Abstract
Background In high-income countries, it is standard practice to give antibiotics to women with pre-term, pre-labour rupture of membranes (pPROM) to delay birth and reduce the risk of infection. In low and middle-income settings, where some 2 million neonatal deaths occur annually due to complications of pre-term birth or infection, many women do not receive antibiotic therapy for pPROM.
Objectives To review the evidence for and estimate the effect on neonatal mortality due to pre-term birth complications or infection, of administration of antibiotics to women with pPROM, in low and middle-income countries.
Methods We performed a systematic review to update a Cochrane review. Standardized abstraction forms were used. The quality of the evidence provided by individual studies and overall was assessed using an adapted GRADE approach.
Results Eighteen RCTs met our inclusion criteria. Most were from high-income countries and provide strong evidence that antibiotics for pPROM reduce the risk of respiratory distress syndrome [risk ratio (RR) = 0.88; confidence interval (CI) 0.80, 0.97], and early onset postnatal infection (RR = 0.61; CI 0.48, 0.77). The data are consistent with a reduction in neonatal mortality (RR = 0.90; CI 0.72, 1.12).
Conclusion Antibiotics for pPROM reduce complications due to pre-term delivery and post-natal infection in high-income settings. There is moderate quality evidence that, in low-income settings, where access to other interventions (antenatal steroids, surfactant therapy, ventilation, antibiotic therapy) may be low, antibiotics for pPROM could prevent 4% of neonatal deaths due to complications of prematurity and 8% of those due to infection.
Keywords: Neonatal mortality, newborn care, antibiotics, pre-term birth, prematurity, infection, respiratory distress syndrome
Background
Each year almost 4 million newborns die.1 Complications of pre-term birth and infections account for more than half of these deaths. Pre-term, pre-labour rupture of membranes (pPROM) occur when the amniotic sac enclosing the fetus ruptures before 37 weeks of gestation (pre-term) and prior to the onset of labour (pre-labour). It is associated with about one-third of pre-term deliveries in high-income countries, and is associated with increased rates of neonatal and maternal infection.2 Infection may be a cause of or result from pPROM.3 Empiric antibiotic therapy following pPROM has been demonstrated in high-income countries to increase the time period between pPROM and delivery, and reduce the risks of maternal and neonatal infection.2 Neonatal infections and prematurity are important causes of neonatal death in low and middle-income countries (LMICs). We therefore reasoned that empiric antibiotic therapy following pPROM may be an efficacious and cost-effective intervention to prevent infection and improve neonatal outcomes in LMICs as well.
A systematic review of randomized trials of antibiotics for pPROM has been published in the Cochrane database.3 This included 22 trials including over 6000 women and concluded that antibiotic administration following pPROM delays delivery and reduces major markers of neonatal morbidity. The authors conclude that the data support the routine use of antibiotics in pPROM but counsel against the use of co-amoxiclav as this was associated with increased risk of neonatal necrotizing enterocolitis.3 In this article, we present an update of this review.
Objective
To review the evidence for, and estimate the effect on neonatal mortality due to the direct complications of pre-term birth and due to infections, of antibiotics administered to women with pPROM, compared with no treatment, for low and middle-income countries.
Methods
Searches
We reviewed the 22 trials included in the Cochrane review3 and abstracted data on the outcomes of interest, namely neonatal mortality, and the incidence of severe morbidity relating to pre-term delivery or infection: necrotizing enterocolitis, intra-ventricular haemorrhage, respiratory distress syndrome (RDS), confirmed sepsis. We excluded two trials in which all women received antibiotic treatment for 3 or 7 days.4,5 We also excluded three trials which compared co-amoxiclav with placebo or no antibiotic treatment6–8 and the co-amoxiclav arm of another trial[9] because of the evidence that co-amoxiclav is associated with increased risk of necrotizing enterocolitis. We therefore included 17 trials from the Cochrane review.9–25
We also undertook systematic searches in multiple databases to identify studies of antibiotics for pPROM. We searched PubMed, Cochrane Libraries and all World Health Organization Regional Databases, and included publications in any language. Search terms included: ‘preterm/premature’, ‘membranes’, ‘rupture,’ ‘antibiotics’. For PubMed, we further restricted the search to randomized/clinical trials. Studies were included if data were reported on mortality or severe morbidity (see above) during the neonatal period.
Inclusion/exclusion criteria, abstraction
We included randomized trials from any setting and observational studies from low and middle-income settings. Studies were included if antibiotics were given alone or in combination with antenatal steroids and surfactants. For all outcomes, the denominator used was the number of liveborn babies. Data from all studies which met the inclusion criteria were abstracted using a standardized form. We abstracted key variables with regard to the study identifiers and context, study design and limitations, the intervention, application of intention-to-treat analysis and the outcomes of interest (neonatal mortality and the incidence of severe morbidity relating to pre-term delivery or infection). We assessed the quality of the evidence provided by each of these studies using a standard table (webtable) employing an adapted version of GRADE26 developed by the Child Health Epidemiology Reference Group (CHERG).27
Analysis and summary measures
Statistical analyses to summarize results across studies were performed using Stata version 10 software (http://www.stata.com). We performed and presented the results from fixed effects meta-analyses for those outcomes for which the evidence of heterogeneity between studies was weak (P > 0.1). Otherwise, we performed and presented the results of random effects meta-analyses. We summarized the overall quality of evidence for each outcome using an adapted version of the GRADE approach.27
Results
We identified 498 titles for screening and reviewed the full text of 19 papers (Supplementary Table 1). The search yielded one randomized trial which had been published since the Cochrane review28 and one observational study from a low-income setting.29
Randomized trials
We analysed data from 18 randomized trials and 4581 newborns (Table 1). Most trials were from high-income settings. The exceptions were one trial from Turkey (31 newborns),12 one trial from Mozambique (106 newborns)10 and one trial in Chile (85 newborns).23 Most (14) trials were blinded through the use of a placebo, but most were small with half involving fewer than 100 women. The largest trial9 contributed more than half of the total mothers (2415) after exclusion of the arms receiving co-amoxiclav. The studies are summarized in Table 1 and described in more detail in the Supplementary table.
Table 1.
Summary of individual studies included in primary analyses
| First author | Year of publication | Country | Study design | Study participants | Antibiotics used | Sample size | Reference |
|---|---|---|---|---|---|---|---|
| Almeida | 1996 | Mozambique | RCT | 30–36 weeks gestation | Amoxicillin | 110 | [10] |
| Amon | 1988 | USA | RCT | 20–34 weeks gestation, singleton pregnancies | Ampicillin | 78 | [11] |
| Camli | 1997 | Turkey | RCT | 28–34 weeks gestation, singleton pregnancies | Ampicillin | 31 | [12] |
| Ernest | 1994 | USA | RCT | 21–37 weeks gestation | Benzylpenicillin, potassium phenoxymethyl penicillin | 144 | [13] |
| Fuhr | 2006 | Germany | RCT | 24.0–32.9 weeks gestation | Mezlocillin | 105 | [28] |
| Garcia- Burguillo | 1996 | Spain | RCT | <36 weeks gestation | Erythromycin | 58 | [14] |
| Grable | 1996 | USA | RCT | ≤35 weeks gestation, singleton pregnancies | Ampicillin | 60 | [15] |
| Johnston | 1990 | USA | RCT | 20–34 weeks gestation, singleton pregnancies | Mezlocillin and ampicillin | 84 | [16] |
| Kenyon | 2001 | UK and others | RCT | <37 weeks gestation, multiple pregnancies included | Erythromycin | 2415 | [9] |
| Kurki | 1992 | Finland | RCT | 23–36 weeks gestation, | Penicillin | 115 | [17] |
| Lockwood | 1993 | USA | RCT | 20–34.9 weeks gestation, singleton pregnancies | Piperacillin sodium | 70 | [18] |
| McGregor | 1991 | USA | RCT | 23–34 weeks gestation, singleton pregnancies | Erythromycin | 54 | [19] |
| Mercer | 1992 | USA | RCT | 20–34.9 weeks gestation, singleton | Erythromycin | 216 | [21] |
| Mercer | 1997 | USA | RCT | 24–32.0 weeks gestation, included multiple pregnancies | Ampicillin, erythromycin, amoxicillin | 611 | [20] |
| Morales | 1989 | USA | RCT | 26–34 weeks, singleton pregnancies | Ampicillin | 165 | [22] |
| Ovalle Salas | 1997 | Chile | RCT | 24–34 weeks gestation | Clindamycin and gentamycin | 85 | [23] |
| Owen | 1993 | USA | RCT | 24–33.9 weeks gestation, singleton | Ampicillin | 117 | [24] |
| Svare | 1997 | Denmark | RCT | 26 + 0–33.6 weeks gestation, singleton pregnancies | Ampicillin, pivampicillin, metronidazole | 67 | [25] |
| Al-Qa'Qa | 2005 | Pakistan | Observational | 28–42 weeks gestation | Unclear | 225 | [29] |
Description of the intervention
Women with pre-term rupture of membranes were treated with antibiotics. The antibiotic used, dose and duration varied between trials. Six trials used ampicillin, four erythromycin, two penicillin and one each used amoxicillin, mezlocillin, piperacillin. Two studies used combinations of two or more of these antibiotics, whereas one trial used a combination of clindamycin and gentamycin.
Effect on all-cause neonatal mortality
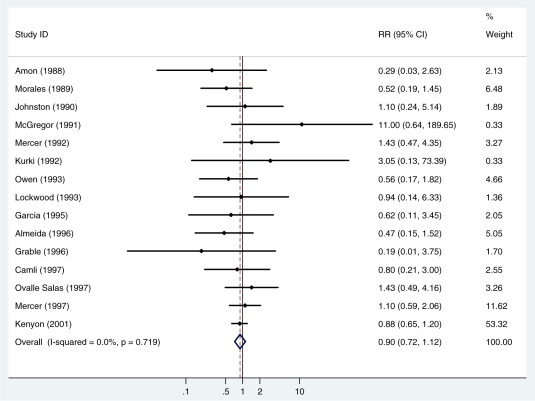
Fifteen studies (4265 newborns) reported on neonatal mortality (or mortality prior to discharge). A total of 284 deaths were reported (NMR = 66/1000 livebirths). There was no evidence of heterogeneity between studies (I2 =0%, P = 0.7). The summary, fixed effects, risk ratio (RR) was 0.90 [95% confidence interval (CI) 0.72, 1.12; P = 0.33] (Figure 1, Table 2).
Box 1 Cause-specific mortality effect and quality grade of the estimate for antibiotics given to women with pPROM.
Causes of mortality to act on:
Direct complications of prematurity and infections.
Cause-specific effect and range:
Twelve percent reduction in incidence of RDS (95% CI 3–20%).
Thirty-nine percent reduction in incidence of neonatal sepsis (95% CI 22–52%)
We assume that these reductions in incidence of severe morbidity translate into similar reductions in mortality amongst babies born to mothers with pPROM.
We assume that one-third of pre-term births are related to pPROM and that ∼60% of infection deaths are related to prematurity.
Thus, we estimate that giving antibiotics to mothers with pPROM could reduce neonatal deaths due to the direct complications of pre-term birth by 4% and neonatal deaths due to sepsis by 8%.
Quality of input evidence:
Moderate
18 RCTs, but mainly from high-income settings. Evidence strong for an effect on severe morbidity but less strong for an effect on mortality. The few data available from low/middle-income countries are consistent with an effect on mortality.
Proximity of the data to cause specific mortality effect:
Moderate (severe morbidity)
Limitations:
The control groups in most or all of these studies will have had access to a high level of routine care whereas the group of interest for programmes in low-income countries are newborns currently receiving little or no medical care.
Possible adverse effects:
Co-amoxiclav is not recommended as it has been associated with an increased risk of necrotizing enterocolitis.
Figure 1.
Fixed effects meta-analysis of the effect of antibiotics for pPROM on neonatal mortality
Table 2.
Assessment of the quality of the evidence with respect to the effect of antibiotics for pPROM on severe neonatal morbidity and mortality in low and middle-income settings
|
Assessment of quality of evidence |
Summary of Findings |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. of studies | Design | Limitations | Consistency | Directness |
No. of events in total |
RR (95% CI) | ||
| Generalizability to low/middle- income settings | Generalizability to mortality outcomes | |||||||
| Inter- vention | Control | |||||||
| Neonatal mortality: Quality of evidence: low | ||||||||
| 15 | RCT | No major | Yes | Low, mostly high-income | Direct (all-cause) | 133 | 151 | RR = 0.90 (0.72, 1.12) |
| Severe morbidity (RDS): Quality of evidence: moderate | ||||||||
| 13 | RCT | No major | Yes | Low, mostly high-income | Indirect (severe morbidity) | 509 | 596 | RR = 0.88 (0.80, 0.97) |
| Severe morbidity (necrotizing enterocolitis): Quality of evidence: low | ||||||||
| 13 | RCT | No major | Yes | Low, mostly high-income | Indirect (severe morbidity) | 63 | 85 | RR = 0.76 (0.56, 1.05) |
| Severe morbidity (intra-ventricular haemorrhage): Quality of evidence: moderate | ||||||||
| 11 | RCT | No major | Yes | Low, mostly high-income | Indirect (severe morbidity) | 59 | 90 | RR = 0.67 (0.49, 0.92) |
| Severe morbidity (confirmed sepsis): Quality of evidence: moderate | ||||||||
| 13 | RCT | No major | Yes | Low, mostly high-income | Indirect (severe morbidity) | 97 | 163 | RR = 0.61 (0.48, 0.78) |
Effects on severe morbidity
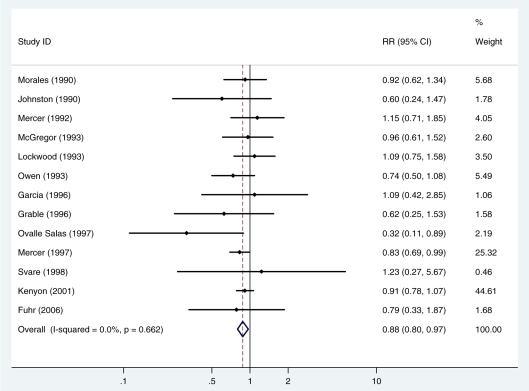
Thirteen studies (4104 newborns) reported on the occurrence of respiratory distress syndrome (1105 cases). There was no evidence of heterogeneity between studies (I 2=0%, P = 0.7). The summary, fixed effects, risk ratio (RR) was 0.88 (95% CI 0.80, 0.97; P = 0.009) (Figure 2, Table 2).
Figure 2.
Fixed effects meta-analysis of the effect of antibiotics for pPROM on the risk of respiratory distress syndrome
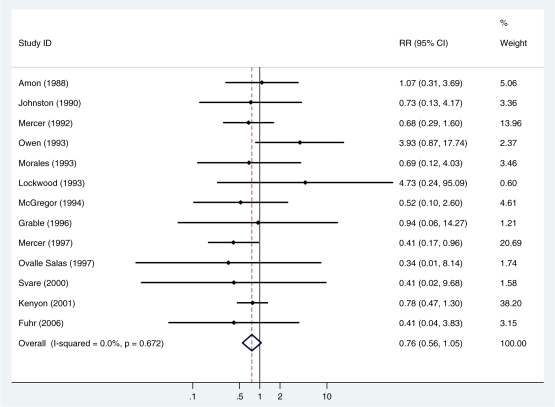
Thirteen studies (4126 newborns) reported on the risk of necrotizing enterocolitis (148 cases). There was no evidence of heterogeneity between studies (I2 =0%, P = 0.7). The summary, fixed effects, risk ratio was 0.76 (95% CI 0.56, 1.05; P = 0.09) (Figure 3, Table 2).
Figure 3.
Fixed effects meta-analysis of the effect of antibiotics for pPROM on the risk of necrotizing enterocolitis
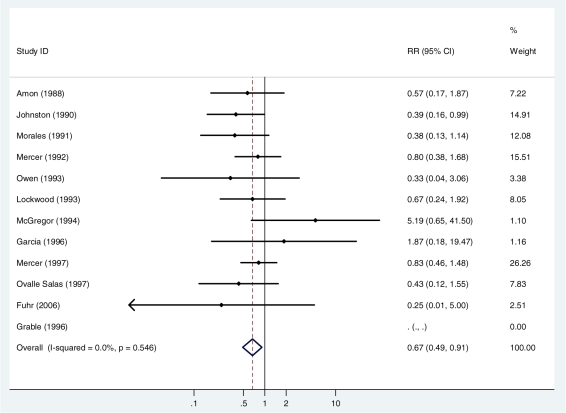
Twelve studies (1702 newborns) reported on the risk of intra-ventricular haemorrhage (149 cases). One study15 reported no cases and did not contribute to the analysis. There was no evidence of heterogeneity between studies (I2 =0%, P = 0.6). The summary, fixed effects, risk ratio was 0.67 (95% CI 0.49, 0.92; P = 0.01) (Figure 4, Table 2).
Figure 4.
Fixed effects meta-analysis of the effect of antibiotics for pPROM on the risk of intra-ventricular haemorrhage
Thirteen studies (3693 newborns) reported on the risk of confirmed sepsis (260 cases). There was no evidence of heterogeneity between studies (I2 = 0%, P = 0.6). The summary, fixed effects, risk ratio was 0.61 (95% CI 0.48, 0.77; P < 0.001) (Figure 5, Table 2).
Figure 5.
Fixed effects meta-analysis of the effect of antibiotics for pPROM on the risk of confirmed sepsis
In 6 of the 18 trials (2860 babies),9,10,12,14,19,21 only oral antibiotics were used. In meta-regression analyses, there was no strong evidence that any of the above effects differed between trials using IV antibiotics and those using oral antibiotics only (P > 0.15 for all outcomes).
Studies in low and middle-income countries
Very few data are available from low and middle-income countries. One trial in Mozambique10 included 106 newborns and reported 11 deaths and 12 cases of confirmed sepsis. Both the risk of death (RR = 0.47) and risk of sepsis (RR = 0.17) appeared lower in the treated arm. A trial in a middle-income setting (Turkey,12) included 31 newborns and reported a total of seven deaths and seven cases of confirmed sepsis. Again, both the risk of death (RR = 0.80) and the risk of sepsis (RR = 0.43) appeared lower in the treated arm. Excluding one death which occurred 10 min after delivery in a baby with multiple malformations, the trial in Chile23 reported 12 neonatal deaths, 7 of which occurred in the group receiving antibiotics (RR = 1.43). RDS (17 cases, RR = 0.32), necrotizing enterocolitis (1 case, control arm), intra-ventricular haemorrhage (10 cases, RR = 0.43) and early onset sepsis (4 cases, RR = 0.34) were all, if anything, less common in the intervention arm.
These data are consistent with one trial and one observational study from low and middle-income countries that we excluded from our primary analysis. We excluded from our main analyses a trial conducted in Zimbabwe8 because it used co-amoxiclav, This trial included 168 newborns and reported 19 deaths and 34 cases of unconfirmed clinical sepsis. Both the risk of death (RR = 0.76) and the risk of unconfirmed sepsis (RR = 0.65) appeared lower in the treated arm. In an observational study conducted in Pakistan,29 225 newborns were studied, of whom 140 were pre-term. It is not clear what antibiotics mothers received though all newborns received antibiotics until a negative blood culture was obtained. The risks of a positive blood culture (9% vs 30%), RDS (25% vs 44%), necrotizing enterocolitis (3% vs 11%) and intra-ventricular haemorrhage (7% vs 12%) were all lower in babies born to mothers who had received antibiotics. Twenty neonatal deaths are reported but it is not clear how these were distributed between mothers who did or did not receive antibiotics.
Discussion
There is high-quality evidence that antibiotics for pPROM reduce the risk of complications due to prematurity and risk of neonatal infection in high-income settings. The evidence for a reduction in neonatal mortality is less strong. Our meta-analysis of 14 trials estimates a 10% reduction in all-cause neonatal mortality but with a wide CI that included no effect. However, there is a dearth of data from low-income countries, where newborns have less access to other forms of care including antenatal steroids, surfactant therapy, ventilation and even antibiotic treatment for infection. In the absence of these other interventions, the use of antibiotics for pPROM will certainly prevent neonatal deaths by preventing RDS, which is the most common cause of death due to complications of prematurity. Similarly, in settings in which newborns do not have easy access to antibiotic therapy for neonatal infections, the prevention of sepsis cases through the use of antibiotics for pPROM will certainly prevent infection deaths. We did not observe strong evidence that oral antibiotics were less effective than IVl antibiotics though our power to detect any such difference is limited by the small number of studies using oral antibiotics only. Clearly, in low-income settings, oral-only regimens will be easier to deliver than regimens involving intravenous administration of the antibiotics.
There is evidence (P = 0.01) of a reduction in the incidence of RDS (estimated reduction 12%, 95% CI 3–20%) with larger reductions in intra-ventricular haemorrhage (33%, 95% CI 8–51%, P = 0.01) and necrotizing enterocolitis (24%, 95% CI 5–44%, P = 0.09) although the evidence for an effect on necrotizing enterocolitis is relatively weak. Given that RDS is the most common complication and has the smallest effect estimate, we propose assuming that antibiotics for pPROM can reduce deaths due to complications of prematurity by 12% (uncertainty range 3–20%) among newborns born following pPROM. About one-third of pre-term deliveries are associated with pPROM,2 so we propose an affected fraction of 33% of all pre-term deaths. Thus, we estimate that increasing coverage with antibiotics for pPROM from 0% to 100% might prevent 4% of all pre-term deaths.
Our analysis suggests a 39% reduction in incidence of neonatal sepsis (95% CI 23–52%, P < 0.001) with antibiotic therapy for pPROM among newborns born following pPROM. In order to estimate by how much this intervention might reduce sepsis deaths as a whole, we need to take into account how many sepsis deaths occur in pre-term babies and the proportion of pre-term births with pPROM (assumed to be one-third, see above). If 60% of neonatal sepsis deaths occur in pre-term babies,30 one-third of which occur after pPROM, then this implies that 20% of sepsis deaths occur in pPROM babies. Thus, antibiotics for pPROM might reduce sepsis deaths by about 8% overall (i.e. 39% reduction among 20% of deaths).
Potential benefits of empiric antibiotic therapy following pPROM must be weighed against possible adverse consequences related to antibiotic administration. Most data have been reassuring, with no increased adverse sequelae following empiric antibiotic therapy for pPROM.2,9,31 The ORACLE I trial, the single largest reported, of nearly 5000 women with pPROM randomized to either placebo or one of several antibiotic regimens found neither increases in immediate neonatal adverse sequelae related to antibiotics nor differences in neurodevelopmental outcome at 7 years of age.9,31 However, in a related concurrent trial, empiric antibiotic therapy for pre-term labour, without rupture of membranes, was associated with an increased risk of neonatal necrotizing enterocolitis (associated with co-amoxiclav) and of subsequent neurodevelopmental functional impairment (associated with erythromycin) without any significant prolongation in pregnancy or improvement in other neonatal comorbidities.32,33 The reasons for increases in neonatal morbidity following antibiotic therapy in pre-term labour remain speculative but may relate to disruptions in the microbial colonization of the neonatal gut by low- virulence microflora that has recently been demonstrated to begin in utero, even with intact fetal membranes, allowing subsequent colonization by more virulent micro-organisms following birth.34 In contrast, antibiotic therapy following pPROM may prevent the ascending colonization of amniotic fluid by more virulent strains arising from the maternal lower genital tract, thereby reducing neonatal infections.
Empiric antibiotic therapy following pPROM reduces risk of neonatal respiratory distress, intra-ventricular haemorrhage and confirmed neonatal sepsis. Whereas most of the data supporting this conclusion derive from studies performed in high-income countries, the limited data from low and middle-income countries suggest similar reductions in neonatal morbidity. Empiric antibiotic therapy following pPROM represents an efficacious and low cost intervention to improve neonatal outcomes. Studies to assess translational feasibility of this intervention and to track its coverage in low and middle-income countries are urgently needed.
Conclusion
Although there are many trials and the results of these trials are quite consistent, we have graded the overall evidence quality as moderate because of the limited evidence from low and middle-income settings, and the fact that the strong evidence of effectiveness is for severe morbidity rather than mortality. In order to estimate mortality effects, we have had to make certain assumptions and in doing so we have tried to be conservative. In low-income settings where infections in pregnancy are more prevalent, yet the available care for mothers and for babies is much more limited, the impact of antibiotic therapy may be greater than in high-income settings. Nevertheless, we propose that, amongst births complicated by pPROM, empiric antibiotic therapy could reduce deaths due to complications of prematurity by 12% and deaths due to sepsis by 39%. Taking account of the proportion of births affected by pPROM and the proportion of sepsis deaths that may occur in pre-term babies in low-income settings, these effects translate into a 4% reduction in all deaths due to complications of prematurity and an 8% reduction in all deaths due to neonatal sepsis.
Supplementary data
Supplementary data are available at IJE online.
Funding
This work was supported in part by a grant to the US Fund for UNICEF from the Bill & Melinda Gates Foundation (grant 43 386) to ‘Promote evidence-based decision making in designing maternal, neonatal and child health interventions in low- and middle-income countries’, and by a grant to Save The Children USA from the Bill & Melinda Gates Foundation ( Grant 50 124) for ‘Saving Newborn Lives’. We also acknowledge the Global Alliance for Prevention of Prematurity and Stillbirths (GAPPS) (http://www.gapps.org/)
Acknowledgements
We thank all members of the Child Health Epidemiology Reference Group for helpful comments and feedback on this work. In particular, we thank Rajiv Bahl for his careful and thoughtful review of earlier versions of this article.
Conflict of interest: None declared.
References
- 1.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: When? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 2.Mercer B, Arheart K. Antimicrobial therapy in expectant management of preterm premature rupture of the membranes. Lancet. 1995;346:1271–79. doi: 10.1016/s0140-6736(95)91868-x. [DOI] [PubMed] [Google Scholar]
- 3.Kenyon S, Boulvain M, Neilson JP. Antibiotics for preterm rupture of membranes. Cochr Database Syst Rev. 2003 doi: 10.1002/14651858.CD001058. doi: 10.1002/14651858.CD001058. [DOI] [PubMed] [Google Scholar]
- 4.Lewis DF, Adair D, Robichaux AG, et al. Antibiotic therapy in preterm premature rupture of membranes: are seven days necessary? A preliminary, randomized clinical trial. Am J Obst Gynecol. 2003;188:1413–17. doi: 10.1067/mob.2003.382. [DOI] [PubMed] [Google Scholar]
- 5.Segel SY, Miles AM, Clothier B, et al. Duration of antibiotic therapy after preterm premature rupture of fetal membranes. Am J Obst Gynecol. 2003;189:799–802. doi: 10.1067/s0002-9378(03)00765-8. [DOI] [PubMed] [Google Scholar]
- 6.Christmas JT, Cox SM, Andrews W, et al. Expectant management of preterm ruptured membranes: effects of antimicrobial therapy. Obstet Gynecol. 1992;80:759–62. [PubMed] [Google Scholar]
- 7.Cox SM, Leveno KJ, Sherman ML, et al. Ruptured membranes at 24–29 weeks: a randomized double blind trial of antimicrobials versus placebo. Am J Obst Gynecol. 1995;172:412. [Google Scholar]
- 8.Magwali TL, et al. Prophylactic augmentin in prelabor preterm rupture of the membranes. Int J Gynecol Obst. 1999;65:261–65. doi: 10.1016/s0020-7292(99)00036-3. [DOI] [PubMed] [Google Scholar]
- 9.Kenyon SL, et al. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE 1 randomised trial. Lancet. 2001;357:979–88. doi: 10.1016/s0140-6736(00)04233-1. [DOI] [PubMed] [Google Scholar]
- 10.Almeida L, Schmauch A, Bergström S. A randomised study on the impact of peroral amoxicillin in women with prelabour rupture of membranes preterm. Gynecol Obstet Invest. 1996;41:82–84. doi: 10.1159/000292046. [DOI] [PubMed] [Google Scholar]
- 11.Amon E, Lewis SV, Sibai BM, et al. Ampicillin prophylaxis in preterm premature rupture of the membranes: a prospective randomized study. Am J Obst Gynecol. 1988;159:539–534. doi: 10.1016/s0002-9378(88)80002-4. [DOI] [PubMed] [Google Scholar]
- 12.Camli L, Mavunagacioglu S, Bostanci A, et al. Preterm erken membran rupturunde antibiyotik kullanimi: latent periyoda ve enfeksiyoz morbiditeye etkisi var mi? Jinekoloji ve Obstetrik Dergisi. 1997;11:138–42. [Google Scholar]
- 13.Ernest JM, Givener LB. A prospective, randomize, placebo-controlled trial of penicillin in preterm premature rupture of membranes. Am J Obst Gynecol. 1994;170:516–21. doi: 10.1016/s0002-9378(94)70220-9. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Burguillo A, Hernandez-Garcia JM, de la Fuente P. Profilaxis con eritromicina en getsaciones pretérmino con routra prematura de las membranes amnioticas. Clin Invest Gin Obst. 1996;23:34–38. [Google Scholar]
- 15.Grable IA, Garcia PM, Perry D, et al. Group B Streptococcus and preterm premature rupture of membranes: a randomized, double-blind clinical trial of antepartum ampicillin. Am J Obst Gynecol. 1996;175:1036–42. doi: 10.1016/s0002-9378(96)80049-4. [DOI] [PubMed] [Google Scholar]
- 16.Johnston MM, Sanchez-Ramos L, Vaughn AJ, et al. Antibiotic therapy in preterm premature rupture of membranes: a randomized, prospective, double-blind trial. Am J Obst Gynecol. 1990;163:743–47. doi: 10.1016/0002-9378(90)91060-p. [DOI] [PubMed] [Google Scholar]
- 17.Kurki T, Hallman M, Zilliacus R, et al. Premature rupture of the membranes: effect of penicillin prophylaxis and long-term outcome of the children. Am J Perinatol. 1992;9:11–16. doi: 10.1055/s-2007-994661. [DOI] [PubMed] [Google Scholar]
- 18.Lockwood CJ, Costigan K, Ghidini A, et al. Double-blind, placebo-controlled trial or piperacillin prophylaxis in preterm membrane rupture. Am J Obst Gynecol. 1993;169:970–76. doi: 10.1016/0002-9378(93)90037-j. [DOI] [PubMed] [Google Scholar]
- 19.McGregor JA, French JI, Seo K. Antimicrobial therapy in preterm premature rupture of membranes: results of a prospective, double-blind, placebo-controlled trial of erythromycin. Am J Obst Gynecol. 1991;165:632–40. doi: 10.1016/0002-9378(91)90299-7. [DOI] [PubMed] [Google Scholar]
- 20.Mercer BM, Miodovnik M, Thurnau GR, et al. Antibiotic therapy for the reduction of infant morbidity after preterm premature rupture of the membranes. J Am Med Assoc. 1997;278:989–95. [PubMed] [Google Scholar]
- 21.Mercer BM, Moretti ML, Prevost RR, et al. Erythromycin therapy in preterm premature rupture of the membranes: a prospetcive, randomised trial of 220 patients. Am J Obst Gynecol. 1992;166:794–802. doi: 10.1016/0002-9378(92)91336-9. [DOI] [PubMed] [Google Scholar]
- 22.Morales WJ, Angel JL, O'Brien WF, et al. Use of ampicillin and corticosteroids in premature rupture of membranes: a randomised study. Obstet Gynecol. 1989;73:721–26. [PubMed] [Google Scholar]
- 23.Ovalle-Salas A, Gomes R, Martinez MA, et al. Antibiotic therapy in patients with pretermpremature rupture of membranes: a prospective, randomized, placebo-controlled study with microbiological assessment of the amniotic cavity and lower genital tract. Prenatal Neonatal Med. 1997;2:213–22. [Google Scholar]
- 24.Owen J, Groome LJ, Hauth JC. Randomized trial of prophylactic antibiotic therapy after preterm amnion rupture. Am J Obst Gynecol. 1993;169:976–81. doi: 10.1016/0002-9378(93)90038-k. [DOI] [PubMed] [Google Scholar]
- 25.Svare J. Department of Obstetrics and Gynecology Rigshospitalet. Copenhagen: University of Copenhagen; 1997. Preterm delivery and subclinical uro-genital infection. [Google Scholar]
- 26.GRADE working Group. Grading quality of evidence and strength of recommendations. Br Med J. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. doi:10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker N, Fischer-Walker C, Bryce J, Bahl R, Cousens S writing for the CHERG Review Groups on Intervention Effects. Standards for CHERG reviews of intervention effects on child survival. Int J Epidemiol. 2010;39(Suppl 1):i21–31. doi: 10.1093/ije/dyq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuhr NA, Becker C, van Baalen A, et al. Antibiotic therapy for preterm premature rupture of membranes - results of a multicenter study. J Perinatal Med. 2006;34:203–06. doi: 10.1515/JPM.2006.035. [DOI] [PubMed] [Google Scholar]
- 29.Al-Qa'Qa K, Al-Awayeshi F. Neonatal outcome and prenatal antibiotic treatment in premature rupture of membranes. Pak J Med Sci. 2005;21:441–44. [Google Scholar]
- 30.Bang AT, Reedy HM, Bang RA, et al. Why do neonates die in rural Gadchiroli, India? (Part II): estimating population attributable risks and contribution of multiple morbidities for identifying a strategy to prevent deaths. J Perinatol. 2005;25(Suppl 1):S35–43. doi: 10.1038/sj.jp.7211270. [DOI] [PubMed] [Google Scholar]
- 31.Kenyon S, Pike K, Jones DR, et al. Childhood outcomes after prescription of antibiotics to pregnant women with preterm rupture of the membranes: 7-year follow-up of the ORACLE I trial. Lancet. 2008;372:1310–18. doi: 10.1016/S0140-6736(08)61202-7. [DOI] [PubMed] [Google Scholar]
- 32.Kenyon S, Pike K, Jones DR, et al. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7-year follow-up of the ORACLE II trial. Lancet. 2008;372:1319–27. doi: 10.1016/S0140-6736(08)61203-9. [DOI] [PubMed] [Google Scholar]
- 33.Kenyon SL, Taylor DJ, Tarnow-Mordi W, et al. Broad-spectrum antibiotics for spontaneous preterm labour; the ORACLE II randomised trial. Lancet. 2001;357:989–94. doi: 10.1016/s0140-6736(00)04234-3. [DOI] [PubMed] [Google Scholar]
- 34.Jimenez E, Fernandez L, Marin ML, et al. Isolation of commensal bacteria from umbilical cord blood of health neonates born by caesarean section. Curr Microbiol. 2005;51:270–74. doi: 10.1007/s00284-005-0020-3. [DOI] [PubMed] [Google Scholar]