studies; 1457 infants).10 In addition, β-methasone did not increase puerperal sepsis; whereas dexamethasone was associated with a significant increase (RR = 1.74; 95% CI 1.04–2.89; four studies; 536 women).10 However, another Cochrane review in the same year concluded that dexamethasone was associated with not only a higher rate of NICU admission but also a lower incidence of intraventricular haemorrhage compared with β-methasone (RR = 0.44; 95% CI 0.21–0.92; four studies; 549 infants).40 More trials of commonly used corticosteroids are required in order to make definitive recommendations on the choice of steroid.
Timing of doses
Antenatal steroids administration appears to be effective when there is birth within 24 or 48 h of treatment, if there is birth in >7 days after treatment (RR = 1.45; 95% CI 0.75–2.8; 561 babies). This may reflect the increasing maturity of such babies.26
Number of doses
In 18 RCTs examined, eight had a protocol for a repeated course of treatment each week until birth. In their study, comparing repeated steroid doses to women at risk of preterm labour, Crowther et al.41 found that babies born to women who had received repeat corticosteroids were less likely to have respiratory problems after birth. In addition, babies that did develop respiratory problems had less severe episodes and lower requirement for ventilation.41 However, the benefit of a single and/or incomplete dose of antenatal corticosteroids on mortality and morbidity related to preterm infants is clear.42,43
Effects of the intervention on neonatal deaths due to direct complications of preterm birth
In the Cochrane review, 18 studies with mortality outcomes involving 3956 infants were included.10 Antenatal steroid treatment was associated with reductions in neonatal mortality in very preterm babies (RR = 0.69; 95% CI 0.58–0.81; 18 studies; 3956 babies) and morbidity (RDS) (RR = 0.66; 95% CI 0.59–0.73; 21 studies; 4038 babies). No evidence of effects on maternal mortality or stillbirths were identified.10 No long-term complications were observed for surviving children—indeed there were non-significant trends towards reduced cerebral palsy and visual impairment following treatment with antenatal steroids. A sensitivity meta-analysis of the Cochrane review, but with two excluded studies,36,37 also showed an association between antenatal steroid treatment and a reduction in neonatal mortality among very preterm babies (RR = 0.66; 95% CI 0.56–0.78; 20 studies; 4143 babies).
Of the 18 studies with mortality outcomes, 14 are from HICs with current neonatal mortality rates (NMR) of less than 5 per 1000 and universal coverage of intensive care with ventilation for all babies and surfactant for all babies since mid-1990s. In contrast, in sub-Saharan Africa, the average NMR is 30 per 1000. Hence, the generalizability of these results to low- and middle-income countries is unclear. The mortality effect estimated by the Cochrane review might substantially underestimate the effect that could be expected in a setting in which preterm newborns currently receive little or no basic neonatal medical care, let alone intensive care.
Variation of mortality effect size in the pre-surfactant era and during testing and post-surfactant era
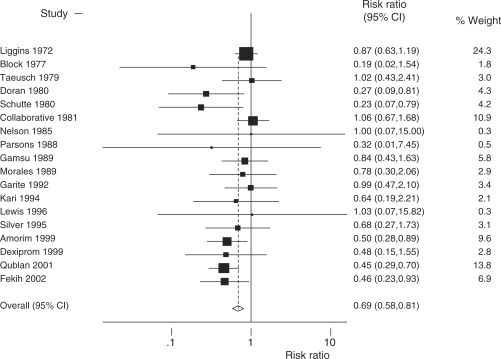
We, therefore, hypothesized that earlier studies, in which the control groups received no surfactant and less complex intensive care and thus more closely resemble current conditions in low- and middle-income countries, might report larger effects than more recent studies. Reordering the 18 studies in the Cochrane review by date, instead of author names in alphabetical order, does not reveal a clear trend, with relatively large effects seen in the four most recent studies (Figure 3). However, the last four studies were from middle-income countries and so may confound any time trend in high-income countries. After excluding these four studies, there was no evidence of a time trend in the effect of steroids (P = 0.9). We undertook sub-analyses to see if earlier studies in the pre-surfactant era, and when intensive care was less complex, would indicate a greater effect size that may be more applicable for current low-income country settings (meta-analysis not shown). As the first surfactant trial was in Japan in 1980,44,45 we defined the pre-surfactant era as pre-1980 (RR = 0.71; 95% CI 0.54–0.93; five studies; 1615 babies), the surfactant testing era from 1980 to 1990 (RR = 0.94; 95% CI 0.66–1.33; five studies; 1245 babies) and the post-surfactant era after 1991, excluding MICs (RR = 0.80; 95% CI 0.48–1.35; four studies; 425 babies). There is no evidence that the mortality effect varied across these three periods (P = 0.50). It is interesting to note that new studies were not instituted in HICs after the NIH Consensus statement on antenatal steroid use.15
Figure 3.
A meta-analysis (fixed effects) of 18 RCTs comparing administration of antenatal steroids for preterm labour with placebo and showing effect on preterm cause-specific mortality outcome. Total events = 491; heterogeneity χ2 = 21.54 (df = 17); P = 0.203; test of RR = 1; z = 4.50; P = 0.000. Fixed effect meta-analysis. Note: 18 RCTs the same inputs as Cochrane review10 but meta-analysis revised to order by date of study instead of author alphabetical order
Variation of mortality effect size in low- and middle-income countries
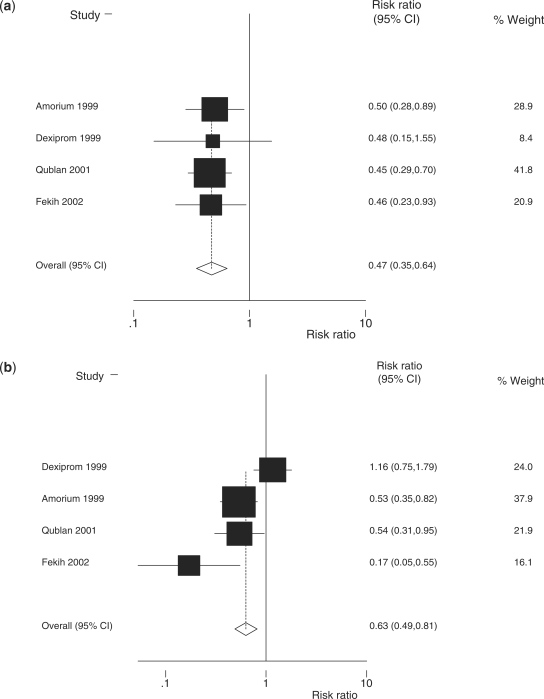
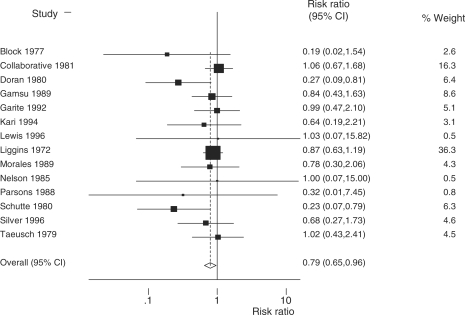
We then performed a separate meta-analysis restricted to four RCTs from middle-income countries (Table 1). There is evidence (P = 0.008) of a larger reduction in neonatal mortality in middle-income settings (Figure 4a; RR = 0.47; 95% CI 0.35–0.64; four studies; 672 babies) than in high-income settings (Figure 5; RR = 0.79; 95% CI 0.65–0.96; 14 studies; 3284 babies).10 A meta-analysis (Figure 4b) of morbidity (RDS) in middle-income countries produces an effect estimate (RR = 0.63; 95% CI 0.49–0.81; four studies; 668 babies) similar to the effect estimate reported by the Cochrane review (RR = 0.66; 95% CI 0.59–0.73; 21 studies; 4038 babies).
Table 1.
RCTs from middle-income countries comparing administration of antenatal steroids for preterm labour with placebo
| No. | Reference | Country | Case definition (drug used) | Number of events in total |
Effect size RR (95% CI) | |
|---|---|---|---|---|---|---|
| Intervention group | Control group | |||||
| 1 | Amorim et al.31,a | Brazil | All women with singleton live fetus, 26–34 weeks and severe pre-eclampsia (Betamethasone) | 14 in 100 | 28 in 100 | 0.50 (0.28–0.89) |
| 2 | Dexiprom, 1999a | South Africa | Women with preterm rupture of membranes or estimated fetal weight 1000–2000 g when going into labour (Dexamethasone) | 4 in 105 | 8 in 101 | 0.48 (0.15–1.55) |
| 3 | Qublan et al.33,a | Jordan | Women with singleton pregnancies and preterm rupture of membranes (Dexamethasone) | 19 in 70 | 39 in 65 | 0.45 (0.29–0.70) |
| 4 | Fekih et al.34,a | Tunisia | Women in Preterm labour (Betamethasone) | 9 in 63 | 21 in 68 | 0.46 (0.23–0.93) |
aIncluded in Cochrane review10; RCT: randomised control trial; RR: relative risk.
Figure 4.
Meta-analysis of four RCTs from middle-income countries comparing administration of antenatal steroids for preterm labour with placebo: (a) effect size on preterm cause-specific mortality outcome [total events = 142; heterogeneity χ2 = 0.08 (d.f. = 3); P = 0.994; test of RR = 1; z = 4.87; P = 0.000; fixed effect meta-analysis]; and (b) effect size on RDS (severe morbidity outcome) [total events = 185; heterogeneity χ2 = 13.40 (d.f. = 3); P = 0.004; test of RR = 1; z = 3.58; P = 0.000; random effects meta-analysis]
Figure 5.
Fixed effects meta-analysis of 14 RCTs from high-income countries comparing administration of antenatal steroids for preterm labour with placebo showing effect size on neonatal mortality outcome. Total events = 349; fixed effect meta-analysis; heterogeneity χ2 = 12.48 (df = 13); P = 0.489; test of RR = 1; z = 2.33; P = 0.020
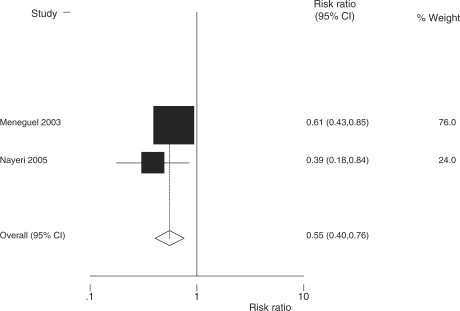
Finally, we undertook a meta-analysis of the two identified observational studies from middle-income countries, which gave a summary risk ratio of 0.55 (95% CI 0.40–0.76) for mortality (Figure 6).
Figure 6.
Fixed effect meta-analysis of two observational studies from low/middle-income countries comparing administration of antenatal steroids for preterm labour with placebo showing effect size on neonatal mortality outcome. Total events = 135; fixed effect meta-analysis; heterogeneity χ2 = 1.09 (df = 1); P = 0.297; test of RR = 1; z = 3.70; P = 0.000
Overview of the findings and the quality of evidence
In summary, there is high-quality evidence of a substantial mortality effect of antenatal steroids, and this effect is greater in MICs than in high-income settings (Table 2). However, there is a dearth of data from low-income countries (Box 1).
Table 2.
Quality assessment grade table of the effect of antenatal steroids for preterm labour on neonatal mortality due to direct complications of preterm birth
| Quality assessment | Summary of findings | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of studies | Design | Limitations | Consistency | Directness | No. of events in total | RR (95% CI) | ||
| Generalizability to population of interest | Generalizability to intervention of interest | Intervention | Control | |||||
| Cause-specific mortalitya (evidence GRADE high quality) | ||||||||
| Eighteeen | RCT | None or few, e.g. small sample sizes, no randomization method and no allocation concealment | Yes | Mostly high income | Direct | 200 events in 1988 | 291 events in 1968 | 0.69 (0.58–0.81) |
| Four | RCT | None or few, e.g. small sample sizes, no randomization method and no allocation concealment | Very | All middle income | Direct | 46 events in 338 | 96 events in 334 | 0.47 (0.35–0.64) |
| Two from MICs | Observational | Neither was a cohort study, therefore temporality an issue | Yes | Also 14 from HIC not abstracted | Direct | 48 events in 345 | 87 events in 347 | 0.55 (0.40–0.76) |
| Severe morbidity (evidence GRADE high-quality evidence, distal mortality effect) | ||||||||
| Twenty-one | RCT | None or few, e.g. small sample sizes, no randomization method and no allocation concealment | Yes | Low as mostly studies from HIC | Indirect (severe morbidity, i.e. RDS) | 351 events in 2030 | 523 events in 2008 | 0.66 (0.59–0.73) |
| Four | RCT | None or few, e.g. small sample size and unclear allocation concealment | Yes | All middle income | Indirect (severe morbidity, i.e. RDS) | 72 events in 335 | 113 events in 333 | 0.63 (0.49–0.81) |
aAll cause neonatal mortality but no mortality effect >36 weeks gestation maximum at 31–32 weeks, so effect is on neonatal deaths due preterm birth direct complication. HIC, high-income country.
Box 1: Key messages for the cause-specific mortality effect and quality grade for the effect of antenatal steroids given to women in preterm labour.
Cause-specific mortality to act on: preterm direct complications (within neonatal period).
Cause-specific effect and range: 53% (36–65%) based on four RCT meta-analysis. Consistent with meta-analysis of two observational studies from middle-income countries (45%, 24–68%).
Quality of input evidence: High, given 18 RCTs in Cochrane and four RCTs in low/middle-income countries. Mortality and morbidity data consistent. Observational study mortality data also consistent.
Proximity of the data to cause-specific mortality effect: high (cause-specific mortality).
Limitations: The control group in all these studies was routine care, including ventilation and in many cases surfactant. In low-income countries, many preterm babies currently receive little or no medical care. It is plausible that the effect of antenatal steroids may be even greater than that estimated above in settings where little other care is available.
Discussion
Preterm birth is the leading cause of neonatal mortality and morbidity in both high- and low-income countries. The recent Cochrane review shows very substantial benefit of antenatal steroid therapy on preterm neonatal mortality (31%) and morbidity outcomes (34% reduction in RDS). Our meta-analysis, restricted to four RCTs from middle-income countries, suggests an even larger effect: namely a halving of deaths due to complications of preterm birth (53% reduction with a CI of 36–65%). Given that there are >1 million preterm deaths each year,1 most of whom do not currently benefit from antenatal steroids, this intervention has the potential to prevent up to 500 000 neonatal deaths each year.
The clearest evidence for an effect is for babies born between 31 weeks and 36 weeks gestation but, surprisingly, there may be benefit at even lower gestational ages, although it should be noted that in all these studies mechanical ventilation was routinely available in addition to antenatal steroids (Figure 1). Importantly, the trials from settings with neonatal intensive care may underestimate the effect in low-income countries where there is little or no care for preterm neonates because neonatal intensive care was standard practice for the control group in all these trials (Box 1). Even if only one dose is given <24 h before birth, the effect is high at 47% reduction.10 The benefits of corticosteroids given to women with preterm premature rupture of membranes have also been demonstrated, lessening the rate of bronchopulmonary dysplasia in the offspring of these women.46 Use of surfactant along with corticosteroid administration may enhance the benefits,47 although the large trial size required to prove synergy means that this has not yet been convincingly demonstrated.
The quality of estimate has a high evidence grade given the four RCTs from the middle-income countries and a large and consistent effect size. Considerably, more than 50 deaths are included in all these analysis. The searches and abstraction of studies for this review were done by one author and checked by another author. We acknowledge this as a limitation compared with double abstraction. The generalizability is moderate because there are no RCTs reported from low-income countries or any from South Asia.
There has been some uptake of antenatal steroid therapy in middle-income countries such as South Africa33 and Thailand.48 However, the mean coverage in 75 countries with >90% of maternal, newborn and child deaths was estimated at around only 10%.49 Even in middle-income countries, antenatal steroids may not be routinely administered. For example, in 22 hospitals in Mexico City and 18 in the Northeast region of Thailand, <20% of those indicated to receive antenatal steroids did so.50
Conclusions
Based on the Cochrane10 review and additional meta-analyses for middle-income countries, there is high grade evidence that antenatal steroids are extremely effective in reducing deaths from direct complications of preterm birth. An injection to a woman in preterm labour of a drug that costs several dollars should be highly cost-effective as well as feasible. In addition, there are minimal if at all any adverse effects on the mother, fetus or child.10 While this intervention has played a major role in altering the profile of complications for preterm babies in high-income countries, in low-income countries where preterm labour is more common and where care for preterm babies is largely lacking, the use of antenatal steroids remains a missed opportunity in many facility births and a total gap for 60 million births outside facilities each year.
Supplementary Data
Supplementary data are available at IJE online.
Funding
US Fund for UNICEF from the Bill and Melinda Gates Foundation (Grant 43386 to ‘Promote evidence-based decision making in designing maternal, neonatal and child health interventions in low- and middle-income countries’) and Save The Children USA from the Bill and Melinda Gates Foundation (Grant 50124 for ‘Saving Newborn Lives’).
Acknowledgements
We thank Rajiv Bahl of WHO for technical review of this article. We also acknowledge the Global Alliance for Prevention of Prematurity and Stillbirths (www.gappseattle.org).
Conflict of interest: None declared.
References
- 1.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 2.Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. Am J Dis Child. 1959;97:517–23. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- 3.Whitsett JA, Pryhuber GS, Rice WR, Warner BB, Wert SE. Acute respiratory disorders. In: Avery GB, Fletcher MA, MacDonald MG, editors. Neonatology: Pathophysiology and Management of the Newborn. 4th. Philadelphia: J.B. Lippincott Company; 1994. pp. 429–52. [Google Scholar]
- 4.Hjalmarson O. Epidemiology and classification of acute neonatal respiratory disorders. A prospective study. Acta Paediatr Scand. 1981;70:773–83. doi: 10.1111/j.1651-2227.1981.tb06228.x. [DOI] [PubMed] [Google Scholar]
- 5.Farrell PM, Avery ME. Hyaline membrane disease. Am Rev Respir Dis. 1975;111:657–85. doi: 10.1164/arrd.1975.111.5.657. [DOI] [PubMed] [Google Scholar]
- 6.Fedrick J, Butler NR. Hyaline membrane disease. Lancet. 1972;2:768–69. doi: 10.1016/s0140-6736(72)92067-3. [DOI] [PubMed] [Google Scholar]
- 7.Costeloe K. EPICure: facts and figures: why preterm labour should be treated. BJOG. 2006;113(Suppl. 3):10–12. doi: 10.1111/j.1471-0528.2006.01118.x. [DOI] [PubMed] [Google Scholar]
- 8.Yasmin S, Osrin D, Paul E, Costello A. Neonatal mortality of low-birth-weight infants in Bangladesh. Bull World Health Organ. 2001;79:608–614. [PMC free article] [PubMed] [Google Scholar]
- 9. http://go.worldbank.org/K2CKM78CC0. [Google Scholar]
- 10.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2008;3:CD004454. doi: 10.1002/14651858.CD004454.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballard PL, Ballard RA. Scientific basis and therapeutic regimes for use of antenatal glucocorticoids. Am J Obstet Gynecol. 1995;173:254–62. doi: 10.1016/0002-9378(95)90210-4. [DOI] [PubMed] [Google Scholar]
- 12.Christensen HD, Sienko AE, Rayburn WF, Gonzalez CL, Coleman FH. A placebo-controlled, blinded comparison between betamethasone and dexamethasone to enhance lung maturation in the fetal mouse. J Soc Gynecol Invest. 1997;4:130–34. doi: 10.1016/s1071-5576(97)00021-x. [DOI] [PubMed] [Google Scholar]
- 13.Rayburn WF, Christensen HD, Gonzalez CL. A placebo controlled comparison between betamethasone and dexamethasone for fetal maturation: differences in neurobehavioral development of mice offspring. Am J Obstet Gynaecol. 1997;176:842–51. doi: 10.1016/s0002-9378(97)70609-4. [DOI] [PubMed] [Google Scholar]
- 14.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–25. [PubMed] [Google Scholar]
- 15.National Institutes of Health. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Statement. 1994;12:1–24. [PubMed] [Google Scholar]
- 16.Suguihara C, Lessa AC. Strategies to minimize lung injury in extremely low birth weight infants. J Pediatr. 2005;81(Suppl. 1) doi: 10.2223/1304. [DOI] [PubMed] [Google Scholar]
- 17.Schunemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. Br Med J. 2008;336:1106–10. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.STATA/IC 10.1. College Station, TX: STATA Corporation; 2008. Statistical Program. [Google Scholar]
- 19.Block MF, Kling OR, Crosby WM. Antenatal glucocorticoid therapy for the prevention of respiratory distress syndrome in the premature infant. Obstetr Gynecol. 1977;50:186–90. [PubMed] [Google Scholar]
- 20.Schutte MF, Treffers PE, Koppe JG, Breur W. The inuence of betamethasone and orciprenaline on the incidence of respiratory distress syndrome in the newborn after preterm labour. Br J Obstetr Gynaecol. 1980;87:127–31. doi: 10.1111/j.1471-0528.1980.tb04505.x. [DOI] [PubMed] [Google Scholar]
- 21.Taeusch HW, Jr, Frigoletto F, Kitzmiller J, et al. Risk of respiratory distress syndrome after prenatal dexamethasone treatment. Pediatrics. 1979;63:64–72. [PubMed] [Google Scholar]
- 22.Doran TA, Swyer P, MacMurray B, et al. Results of a double blind controlled study on the use of betamethasone in the prevention of respiratory distress syndrome. Am J Obstetr Gynecol. 1980;136:313–20. doi: 10.1016/0002-9378(80)90855-8. [DOI] [PubMed] [Google Scholar]
- 23.Collaborative Group on Antenatal Steroid Therapy. Effect of antenatal dexamethasone administration on the prevention of respiratory distress syndrome. Am J Obstetr Gynecol. 1981;141:276–87. [PubMed] [Google Scholar]
- 24.Nelson LH, Meis PJ, Hatjis CG, Ernest JM, Dillard R, Schey HM. Premature rupture of membranes: a prospective randomized evaluation of steroids, latent phase and expectant management. Obstetr Gynecol. 1985;66:55–58. [PubMed] [Google Scholar]
- 25.Parsons MT, Sobel D, Cummiskey K, Constantine L, Roitman J. Proceedings of the 8th Annual Meeting of the Society of Perinatal Obstetricians. Las Vegas: Nevada; 1988. Steroid, antibiotic and tocolytic vs no steroid, antibiotic and tocolytic management in patients with preterm PROM at 25–32 weeks; p. 44. [Google Scholar]
- 26.Gamsu HR, Mullinger BM, Donnai P, Dash CH. Antenatal administration of betamethasone to prevent respiratory distress syndrome in preterm infants: report of a UK multicentre trial. Br J Obstetr Gynaecol. 1989;96:401–10. doi: 10.1111/j.1471-0528.1989.tb02413.x. [DOI] [PubMed] [Google Scholar]
- 27.Morales WJ, Angel JL, O’Brien WF, Knuppel RA. Use of ampicillin and corticosteroids in premature rupture of membranes: a randomized study. Obstetr Gynecol. 1989;73:721–26. [PubMed] [Google Scholar]
- 28.Garite TJ, Rumney PJ, Briggs GG, et al. A randomized placebo-controlled trial of betamethasone for the prevention of respiratory distress syndrome at 24-28 weeks gestation. Am J Obstetr Gynecol. 1992;166:646–51. doi: 10.1016/0002-9378(92)91691-3. [DOI] [PubMed] [Google Scholar]
- 29.Kari MA, Hallman M, Eronen M, et al. Prenatal dexamethasone treatment in conjunction with rescue therapy of human surfactant: a randomised placebo-controlled multicenter study. Pediatrics. 1994;93:730–36. [PubMed] [Google Scholar]
- 30.Lewis D, Brody K, Edwards M, Brouillette RM, Burlison S, London SN. Preterm premature ruptured membranes: a randomized trial of steroids after treatment with antibiotics. Obstetr Gynecol. 1996;88:801–5. doi: 10.1016/0029-7844(96)00319-5. [DOI] [PubMed] [Google Scholar]
- 31.Silver RK, Vyskocil CR, Solomon SL, Ragin A, Neerhof MG, Farrell EE. Randomized trial of antenatal dexamethasone in surfactant treated infants delivered prior to 30 weeks of gestation. Obstetr Gynecol. 1996;87:683–91. doi: 10.1016/0029-7844(96)00033-6. [DOI] [PubMed] [Google Scholar]
- 32.Amorim MM, Santos LC, Faundes A. Corticosteroid therapy for prevention of respiratory distress syndrome in severe preeclampsia. Am J Obstetr Gynecol. 1999;180:1283–88. doi: 10.1016/s0002-9378(99)70630-7. [DOI] [PubMed] [Google Scholar]
- 33.Pattinson RC, Makin JD, Funk M, Delport SD, Macdonald AP, Norman K. The use of dexamethasone in women with preterm premature rupture of membranes: a multicentre double blind, placebo controlled randomised trial. South African Med J. 1999;89:865–70. [PubMed] [Google Scholar]
- 34.Qublan H, Malkawi H, Hiasat M, et al. The effect of antenatal corticosteroid therapy on pregnancies complicated by premature rupture of membranes. Clin Exp Obstetr Gynecol. 2001;28:183–86. [PubMed] [Google Scholar]
- 35.Fekih M, Chaieb A, Sboui H, Denguezli W, Hidar S, Khairi H. Value of prenatal corticotherapy in the prevention of hyaline membrane disease in premature infants. Randomized prospective study. Tunisie Medicale. 2002;80:260–65. [PubMed] [Google Scholar]
- 36.Morrison JC, Whybrew WD, Bucovaz ET, Scheiner JM. Injection of corticosteroids into mother to prevent neonatal respiratory distress syndrome. Am J Obstetr Gynecol. 1978;131:358–66. doi: 10.1016/0002-9378(78)90408-8. [DOI] [PubMed] [Google Scholar]
- 37.Papageorgiou AN, Desgranges MF, Masson M, Colle E, Shatz R, Gelfand MM. The antenatal use of betamethasone in the prevention of respiratory distress syndrome: a controlled blind study. Pediatrics. 1979;63:73–79. [PubMed] [Google Scholar]
- 38.Nayeri F, Movaghar-Nezhad K, Assar-Zadegan F. Effects of antenatal steroids on the incidence and severity of respiratory distress syndrome in an Iranian hospital. Eastern Mediterranean Health J. 2005;11:716–22. [PubMed] [Google Scholar]
- 39.Meneguel JF, Guinsburg R, Miyoshi MH, et al. Antenatal treatment with corticosteroids for preterm neonates: Impact on the incidence of respiratory distress syndrome and intra-hospital mortality. Sao Paulo Med J, 2003;121:45–52. doi: 10.1590/S1516-31802003000200003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brownfoot FC, Crowther CA, Middleton P. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2008;4:CD006764. doi: 10.1002/14651858.CD006764.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crowther C, Haslam RR, Hiller JE, Doyle LW, Robinson JS. Australasian Collaborative Trial of Repeat Doses of Steroids (ACTORDS) Study Group Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomized controlled trial. Lancet. 2006;367:9526,1913–19. doi: 10.1016/S0140-6736(06)68846-6. [DOI] [PubMed] [Google Scholar]
- 42.Elimian A, Figueroa R, Spitzer AR, et al. Antenatal corticosteroids: are incomplete courses beneficial? Obstetr Gynecol. 2003;102:352–55. doi: 10.1016/s0029-7844(03)00485-x. [DOI] [PubMed] [Google Scholar]
- 43.Costa S, Zecca E, De Luca D, De Carolis MP, Romagnoli C. Efficacy of a single dose of antenatal corticosteroids on morbidity and mortality of preterm infants. Eur J Obstet Gynecol Reprod Biol. 2007;131:154–57. doi: 10.1016/j.ejogrb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Fujiwara T, Adams FH. Surfactant for hyaline membrane disease. Pediatrics. 1980;66:795–98. [PubMed] [Google Scholar]
- 45.Halliday HL. Surfactants: past, present and future (Review) J Perinatol. 2008;(Suppl 1):S47–S56. doi: 10.1038/jp.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tucker L, Hoff C, Peevy K, Brost B, Holland RNCS, Calhoun BC. The effects of antenatal steroid use in premature rupture of membranes. ANZJOG. 1980;35:390–92. doi: 10.1111/j.1479-828x.1995.tb02149.x. [DOI] [PubMed] [Google Scholar]
- 47.Jobe AH, Mitchell BR, Gunkel JH. Beneficial effects of the combined use of prenatal corticosteroids and postnatal surfactant on preterm infants. Am J Obstet Gynecol. 1993;168:508–13. doi: 10.1016/0002-9378(93)90483-y. [DOI] [PubMed] [Google Scholar]
- 48.Saengwaree P, Liabsuetrakul T. Changing practice on corticosteroids. J Med Assoc Thailand. 2005;88:307–13. [PubMed] [Google Scholar]
- 49.Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, De Bernis L. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005;365:977–88. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- 50.Gülmezoglu AM, Langer A, Piaggio G, Lumbiganon P, Villar J, Grimshaw J. Cluster randomised trial of an active, multifaceted educational intervention based on the WHO Reproductive Health Library to improve obstetric practices. BJOG. 2007;114:16–23. doi: 10.1111/j.1471-0528.2006.01091.x. [DOI] [PubMed] [Google Scholar]